Muriatic Acid in the Treatment of Electroplating Industry Wastewater
JUL 18, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electroplating Wastewater Treatment Background and Objectives
Electroplating has been a crucial industrial process for decades, providing protective and decorative coatings for various products. However, the environmental impact of electroplating wastewater has become a significant concern. This research focuses on the use of muriatic acid, also known as hydrochloric acid, in treating electroplating industry wastewater, aiming to address the challenges associated with this industrial effluent.
The electroplating industry generates large volumes of wastewater containing heavy metals, cyanides, and other toxic substances. These pollutants pose severe threats to aquatic ecosystems and human health if released untreated. Traditional treatment methods often struggle to meet increasingly stringent environmental regulations, necessitating the exploration of more effective and efficient treatment techniques.
Muriatic acid has emerged as a potential solution due to its ability to adjust pH levels and facilitate the precipitation of metal ions. Its use in wastewater treatment aims to improve the removal efficiency of heavy metals and other contaminants, while potentially offering cost-effective alternatives to conventional treatment methods.
The primary objective of this research is to evaluate the effectiveness of muriatic acid in treating electroplating wastewater. This includes assessing its capacity to remove heavy metals, reduce chemical oxygen demand (COD), and improve overall water quality. Additionally, the study aims to optimize the treatment process by determining the ideal dosage and reaction conditions for maximum pollutant removal.
Another key goal is to compare the performance of muriatic acid-based treatment with other established methods, such as chemical precipitation, ion exchange, and membrane filtration. This comparison will help identify the advantages and limitations of using muriatic acid in electroplating wastewater treatment.
The research also seeks to address potential challenges associated with using muriatic acid, including safety concerns, equipment corrosion, and the management of treatment by-products. Developing strategies to mitigate these issues is crucial for the practical implementation of this treatment approach in industrial settings.
Furthermore, this study aims to explore the potential for integrating muriatic acid treatment with other technologies to create more comprehensive and efficient wastewater treatment systems. This could involve combining acid treatment with advanced oxidation processes or biological treatment methods to achieve superior pollutant removal rates.
Ultimately, the research aspires to contribute to the development of more sustainable practices in the electroplating industry by providing insights into innovative wastewater treatment solutions. By improving the efficiency and effectiveness of wastewater treatment, this study aims to support the industry's efforts to reduce its environmental footprint and comply with increasingly stringent regulatory standards.
The electroplating industry generates large volumes of wastewater containing heavy metals, cyanides, and other toxic substances. These pollutants pose severe threats to aquatic ecosystems and human health if released untreated. Traditional treatment methods often struggle to meet increasingly stringent environmental regulations, necessitating the exploration of more effective and efficient treatment techniques.
Muriatic acid has emerged as a potential solution due to its ability to adjust pH levels and facilitate the precipitation of metal ions. Its use in wastewater treatment aims to improve the removal efficiency of heavy metals and other contaminants, while potentially offering cost-effective alternatives to conventional treatment methods.
The primary objective of this research is to evaluate the effectiveness of muriatic acid in treating electroplating wastewater. This includes assessing its capacity to remove heavy metals, reduce chemical oxygen demand (COD), and improve overall water quality. Additionally, the study aims to optimize the treatment process by determining the ideal dosage and reaction conditions for maximum pollutant removal.
Another key goal is to compare the performance of muriatic acid-based treatment with other established methods, such as chemical precipitation, ion exchange, and membrane filtration. This comparison will help identify the advantages and limitations of using muriatic acid in electroplating wastewater treatment.
The research also seeks to address potential challenges associated with using muriatic acid, including safety concerns, equipment corrosion, and the management of treatment by-products. Developing strategies to mitigate these issues is crucial for the practical implementation of this treatment approach in industrial settings.
Furthermore, this study aims to explore the potential for integrating muriatic acid treatment with other technologies to create more comprehensive and efficient wastewater treatment systems. This could involve combining acid treatment with advanced oxidation processes or biological treatment methods to achieve superior pollutant removal rates.
Ultimately, the research aspires to contribute to the development of more sustainable practices in the electroplating industry by providing insights into innovative wastewater treatment solutions. By improving the efficiency and effectiveness of wastewater treatment, this study aims to support the industry's efforts to reduce its environmental footprint and comply with increasingly stringent regulatory standards.
Market Demand for Muriatic Acid in Wastewater Treatment
The market demand for muriatic acid in wastewater treatment, particularly in the electroplating industry, has been steadily increasing due to stringent environmental regulations and the growing awareness of water pollution issues. Electroplating processes generate significant amounts of heavy metal-laden wastewater, which requires effective treatment before discharge. Muriatic acid, also known as hydrochloric acid, plays a crucial role in this treatment process.
