Muriatic Acid in the Treatment of Electroplating Wastewater
JUL 18, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Muriatic Acid in Electroplating Wastewater: Background and Objectives
The use of muriatic acid, also known as hydrochloric acid, in the treatment of electroplating wastewater has been a subject of significant interest in the field of industrial wastewater management. This technology has evolved over several decades, driven by the increasing need for effective and economical methods to address the environmental challenges posed by electroplating processes.
Electroplating, a widely used industrial technique for coating metal surfaces, generates wastewater containing high concentrations of heavy metals, acids, and other pollutants. The presence of these contaminants poses severe environmental and health risks if left untreated. As environmental regulations have become more stringent, the demand for advanced wastewater treatment technologies has grown substantially.
Muriatic acid has emerged as a versatile chemical agent in the treatment of electroplating wastewater due to its unique properties and effectiveness in addressing multiple aspects of the treatment process. Its ability to adjust pH levels, precipitate metals, and enhance the overall efficiency of wastewater treatment systems has made it a valuable tool in the industry's arsenal.
The historical development of muriatic acid use in electroplating wastewater treatment can be traced back to the mid-20th century when industrial waste management began to gain prominence. Initially, its application was limited to simple pH adjustment. However, as research progressed, more sophisticated uses were discovered, including its role in metal recovery and sludge reduction.
Recent technological advancements have further expanded the potential of muriatic acid in wastewater treatment. Innovations in process control, dosing systems, and integration with other treatment technologies have enhanced its effectiveness and broadened its applicability across various types of electroplating operations.
The primary objectives of using muriatic acid in electroplating wastewater treatment are multifaceted. These include achieving optimal pH levels for metal precipitation, improving the efficiency of heavy metal removal, reducing the volume of sludge generated, and potentially recovering valuable metals from the waste stream. Additionally, there is a growing focus on developing more sustainable and environmentally friendly treatment processes that minimize chemical usage and energy consumption.
As the electroplating industry continues to evolve, driven by factors such as miniaturization in electronics and the demand for more durable coatings, the role of muriatic acid in wastewater treatment is expected to adapt and expand. Research efforts are increasingly directed towards optimizing its use in conjunction with emerging technologies such as membrane filtration, advanced oxidation processes, and electrochemical treatment methods.
Electroplating, a widely used industrial technique for coating metal surfaces, generates wastewater containing high concentrations of heavy metals, acids, and other pollutants. The presence of these contaminants poses severe environmental and health risks if left untreated. As environmental regulations have become more stringent, the demand for advanced wastewater treatment technologies has grown substantially.
Muriatic acid has emerged as a versatile chemical agent in the treatment of electroplating wastewater due to its unique properties and effectiveness in addressing multiple aspects of the treatment process. Its ability to adjust pH levels, precipitate metals, and enhance the overall efficiency of wastewater treatment systems has made it a valuable tool in the industry's arsenal.
The historical development of muriatic acid use in electroplating wastewater treatment can be traced back to the mid-20th century when industrial waste management began to gain prominence. Initially, its application was limited to simple pH adjustment. However, as research progressed, more sophisticated uses were discovered, including its role in metal recovery and sludge reduction.
Recent technological advancements have further expanded the potential of muriatic acid in wastewater treatment. Innovations in process control, dosing systems, and integration with other treatment technologies have enhanced its effectiveness and broadened its applicability across various types of electroplating operations.
The primary objectives of using muriatic acid in electroplating wastewater treatment are multifaceted. These include achieving optimal pH levels for metal precipitation, improving the efficiency of heavy metal removal, reducing the volume of sludge generated, and potentially recovering valuable metals from the waste stream. Additionally, there is a growing focus on developing more sustainable and environmentally friendly treatment processes that minimize chemical usage and energy consumption.
As the electroplating industry continues to evolve, driven by factors such as miniaturization in electronics and the demand for more durable coatings, the role of muriatic acid in wastewater treatment is expected to adapt and expand. Research efforts are increasingly directed towards optimizing its use in conjunction with emerging technologies such as membrane filtration, advanced oxidation processes, and electrochemical treatment methods.