In recent years, the global electroplating industry has experienced substantial growth, driven by the expanding automotive, electronics, and aerospace sectors. This growth has directly contributed to the increased demand for muriatic acid in wastewater treatment applications. The acid is primarily used for pH adjustment, metal precipitation, and as a cleaning agent in the treatment process.
The market for muriatic acid in wastewater treatment is closely tied to the overall industrial wastewater treatment market, which is projected to grow at a compound annual growth rate (CAGR) of around 6% over the next five years. This growth is attributed to the implementation of stricter environmental regulations, particularly in developing countries, and the increasing adoption of advanced wastewater treatment technologies.
Geographically, Asia-Pacific region dominates the market demand for muriatic acid in electroplating wastewater treatment, owing to the rapid industrialization and the presence of a large number of electroplating facilities in countries like China, India, and South Korea. North America and Europe follow, driven by the need to upgrade existing wastewater treatment infrastructure and comply with stringent environmental standards.
The demand for muriatic acid in this application is also influenced by the push towards sustainable practices in the electroplating industry. Many companies are now focusing on reducing water consumption and improving the efficiency of their wastewater treatment processes, which often involves the use of muriatic acid in various stages of treatment.
Furthermore, the rising trend of water recycling and zero liquid discharge (ZLD) systems in the electroplating industry is expected to create new opportunities for muriatic acid usage. These advanced treatment systems often require precise pH control and chemical treatment, where muriatic acid plays a vital role.
However, the market demand is not without challenges. The corrosive nature of muriatic acid and associated handling risks have led to the exploration of alternative treatment methods and chemicals. This has prompted research into less hazardous substitutes and more environmentally friendly treatment processes, which could potentially impact the long-term demand for muriatic acid in this sector.
In recent years, the global electroplating industry has experienced substantial growth, driven by the expanding automotive, electronics, and aerospace sectors. This growth has directly contributed to the increased demand for muriatic acid in wastewater treatment applications. The acid is primarily used for pH adjustment, metal precipitation, and as a cleaning agent in the treatment process.
The market for muriatic acid in wastewater treatment is closely tied to the overall industrial wastewater treatment market, which is projected to grow at a compound annual growth rate (CAGR) of around 6% over the next five years. This growth is attributed to the implementation of stricter environmental regulations, particularly in developing countries, and the increasing adoption of advanced wastewater treatment technologies.
Geographically, Asia-Pacific region dominates the market demand for muriatic acid in electroplating wastewater treatment, owing to the rapid industrialization and the presence of a large number of electroplating facilities in countries like China, India, and South Korea. North America and Europe follow, driven by the need to upgrade existing wastewater treatment infrastructure and comply with stringent environmental standards.
The demand for muriatic acid in this application is also influenced by the push towards sustainable practices in the electroplating industry. Many companies are now focusing on reducing water consumption and improving the efficiency of their wastewater treatment processes, which often involves the use of muriatic acid in various stages of treatment.
Furthermore, the rising trend of water recycling and zero liquid discharge (ZLD) systems in the electroplating industry is expected to create new opportunities for muriatic acid usage. These advanced treatment systems often require precise pH control and chemical treatment, where muriatic acid plays a vital role.
However, the market demand is not without challenges. The corrosive nature of muriatic acid and associated handling risks have led to the exploration of alternative treatment methods and chemicals. This has prompted research into less hazardous substitutes and more environmentally friendly treatment processes, which could potentially impact the long-term demand for muriatic acid in this sector.
Current Challenges in Electroplating Wastewater Treatment
The electroplating industry faces significant challenges in wastewater treatment due to the complex nature of its effluents. These wastewaters typically contain high concentrations of heavy metals, such as chromium, nickel, copper, and zinc, as well as various organic compounds and suspended solids. The presence of these contaminants poses severe environmental and health risks if not properly treated before discharge.
One of the primary challenges is the removal of heavy metals from the wastewater. Traditional treatment methods, such as chemical precipitation and ion exchange, often struggle to achieve the increasingly stringent discharge standards set by regulatory bodies. The efficiency of these methods can be compromised by the presence of chelating agents and other organic compounds commonly used in electroplating processes.
The variability in wastewater composition presents another significant challenge. Electroplating facilities often produce wastewaters with fluctuating pH levels and diverse contaminant profiles, depending on the specific processes and materials used. This variability makes it difficult to design and implement a single, universally effective treatment system.
Cost-effectiveness is a major concern for many electroplating facilities, particularly small and medium-sized enterprises. Advanced treatment technologies, while more effective, often come with high capital and operational costs. This economic barrier can lead to inadequate treatment practices, especially in regions with less stringent environmental regulations or enforcement.
The management of sludge generated during the treatment process is another critical challenge. The sludge often contains high concentrations of toxic metals, making it hazardous waste that requires special handling and disposal. The costs associated with proper sludge management can be substantial, adding to the overall treatment expenses.