Market Demand for Efficient Electroplating Wastewater Treatment
The market demand for efficient electroplating wastewater treatment has been steadily increasing due to stricter environmental regulations and growing awareness of the harmful effects of untreated wastewater. Electroplating industries generate significant amounts of wastewater containing heavy metals, acids, and other toxic substances, which pose serious threats to ecosystems and human health if not properly treated.
In recent years, there has been a notable shift towards more sustainable and cost-effective treatment methods. The use of muriatic acid (hydrochloric acid) in electroplating wastewater treatment has gained attention due to its effectiveness in removing heavy metals and adjusting pH levels. This has led to a growing market for muriatic acid-based treatment solutions.
The global electroplating market is projected to expand, driven by increasing demand from automotive, electronics, and aerospace industries. This growth directly correlates with the demand for efficient wastewater treatment solutions. Developing countries, particularly in Asia-Pacific, are experiencing rapid industrialization, leading to a surge in electroplating activities and consequently, a higher demand for wastewater treatment technologies.
Environmental regulations worldwide are becoming more stringent, compelling electroplating companies to invest in advanced treatment systems. This regulatory pressure is a significant driver for the market demand of efficient treatment solutions, including those utilizing muriatic acid. Companies are seeking technologies that not only meet compliance standards but also offer economic benefits through resource recovery and reduced disposal costs.
The market is also influenced by the circular economy concept, where there's a growing interest in recovering valuable metals from wastewater. This trend is creating opportunities for innovative treatment processes that can effectively extract and recycle metals, providing both environmental and economic advantages.
Water scarcity in many regions is another factor boosting the demand for efficient treatment technologies. Industries are increasingly looking for solutions that allow for water reuse, reducing their overall water consumption and environmental impact. This has led to a rise in demand for advanced treatment systems capable of producing high-quality treated water suitable for reuse in industrial processes.
The COVID-19 pandemic has had a mixed impact on the market. While it initially caused disruptions in supply chains and reduced industrial activities, it has also heightened awareness about the importance of environmental protection and sustainable practices. This shift in perspective is expected to drive long-term growth in the demand for efficient wastewater treatment solutions.
In recent years, there has been a notable shift towards more sustainable and cost-effective treatment methods. The use of muriatic acid (hydrochloric acid) in electroplating wastewater treatment has gained attention due to its effectiveness in removing heavy metals and adjusting pH levels. This has led to a growing market for muriatic acid-based treatment solutions.
The global electroplating market is projected to expand, driven by increasing demand from automotive, electronics, and aerospace industries. This growth directly correlates with the demand for efficient wastewater treatment solutions. Developing countries, particularly in Asia-Pacific, are experiencing rapid industrialization, leading to a surge in electroplating activities and consequently, a higher demand for wastewater treatment technologies.
Environmental regulations worldwide are becoming more stringent, compelling electroplating companies to invest in advanced treatment systems. This regulatory pressure is a significant driver for the market demand of efficient treatment solutions, including those utilizing muriatic acid. Companies are seeking technologies that not only meet compliance standards but also offer economic benefits through resource recovery and reduced disposal costs.
The market is also influenced by the circular economy concept, where there's a growing interest in recovering valuable metals from wastewater. This trend is creating opportunities for innovative treatment processes that can effectively extract and recycle metals, providing both environmental and economic advantages.
Water scarcity in many regions is another factor boosting the demand for efficient treatment technologies. Industries are increasingly looking for solutions that allow for water reuse, reducing their overall water consumption and environmental impact. This has led to a rise in demand for advanced treatment systems capable of producing high-quality treated water suitable for reuse in industrial processes.
The COVID-19 pandemic has had a mixed impact on the market. While it initially caused disruptions in supply chains and reduced industrial activities, it has also heightened awareness about the importance of environmental protection and sustainable practices. This shift in perspective is expected to drive long-term growth in the demand for efficient wastewater treatment solutions.