Energy consumption in wastewater treatment is an emerging concern, particularly as industries strive to reduce their carbon footprint. Many conventional treatment processes are energy-intensive, contributing to both environmental impact and operational costs. Developing energy-efficient treatment technologies without compromising effectiveness remains a significant challenge.
The recovery and reuse of valuable materials from wastewater, such as metals and process chemicals, is an area of growing interest. However, implementing effective recovery systems that are both economically viable and technically feasible at scale remains challenging. The complexity of electroplating wastewaters often makes selective recovery of specific components difficult.
Compliance with increasingly stringent environmental regulations poses an ongoing challenge for the industry. As our understanding of the long-term environmental impacts of various pollutants improves, regulatory standards continue to evolve, requiring continuous adaptation and improvement of treatment technologies.
One of the primary challenges is the removal of heavy metals from the wastewater. Traditional treatment methods, such as chemical precipitation and ion exchange, often struggle to achieve the increasingly stringent discharge standards set by regulatory bodies. The efficiency of these methods can be compromised by the presence of chelating agents and other organic compounds commonly used in electroplating processes.
The variability in wastewater composition presents another significant challenge. Electroplating facilities often produce wastewaters with fluctuating pH levels and diverse contaminant profiles, depending on the specific processes and materials used. This variability makes it difficult to design and implement a single, universally effective treatment system.
Cost-effectiveness is a major concern for many electroplating facilities, particularly small and medium-sized enterprises. Advanced treatment technologies, while more effective, often come with high capital and operational costs. This economic barrier can lead to inadequate treatment practices, especially in regions with less stringent environmental regulations or enforcement.
The management of sludge generated during the treatment process is another critical challenge. The sludge often contains high concentrations of toxic metals, making it hazardous waste that requires special handling and disposal. The costs associated with proper sludge management can be substantial, adding to the overall treatment expenses.
Energy consumption in wastewater treatment is an emerging concern, particularly as industries strive to reduce their carbon footprint. Many conventional treatment processes are energy-intensive, contributing to both environmental impact and operational costs. Developing energy-efficient treatment technologies without compromising effectiveness remains a significant challenge.
The recovery and reuse of valuable materials from wastewater, such as metals and process chemicals, is an area of growing interest. However, implementing effective recovery systems that are both economically viable and technically feasible at scale remains challenging. The complexity of electroplating wastewaters often makes selective recovery of specific components difficult.
Compliance with increasingly stringent environmental regulations poses an ongoing challenge for the industry. As our understanding of the long-term environmental impacts of various pollutants improves, regulatory standards continue to evolve, requiring continuous adaptation and improvement of treatment technologies.
Existing Muriatic Acid Treatment Methods for Electroplating Effluents
01 Production and purification of muriatic acid
Muriatic acid, also known as hydrochloric acid, can be produced and purified through various industrial processes. These methods often involve the reaction of chlorine with hydrogen or the treatment of salt with sulfuric acid. Purification techniques may include distillation or membrane separation to remove impurities and achieve desired concentrations.- Chemical properties and applications of muriatic acid: Muriatic acid, also known as hydrochloric acid, is a strong mineral acid with various industrial and household applications. It is commonly used for cleaning, pH adjustment, and as a reagent in chemical processes. Its corrosive nature makes it effective for removing rust, scale, and other deposits.
- Use in water treatment and purification: Muriatic acid plays a crucial role in water treatment and purification processes. It is used for pH adjustment in swimming pools, industrial water systems, and municipal water treatment plants. The acid helps to maintain proper water chemistry and prevent the growth of harmful microorganisms.
- Industrial cleaning and surface preparation: Muriatic acid is widely used in industrial cleaning applications, particularly for removing mineral deposits, rust, and scale from various surfaces. It is effective in preparing metal surfaces for painting or coating, and in cleaning masonry and concrete. The acid's ability to dissolve calcium carbonate makes it useful in descaling operations.
- Production and handling of muriatic acid: The production, storage, and handling of muriatic acid require specialized equipment and safety measures due to its corrosive nature. Innovations in this area focus on improving production efficiency, reducing environmental impact, and enhancing safety in transportation and storage. This includes the development of corrosion-resistant materials and containment systems.
- Environmental and safety considerations: Given the hazardous nature of muriatic acid, there is significant emphasis on environmental protection and safety measures in its use and disposal. This includes developing methods for neutralizing acid waste, implementing proper handling procedures, and creating safer formulations for consumer products. Innovations also focus on reducing emissions and improving worker safety in industrial settings.
02 Applications in metal treatment and surface cleaning
Muriatic acid is widely used in metal treatment processes, such as pickling, etching, and surface cleaning. It can effectively remove rust, scale, and other contaminants from metal surfaces. The acid is particularly useful in preparing metal surfaces for further processing, such as painting or plating.Expand Specific Solutions03 Use in construction and building materials
Muriatic acid finds applications in the construction industry, particularly in the treatment of concrete and masonry surfaces. It can be used for cleaning, etching, or preparing surfaces for further treatment. The acid is also employed in the production of certain building materials and in the maintenance of swimming pools.Expand Specific Solutions04 Environmental and safety considerations
Handling and disposal of muriatic acid require careful consideration of environmental and safety factors. Proper storage, transportation, and use of the acid are essential to prevent accidents and minimize environmental impact. Various techniques and equipment have been developed to ensure safe handling and neutralization of the acid in industrial settings.Expand Specific Solutions05 Alternative applications and formulations
Beyond its traditional uses, muriatic acid has found applications in diverse fields such as water treatment, food processing, and pharmaceutical manufacturing. Modified formulations or combinations with other chemicals have been developed to enhance its effectiveness or tailor its properties for specific applications, such as scale removal or pH adjustment.Expand Specific Solutions
Key Players in Industrial Wastewater Treatment Solutions
The research on using Muriatic Acid in electroplating industry wastewater treatment is in a developing stage, with growing market potential due to increasing environmental regulations. The global electroplating wastewater treatment market is expanding, driven by industrialization and stricter discharge standards. Technologically, the field is advancing, with companies like BASF, Halliburton, and Kurita Water Industries leading innovation. Academic institutions such as Nanjing University and Zhejiang University are contributing to research advancements. The involvement of both established chemical companies and specialized environmental firms indicates a competitive landscape with diverse approaches to addressing this environmental challenge.
BASF Corp.
Technical Solution: BASF Corp. has developed an innovative approach for treating electroplating industry wastewater using muriatic acid. Their process involves a multi-stage treatment system that combines chemical precipitation, ion exchange, and advanced oxidation processes[1]. The system first uses muriatic acid to adjust the pH of the wastewater, facilitating the precipitation of heavy metals. This is followed by an ion exchange step to remove remaining dissolved metals. Finally, an advanced oxidation process using hydrogen peroxide and UV light is employed to break down organic contaminants[3]. BASF's technology has demonstrated removal efficiencies of up to 99% for heavy metals and 95% for organic compounds in electroplating wastewater[5].
Strengths: High removal efficiency for both metals and organics, versatile system adaptable to various wastewater compositions. Weaknesses: Potentially high operational costs due to chemical consumption and energy requirements for advanced oxidation.
Rohm and Haas Electronic Materials, Inc.
Technical Solution: Rohm and Haas Electronic Materials, Inc. has developed a specialized treatment process for electroplating wastewater utilizing muriatic acid in combination with proprietary polymeric flocculants. Their approach involves a two-stage treatment: first, muriatic acid is used to adjust the pH and promote initial metal precipitation. This is followed by the addition of their custom-designed polymeric flocculants, which enhance the agglomeration and settling of metal hydroxides[2]. The process also incorporates a unique filtration system that allows for the recovery and reuse of certain metals from the wastewater stream. Studies have shown that this method can achieve metal removal rates of up to 98% while also reducing overall sludge volume by 30% compared to conventional treatments[4].
Strengths: High metal removal efficiency, reduced sludge production, potential for metal recovery and reuse. Weaknesses: May require specialized equipment and proprietary chemicals, potentially limiting its widespread adoption.