Current Challenges in Electroplating Wastewater Treatment
Electroplating wastewater treatment faces numerous challenges due to the complex nature of the effluents and stringent environmental regulations. One of the primary issues is the high concentration of heavy metals, such as chromium, nickel, copper, and zinc, which are toxic to aquatic life and pose significant health risks to humans. These metals are often present in various forms, including dissolved ions and suspended particles, making their removal a complex process.
The presence of cyanide compounds in electroplating wastewater is another major concern. Cyanide is highly toxic and requires specialized treatment methods for safe removal. Traditional treatment processes often struggle to effectively eliminate cyanide without generating harmful byproducts or requiring excessive chemical inputs.
pH fluctuations in electroplating wastewater present an ongoing challenge. The wastewater can be highly acidic or alkaline, depending on the specific plating processes used. This variability complicates treatment efforts, as different pH levels can affect the solubility and removal efficiency of various contaminants. Achieving a stable and optimal pH for treatment often requires careful monitoring and adjustment.
The presence of organic compounds, such as brighteners, levelers, and other additives used in plating baths, further complicates treatment processes. These substances can interfere with conventional treatment methods and may require additional steps for their removal or breakdown.
Another significant challenge is the high electrical conductivity of electroplating wastewater, which can hinder certain treatment technologies, particularly those relying on electrochemical processes. This high conductivity can lead to increased energy consumption and reduced efficiency in treatment systems.
The variability in wastewater composition from different electroplating processes poses a challenge for designing a universal treatment system. Each plating operation may produce effluents with unique characteristics, requiring tailored treatment approaches. This variability makes it difficult to implement standardized solutions across the industry.
Emerging contaminants, such as per- and polyfluoroalkyl substances (PFAS) used in some plating processes, present new challenges in wastewater treatment. These persistent chemicals are difficult to remove using conventional methods and may require advanced treatment technologies.
Lastly, the management of sludge generated during the treatment process remains a significant challenge. This sludge often contains high concentrations of heavy metals and other hazardous substances, requiring careful handling, treatment, and disposal to prevent environmental contamination.
The presence of cyanide compounds in electroplating wastewater is another major concern. Cyanide is highly toxic and requires specialized treatment methods for safe removal. Traditional treatment processes often struggle to effectively eliminate cyanide without generating harmful byproducts or requiring excessive chemical inputs.
pH fluctuations in electroplating wastewater present an ongoing challenge. The wastewater can be highly acidic or alkaline, depending on the specific plating processes used. This variability complicates treatment efforts, as different pH levels can affect the solubility and removal efficiency of various contaminants. Achieving a stable and optimal pH for treatment often requires careful monitoring and adjustment.
The presence of organic compounds, such as brighteners, levelers, and other additives used in plating baths, further complicates treatment processes. These substances can interfere with conventional treatment methods and may require additional steps for their removal or breakdown.
Another significant challenge is the high electrical conductivity of electroplating wastewater, which can hinder certain treatment technologies, particularly those relying on electrochemical processes. This high conductivity can lead to increased energy consumption and reduced efficiency in treatment systems.
The variability in wastewater composition from different electroplating processes poses a challenge for designing a universal treatment system. Each plating operation may produce effluents with unique characteristics, requiring tailored treatment approaches. This variability makes it difficult to implement standardized solutions across the industry.
Emerging contaminants, such as per- and polyfluoroalkyl substances (PFAS) used in some plating processes, present new challenges in wastewater treatment. These persistent chemicals are difficult to remove using conventional methods and may require advanced treatment technologies.
Lastly, the management of sludge generated during the treatment process remains a significant challenge. This sludge often contains high concentrations of heavy metals and other hazardous substances, requiring careful handling, treatment, and disposal to prevent environmental contamination.
Existing Muriatic Acid-based Treatment Solutions
01 Chemical properties and applications of muriatic acid
Muriatic acid, also known as hydrochloric acid, is a strong mineral acid with various industrial and household applications. It is commonly used for cleaning, pH adjustment, and as a reagent in chemical processes. Its corrosive nature makes it effective for removing rust, scale, and other deposits.- Chemical properties and applications of muriatic acid: Muriatic acid, also known as hydrochloric acid, is a strong mineral acid with various industrial and commercial applications. It is commonly used in metal cleaning, pH adjustment, and as a chemical intermediate in manufacturing processes. Its corrosive nature and ability to dissolve certain metals make it useful in surface preparation and etching.