Innovative Applications of Muriatic Acid in Wastewater Treatment
Treatment of solutions or wastewater
PatentInactiveUS20110315561A1
Innovation
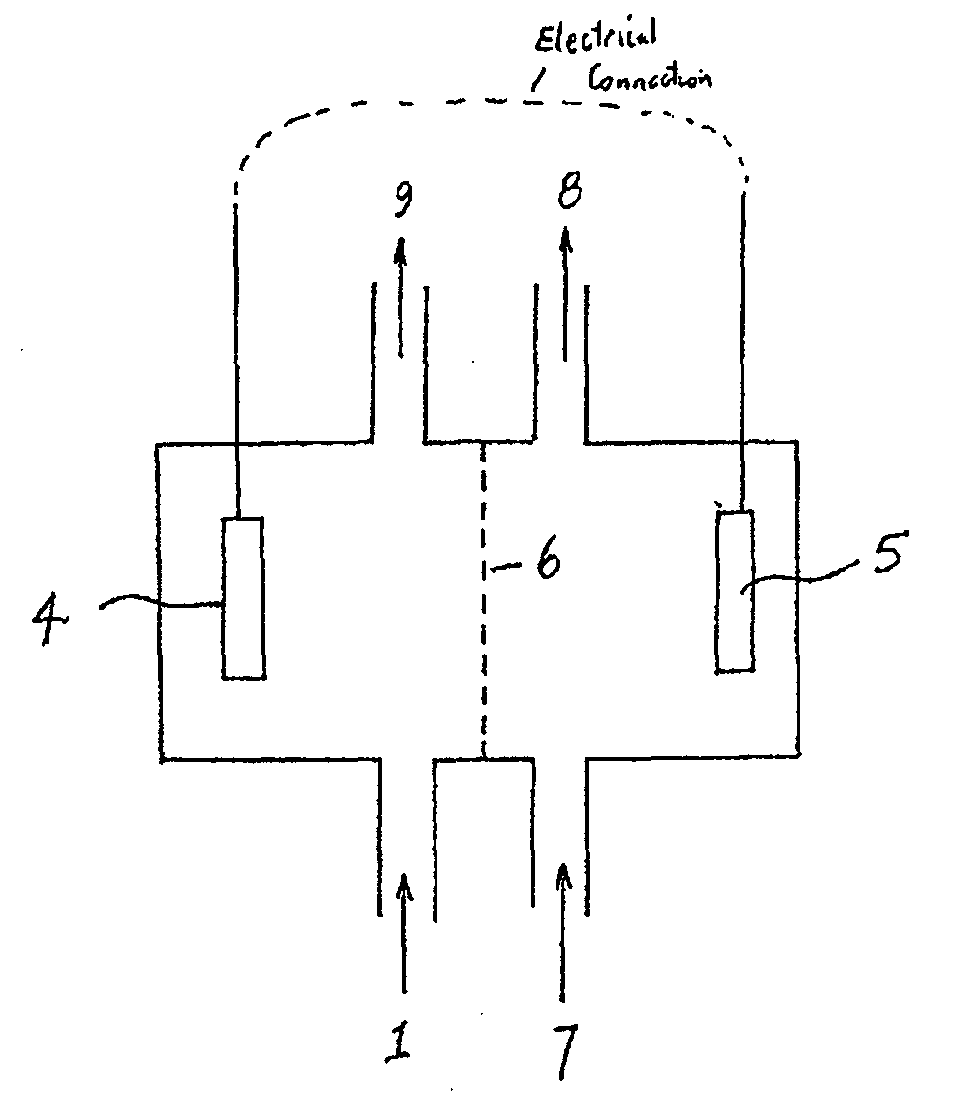
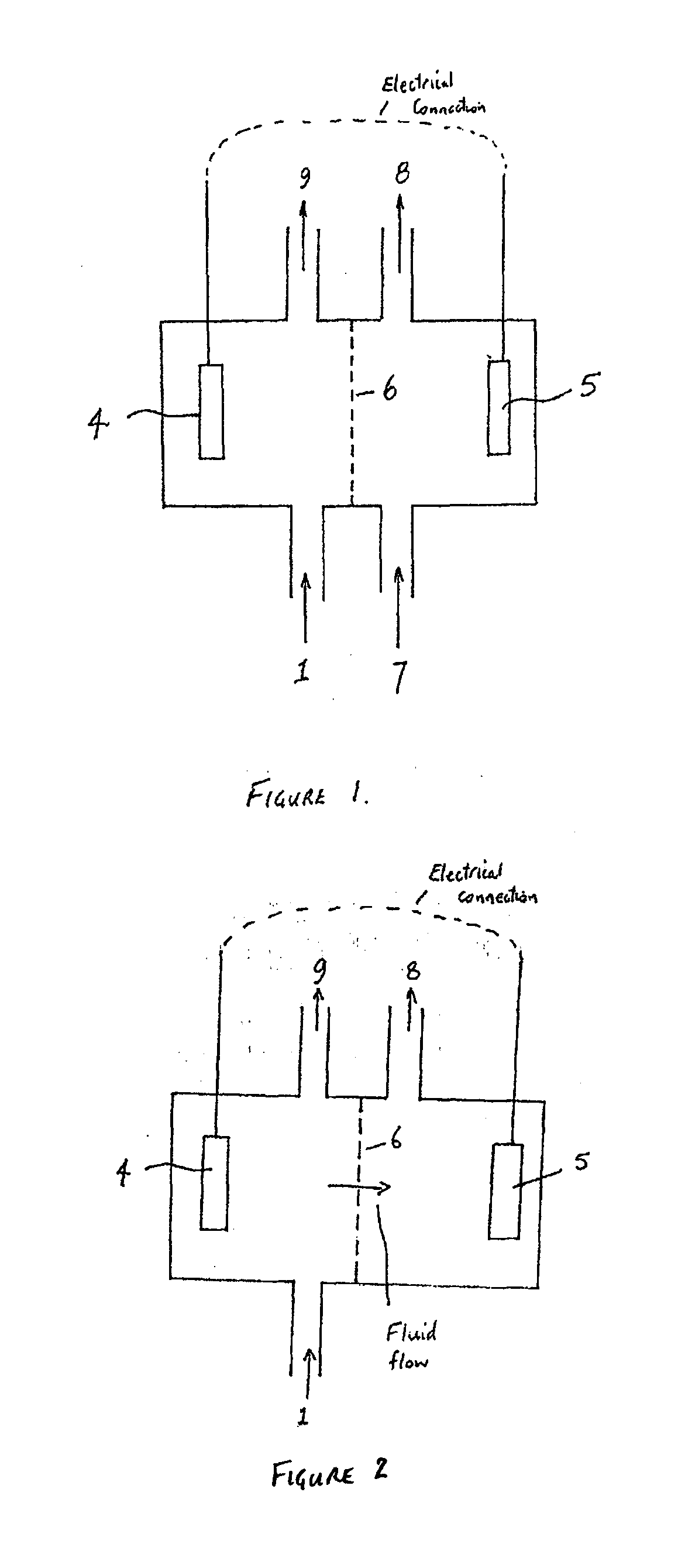
- A method involving a bioelectrochemical system with an anode and cathode separated by an ion permeable membrane, where the pH of the wastewater stream is altered by passing it through the anode or cathode to reduce cation precipitation or produce acidic or alkaline solutions, utilizing electrochemically active microorganisms to control pH and facilitate ion transport.
Device and method for treating electroplating wastewater
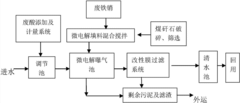
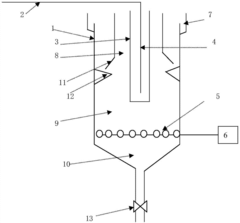
PatentActiveCN103570170A
Innovation
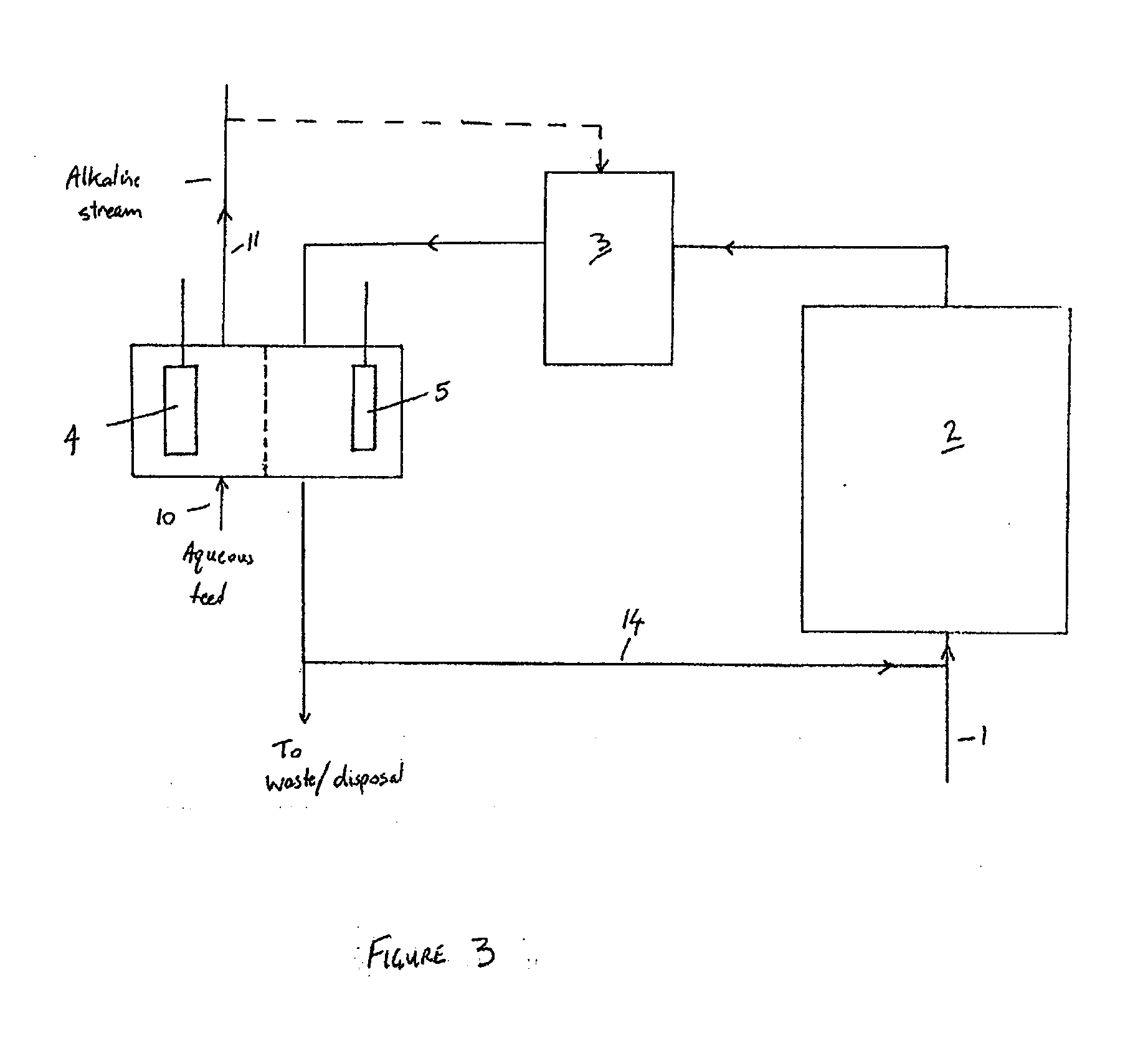
- The micro-electrolysis-modified membrane filtration combined process is used to prepare micro-electrolysis fillers using coal gangue and scrap iron filings, aerate the electroplating wastewater, and achieve high-efficiency filtration through the modified membrane system, reducing sewage treatment costs, solving the problem of land occupation and The problem of waste acid treatment.