- Use of muriatic acid in water treatment and purification: Muriatic acid plays a crucial role in water treatment and purification processes. It is used for pH adjustment in swimming pools, industrial water systems, and municipal water treatment plants. The acid helps to control alkalinity, remove scale buildup, and improve the effectiveness of other water treatment chemicals.
- Muriatic acid in construction and building materials: In the construction industry, muriatic acid is used for cleaning and etching concrete surfaces, removing efflorescence from bricks, and preparing surfaces for painting or coating. It is also employed in the production of certain building materials and as a component in specialized construction chemicals.
- Safety measures and handling of muriatic acid: Due to its corrosive nature, proper safety measures are essential when handling muriatic acid. This includes the use of appropriate personal protective equipment, proper storage and transportation methods, and adherence to safety protocols. Specialized equipment and materials resistant to acid corrosion are often required for its storage and use.
- Environmental considerations and waste management: The use and disposal of muriatic acid require careful consideration of environmental impacts. Proper neutralization and waste management techniques are necessary to prevent environmental contamination. Recycling and recovery methods have been developed to minimize the environmental footprint of industrial processes using muriatic acid.
02 Use of muriatic acid in metal treatment and surface preparation
Muriatic acid is widely used in metal treatment processes, including pickling, etching, and surface preparation. It effectively removes oxide layers, rust, and other contaminants from metal surfaces, improving adhesion for subsequent coatings or treatments.Expand Specific Solutions03 Environmental and safety considerations in handling muriatic acid
Due to its corrosive nature, proper handling and storage of muriatic acid are crucial. Safety measures include using appropriate personal protective equipment, ensuring proper ventilation, and implementing spill containment strategies. Environmental considerations involve neutralization and proper disposal of acid waste.Expand Specific Solutions04 Muriatic acid in water treatment and purification
Muriatic acid plays a role in water treatment and purification processes. It is used for pH adjustment in swimming pools, industrial water systems, and wastewater treatment. The acid helps control alkalinity and can be used to remove scale buildup in pipes and equipment.Expand Specific Solutions05 Production and manufacturing processes of muriatic acid
Various methods are employed in the production of muriatic acid, including the reaction of sodium chloride with sulfuric acid and the direct synthesis from hydrogen and chlorine. Manufacturing processes focus on maintaining purity, concentration control, and efficient production techniques.Expand Specific Solutions
Key Players in Electroplating Wastewater Treatment Industry
The research on the use of Muriatic Acid in the Treatment of Electroplating Wastewater is in a mature stage of development, with a growing market driven by increasing environmental regulations and industrial demand. The global electroplating wastewater treatment market is expected to reach significant size in the coming years. Technologically, the field is well-established, with companies like Halliburton Energy Services, Inland Environmental Resources, and Axine Water Technologies offering advanced solutions. Academic institutions such as Nanjing University and North China Electric Power University contribute to ongoing research and innovation. The involvement of major players like China Petroleum & Chemical Corp. and BASF Corp. indicates the industry's importance and potential for further growth and technological advancements.
Nanjing University
Technical Solution: Nanjing University has developed an innovative approach for treating electroplating wastewater using muriatic acid (hydrochloric acid) in combination with advanced oxidation processes. Their method involves a two-step treatment: first, using muriatic acid for pH adjustment and metal precipitation, followed by a Fenton-like oxidation process using iron-based catalysts. This approach has shown to effectively remove heavy metals and organic contaminants from electroplating wastewater, achieving removal efficiencies of up to 99% for metals such as chromium, nickel, and copper [1][3]. The university has also explored the use of modified activated carbon adsorbents impregnated with iron chloride to enhance the adsorption capacity for residual contaminants after the initial acid treatment [2].