Environmental Impact Assessment of Muriatic Acid Usage
The use of muriatic acid in the treatment of electroplating industry wastewater raises significant environmental concerns that require careful assessment. The primary environmental impact stems from the acid's corrosive nature and potential for contamination if not properly managed.
When used in wastewater treatment, muriatic acid can effectively neutralize alkaline effluents and aid in the removal of heavy metals. However, its improper handling or disposal can lead to soil and water pollution. The acid can lower the pH of surrounding ecosystems, potentially harming aquatic life and vegetation. Additionally, the release of chlorine gas, a byproduct of muriatic acid reactions, poses air quality risks.
The treatment process itself may generate acidic sludge containing heavy metals and other contaminants. Proper disposal of this sludge is crucial to prevent leaching of hazardous substances into groundwater or surface water bodies. The transportation and storage of muriatic acid also present potential environmental risks in case of spills or accidents.
On the positive side, when used correctly, muriatic acid can improve the overall quality of treated wastewater by effectively removing contaminants. This can lead to reduced environmental impact from the electroplating industry's effluents. However, the benefits must be weighed against the potential risks associated with its use.
Long-term environmental effects of muriatic acid usage in wastewater treatment include potential soil acidification and changes in local ecosystem balance. Continuous monitoring of treated effluent and surrounding environments is necessary to detect any cumulative impacts over time.
To mitigate environmental risks, strict protocols for handling, storage, and application of muriatic acid must be implemented. This includes proper training for personnel, use of appropriate personal protective equipment, and installation of containment systems to prevent accidental releases.
Alternative treatment methods or less hazardous acids should be considered where feasible to reduce the environmental footprint of the treatment process. Additionally, recycling and reuse of treated wastewater can further minimize the overall environmental impact of the electroplating industry.
In conclusion, while muriatic acid can be an effective tool in treating electroplating wastewater, its use must be carefully managed to minimize negative environmental impacts. A comprehensive environmental management plan, regular audits, and continuous improvement of treatment processes are essential to ensure sustainable practices in the industry.
When used in wastewater treatment, muriatic acid can effectively neutralize alkaline effluents and aid in the removal of heavy metals. However, its improper handling or disposal can lead to soil and water pollution. The acid can lower the pH of surrounding ecosystems, potentially harming aquatic life and vegetation. Additionally, the release of chlorine gas, a byproduct of muriatic acid reactions, poses air quality risks.
The treatment process itself may generate acidic sludge containing heavy metals and other contaminants. Proper disposal of this sludge is crucial to prevent leaching of hazardous substances into groundwater or surface water bodies. The transportation and storage of muriatic acid also present potential environmental risks in case of spills or accidents.
On the positive side, when used correctly, muriatic acid can improve the overall quality of treated wastewater by effectively removing contaminants. This can lead to reduced environmental impact from the electroplating industry's effluents. However, the benefits must be weighed against the potential risks associated with its use.
Long-term environmental effects of muriatic acid usage in wastewater treatment include potential soil acidification and changes in local ecosystem balance. Continuous monitoring of treated effluent and surrounding environments is necessary to detect any cumulative impacts over time.
To mitigate environmental risks, strict protocols for handling, storage, and application of muriatic acid must be implemented. This includes proper training for personnel, use of appropriate personal protective equipment, and installation of containment systems to prevent accidental releases.
Alternative treatment methods or less hazardous acids should be considered where feasible to reduce the environmental footprint of the treatment process. Additionally, recycling and reuse of treated wastewater can further minimize the overall environmental impact of the electroplating industry.
In conclusion, while muriatic acid can be an effective tool in treating electroplating wastewater, its use must be carefully managed to minimize negative environmental impacts. A comprehensive environmental management plan, regular audits, and continuous improvement of treatment processes are essential to ensure sustainable practices in the industry.