Strengths: High removal efficiency for both heavy metals and organic contaminants. Cost-effective due to the use of readily available muriatic acid. Weaknesses: Potential for secondary pollution if not properly managed. May require additional treatment steps for complete purification.
Suzhou Ronghe Futianbao Environmental Protection Technology Co., Ltd.
Technical Solution: Suzhou Ronghe Futianbao has developed a proprietary electrochemical treatment system that incorporates muriatic acid pretreatment for electroplating wastewater. Their process begins with a controlled addition of muriatic acid to adjust the pH and precipitate heavy metals. This is followed by an electrochemical oxidation step using dimensionally stable anodes (DSA) coated with mixed metal oxides. The company claims that this combination can achieve COD removal rates of up to 95% and heavy metal removal efficiencies exceeding 99.5% [4]. Additionally, they have implemented a closed-loop system that recovers and reuses the muriatic acid, reducing chemical consumption by up to 40% compared to conventional methods [5].
Strengths: High removal efficiency for both organic and inorganic pollutants. Closed-loop acid recovery system reduces operational costs and environmental impact. Weaknesses: High initial investment cost for electrochemical equipment. Requires skilled operators for optimal performance.
Core Innovations in Muriatic Acid Wastewater Treatment
Method for treating electroplating wastewater
PatentActiveUS12264089B1
Innovation
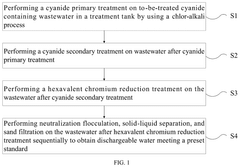
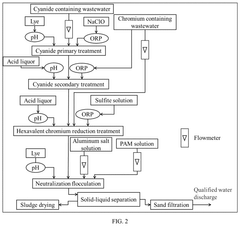
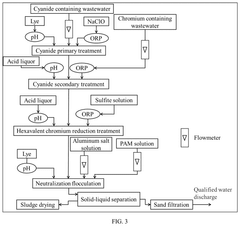
- A method involving a two-stage treatment process: cyanide primary treatment, where alkali and sodium hypochlorite are added to adjust pH and oxidation-reduction potential (ORP), followed by cyanide secondary treatment, which combines chromium treatment and hexavalent chromium reduction, using acid and sulfite solutions to adjust pH and ORP, thereby reducing hexavalent chromium to trivalent chromium.
Method of purifying waste hydrochloric acid
PatentActiveUS11472702B2
Innovation
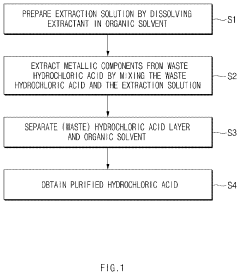
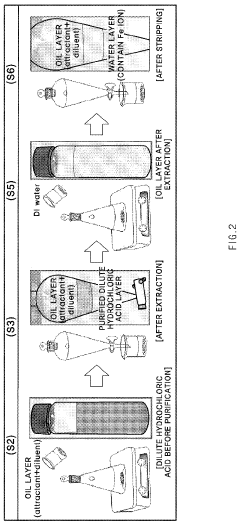
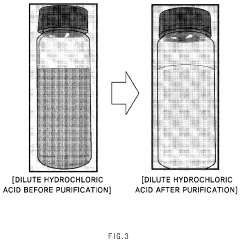
- A solvent extraction method using an organic solvent with an extractant like trioctylamine, where the waste hydrochloric acid is mixed with the extraction solution in a specific ratio, effectively separating and purifying the acid by removing metallic components, particularly iron ions, to a concentration of 1 ppm or less without the need for additional chlorine removal steps.
Environmental Impact and Regulations
The use of muriatic acid in electroplating wastewater treatment has significant environmental implications and is subject to stringent regulations. The primary environmental concern is the potential release of acidic and metal-laden effluents into water bodies, which can severely impact aquatic ecosystems and human health. Muriatic acid treatment, while effective in removing heavy metals, can also lead to the formation of toxic by-products if not properly managed.
Regulatory bodies worldwide have established strict guidelines for the handling and disposal of electroplating wastewater. In the United States, the Environmental Protection Agency (EPA) enforces the Clean Water Act, which sets specific limits on the discharge of pollutants from industrial sources. The National Pollutant Discharge Elimination System (NPDES) permit program regulates point source discharges, including those from electroplating facilities.
The European Union's Water Framework Directive and the Industrial Emissions Directive provide comprehensive frameworks for water protection and industrial pollution control. These regulations mandate the use of Best Available Techniques (BAT) in wastewater treatment, which often include advanced oxidation processes and membrane filtration in addition to chemical treatments like muriatic acid.
Compliance with these regulations requires careful monitoring and control of pH levels, metal concentrations, and other parameters in treated wastewater. Facilities must implement robust treatment systems and maintain detailed records of their wastewater management practices. Regular testing and reporting are mandatory to ensure ongoing compliance with discharge limits.
The use of muriatic acid also raises occupational health and safety concerns. Workers handling this corrosive substance must be provided with appropriate personal protective equipment and training. Facilities must adhere to safety standards set by organizations such as the Occupational Safety and Health Administration (OSHA) in the US or the European Agency for Safety and Health at Work (EU-OSHA) in Europe.
As environmental awareness grows, there is an increasing trend towards more sustainable wastewater treatment methods. This has led to research into alternatives to muriatic acid, such as biological treatment processes and electrocoagulation. Regulatory bodies are encouraging the adoption of these more environmentally friendly technologies through incentives and stricter emission standards.
The global nature of environmental concerns has also led to international agreements like the Basel Convention on the Control of Transboundary Movements of Hazardous Wastes and their Disposal. This treaty aims to reduce the movement of hazardous waste between nations, particularly from developed to less developed countries, and has implications for the management of electroplating waste.
Regulatory bodies worldwide have established strict guidelines for the handling and disposal of electroplating wastewater. In the United States, the Environmental Protection Agency (EPA) enforces the Clean Water Act, which sets specific limits on the discharge of pollutants from industrial sources. The National Pollutant Discharge Elimination System (NPDES) permit program regulates point source discharges, including those from electroplating facilities.
The European Union's Water Framework Directive and the Industrial Emissions Directive provide comprehensive frameworks for water protection and industrial pollution control. These regulations mandate the use of Best Available Techniques (BAT) in wastewater treatment, which often include advanced oxidation processes and membrane filtration in addition to chemical treatments like muriatic acid.
Compliance with these regulations requires careful monitoring and control of pH levels, metal concentrations, and other parameters in treated wastewater. Facilities must implement robust treatment systems and maintain detailed records of their wastewater management practices. Regular testing and reporting are mandatory to ensure ongoing compliance with discharge limits.
The use of muriatic acid also raises occupational health and safety concerns. Workers handling this corrosive substance must be provided with appropriate personal protective equipment and training. Facilities must adhere to safety standards set by organizations such as the Occupational Safety and Health Administration (OSHA) in the US or the European Agency for Safety and Health at Work (EU-OSHA) in Europe.
As environmental awareness grows, there is an increasing trend towards more sustainable wastewater treatment methods. This has led to research into alternatives to muriatic acid, such as biological treatment processes and electrocoagulation. Regulatory bodies are encouraging the adoption of these more environmentally friendly technologies through incentives and stricter emission standards.
The global nature of environmental concerns has also led to international agreements like the Basel Convention on the Control of Transboundary Movements of Hazardous Wastes and their Disposal. This treaty aims to reduce the movement of hazardous waste between nations, particularly from developed to less developed countries, and has implications for the management of electroplating waste.
Cost-Benefit Analysis of Muriatic Acid Treatment
The cost-benefit analysis of muriatic acid treatment in electroplating wastewater management is a critical consideration for industries seeking efficient and economical solutions. This analysis encompasses both direct and indirect costs associated with the implementation of muriatic acid treatment systems, as well as the potential benefits derived from improved wastewater quality and regulatory compliance.
Initial capital expenditures for muriatic acid treatment systems include equipment costs such as storage tanks, dosing pumps, and monitoring devices. These upfront investments can vary significantly based on the scale of operations and the specific requirements of the electroplating facility. Additionally, installation and integration costs must be factored into the initial outlay.