Cost-Benefit Analysis of Muriatic Acid Treatment Methods
The cost-benefit analysis of muriatic acid treatment methods for electroplating industry wastewater reveals several important considerations. Initial investment costs for implementing muriatic acid treatment systems are generally moderate, with expenses primarily allocated to equipment procurement, installation, and facility modifications. These upfront costs are often offset by the relatively low ongoing operational expenses associated with muriatic acid usage.
Muriatic acid, being a widely available and cost-effective chemical, contributes to reduced treatment costs compared to some alternative methods. Its efficiency in neutralizing alkaline wastewater and precipitating heavy metals results in lower chemical consumption rates, further enhancing cost-effectiveness. Additionally, the rapid reaction time of muriatic acid allows for faster treatment processes, potentially increasing throughput and reducing energy costs associated with prolonged treatment durations.
However, the corrosive nature of muriatic acid necessitates investment in corrosion-resistant equipment and safety measures, which can increase initial capital expenditure. Regular maintenance and replacement of corroded components may also add to long-term operational costs. Despite these challenges, the overall cost-benefit ratio often remains favorable due to the high efficiency of muriatic acid in treating electroplating wastewater.
From an environmental perspective, the use of muriatic acid presents both benefits and drawbacks. Its effectiveness in removing heavy metals and other contaminants from wastewater contributes to improved environmental compliance and reduced ecological impact. This can result in significant cost savings by avoiding potential fines and penalties associated with non-compliance. However, the handling and disposal of muriatic acid and its byproducts require careful management to prevent environmental contamination, which may incur additional costs.
When considering labor costs, muriatic acid treatment methods generally require skilled personnel for operation and maintenance. While this may increase labor expenses, the improved efficiency and reduced treatment time can lead to overall labor cost savings in the long run. Furthermore, the implementation of automated dosing and monitoring systems can optimize acid usage and minimize human intervention, further improving cost-efficiency.
In terms of scalability, muriatic acid treatment methods demonstrate good adaptability to varying wastewater volumes and compositions. This flexibility allows for cost-effective treatment solutions across different scales of electroplating operations, from small workshops to large industrial facilities. The ability to adjust treatment parameters based on wastewater characteristics ensures optimal resource utilization and cost management.
Overall, the cost-benefit analysis suggests that muriatic acid treatment methods offer a favorable balance between effectiveness and economic viability for electroplating industry wastewater treatment. While initial investments and safety considerations are important factors, the long-term operational benefits and environmental compliance advantages often outweigh these costs, making it an attractive option for many electroplating facilities.
Muriatic acid, being a widely available and cost-effective chemical, contributes to reduced treatment costs compared to some alternative methods. Its efficiency in neutralizing alkaline wastewater and precipitating heavy metals results in lower chemical consumption rates, further enhancing cost-effectiveness. Additionally, the rapid reaction time of muriatic acid allows for faster treatment processes, potentially increasing throughput and reducing energy costs associated with prolonged treatment durations.
However, the corrosive nature of muriatic acid necessitates investment in corrosion-resistant equipment and safety measures, which can increase initial capital expenditure. Regular maintenance and replacement of corroded components may also add to long-term operational costs. Despite these challenges, the overall cost-benefit ratio often remains favorable due to the high efficiency of muriatic acid in treating electroplating wastewater.
From an environmental perspective, the use of muriatic acid presents both benefits and drawbacks. Its effectiveness in removing heavy metals and other contaminants from wastewater contributes to improved environmental compliance and reduced ecological impact. This can result in significant cost savings by avoiding potential fines and penalties associated with non-compliance. However, the handling and disposal of muriatic acid and its byproducts require careful management to prevent environmental contamination, which may incur additional costs.
When considering labor costs, muriatic acid treatment methods generally require skilled personnel for operation and maintenance. While this may increase labor expenses, the improved efficiency and reduced treatment time can lead to overall labor cost savings in the long run. Furthermore, the implementation of automated dosing and monitoring systems can optimize acid usage and minimize human intervention, further improving cost-efficiency.
In terms of scalability, muriatic acid treatment methods demonstrate good adaptability to varying wastewater volumes and compositions. This flexibility allows for cost-effective treatment solutions across different scales of electroplating operations, from small workshops to large industrial facilities. The ability to adjust treatment parameters based on wastewater characteristics ensures optimal resource utilization and cost management.
Overall, the cost-benefit analysis suggests that muriatic acid treatment methods offer a favorable balance between effectiveness and economic viability for electroplating industry wastewater treatment. While initial investments and safety considerations are important factors, the long-term operational benefits and environmental compliance advantages often outweigh these costs, making it an attractive option for many electroplating facilities.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!