Operational expenses constitute a substantial portion of the ongoing costs. The price of muriatic acid itself is a primary consideration, subject to market fluctuations and supply chain dynamics. Labor costs for system operation and maintenance, as well as energy consumption for pumping and mixing processes, contribute to the recurring expenses. Regular equipment maintenance and potential replacement of components also factor into the long-term cost structure.
On the benefits side, muriatic acid treatment offers several advantages that can offset its costs. Improved wastewater quality leads to reduced environmental impact and lower risk of regulatory non-compliance penalties. This can result in significant cost savings over time, particularly in regions with stringent environmental regulations.
The efficiency of muriatic acid in metal removal from electroplating wastewater can lead to decreased sludge production. This reduction in waste volume translates to lower disposal costs and potentially creates opportunities for metal recovery and recycling, offering additional revenue streams.
Enhanced treatment efficacy may also allow for water reuse within the electroplating process, reducing overall water consumption and associated costs. This aspect becomes increasingly valuable in water-scarce regions or where water prices are high.
When evaluating the cost-benefit ratio, it is essential to consider the long-term environmental and regulatory landscape. As environmental standards become more stringent, investments in effective treatment technologies like muriatic acid systems may prove to be economically advantageous in the long run, despite higher initial costs.
The scalability of muriatic acid treatment systems allows for flexibility in implementation, potentially offering a phased approach to investment that aligns with business growth and changing regulatory requirements. This adaptability can be a significant factor in the overall cost-benefit analysis, particularly for small to medium-sized enterprises.
In conclusion, while the implementation of muriatic acid treatment for electroplating wastewater involves substantial costs, the potential benefits in terms of regulatory compliance, resource efficiency, and environmental stewardship can provide a compelling economic case for its adoption. A thorough, site-specific analysis is crucial for accurate assessment of the cost-benefit ratio in each unique industrial context.
Initial capital expenditures for muriatic acid treatment systems include equipment costs such as storage tanks, dosing pumps, and monitoring devices. These upfront investments can vary significantly based on the scale of operations and the specific requirements of the electroplating facility. Additionally, installation and integration costs must be factored into the initial outlay.
Operational expenses constitute a substantial portion of the ongoing costs. The price of muriatic acid itself is a primary consideration, subject to market fluctuations and supply chain dynamics. Labor costs for system operation and maintenance, as well as energy consumption for pumping and mixing processes, contribute to the recurring expenses. Regular equipment maintenance and potential replacement of components also factor into the long-term cost structure.
On the benefits side, muriatic acid treatment offers several advantages that can offset its costs. Improved wastewater quality leads to reduced environmental impact and lower risk of regulatory non-compliance penalties. This can result in significant cost savings over time, particularly in regions with stringent environmental regulations.
The efficiency of muriatic acid in metal removal from electroplating wastewater can lead to decreased sludge production. This reduction in waste volume translates to lower disposal costs and potentially creates opportunities for metal recovery and recycling, offering additional revenue streams.
Enhanced treatment efficacy may also allow for water reuse within the electroplating process, reducing overall water consumption and associated costs. This aspect becomes increasingly valuable in water-scarce regions or where water prices are high.
When evaluating the cost-benefit ratio, it is essential to consider the long-term environmental and regulatory landscape. As environmental standards become more stringent, investments in effective treatment technologies like muriatic acid systems may prove to be economically advantageous in the long run, despite higher initial costs.
The scalability of muriatic acid treatment systems allows for flexibility in implementation, potentially offering a phased approach to investment that aligns with business growth and changing regulatory requirements. This adaptability can be a significant factor in the overall cost-benefit analysis, particularly for small to medium-sized enterprises.
In conclusion, while the implementation of muriatic acid treatment for electroplating wastewater involves substantial costs, the potential benefits in terms of regulatory compliance, resource efficiency, and environmental stewardship can provide a compelling economic case for its adoption. A thorough, site-specific analysis is crucial for accurate assessment of the cost-benefit ratio in each unique industrial context.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!