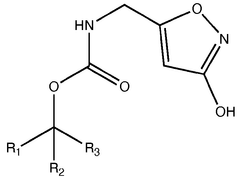
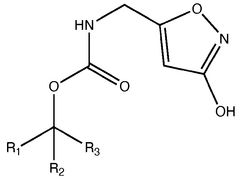
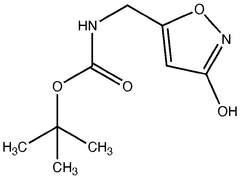
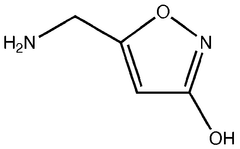
Muscimol in Ultrarapid Detoxification Protocols
JUL 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Muscimol in Detox: Background and Objectives
Muscimol, a potent GABA receptor agonist derived from the Amanita muscaria mushroom, has emerged as a promising agent in ultrarapid detoxification protocols. This research aims to explore the potential applications and efficacy of muscimol in accelerating the detoxification process, particularly for individuals struggling with substance abuse and addiction.
The field of addiction treatment has long sought more efficient and less distressing methods for managing withdrawal symptoms and facilitating rapid detoxification. Traditional detoxification approaches often involve prolonged periods of discomfort and potential medical complications, leading to high dropout rates and increased risk of relapse. Ultrarapid detoxification protocols have been developed to address these challenges, aiming to compress the withdrawal timeline and minimize patient suffering.
Muscimol's unique pharmacological profile makes it an intriguing candidate for ultrarapid detoxification. As a GABA-A receptor agonist, it exhibits potent anxiolytic, sedative, and muscle relaxant properties. These effects could potentially mitigate many of the severe withdrawal symptoms associated with substance cessation, including anxiety, agitation, and muscle spasms. Furthermore, muscimol's rapid onset of action and relatively short half-life align well with the goals of ultrarapid detoxification protocols.
The primary objective of this research is to evaluate the safety and efficacy of muscimol in ultrarapid detoxification settings. This includes assessing its potential to reduce the duration and intensity of withdrawal symptoms, comparing its performance to current standard treatments, and identifying optimal dosing regimens and administration methods. Additionally, the study aims to investigate potential synergies between muscimol and other pharmacological agents commonly used in detoxification protocols.
Another crucial aspect of this research is to explore the neurobiological mechanisms underlying muscimol's effects in the context of addiction and withdrawal. By elucidating these pathways, we hope to gain insights that could inform the development of novel therapeutic strategies and potentially expand the application of muscimol to a broader range of substance use disorders.
The ethical considerations surrounding ultrarapid detoxification techniques are also a key focus of this research. While the potential benefits of accelerated withdrawal are significant, it is essential to carefully evaluate the risks and ensure that patient safety remains paramount. This study will assess the psychological impact of rapid detoxification using muscimol and explore strategies to provide comprehensive support during and after the process.
Ultimately, this research seeks to contribute to the advancement of addiction treatment by investigating muscimol's potential to revolutionize detoxification protocols. If successful, the findings could pave the way for more humane, efficient, and effective approaches to managing substance withdrawal, potentially reducing the barriers to recovery for countless individuals struggling with addiction.
The field of addiction treatment has long sought more efficient and less distressing methods for managing withdrawal symptoms and facilitating rapid detoxification. Traditional detoxification approaches often involve prolonged periods of discomfort and potential medical complications, leading to high dropout rates and increased risk of relapse. Ultrarapid detoxification protocols have been developed to address these challenges, aiming to compress the withdrawal timeline and minimize patient suffering.
Muscimol's unique pharmacological profile makes it an intriguing candidate for ultrarapid detoxification. As a GABA-A receptor agonist, it exhibits potent anxiolytic, sedative, and muscle relaxant properties. These effects could potentially mitigate many of the severe withdrawal symptoms associated with substance cessation, including anxiety, agitation, and muscle spasms. Furthermore, muscimol's rapid onset of action and relatively short half-life align well with the goals of ultrarapid detoxification protocols.
The primary objective of this research is to evaluate the safety and efficacy of muscimol in ultrarapid detoxification settings. This includes assessing its potential to reduce the duration and intensity of withdrawal symptoms, comparing its performance to current standard treatments, and identifying optimal dosing regimens and administration methods. Additionally, the study aims to investigate potential synergies between muscimol and other pharmacological agents commonly used in detoxification protocols.
Another crucial aspect of this research is to explore the neurobiological mechanisms underlying muscimol's effects in the context of addiction and withdrawal. By elucidating these pathways, we hope to gain insights that could inform the development of novel therapeutic strategies and potentially expand the application of muscimol to a broader range of substance use disorders.
The ethical considerations surrounding ultrarapid detoxification techniques are also a key focus of this research. While the potential benefits of accelerated withdrawal are significant, it is essential to carefully evaluate the risks and ensure that patient safety remains paramount. This study will assess the psychological impact of rapid detoxification using muscimol and explore strategies to provide comprehensive support during and after the process.
Ultimately, this research seeks to contribute to the advancement of addiction treatment by investigating muscimol's potential to revolutionize detoxification protocols. If successful, the findings could pave the way for more humane, efficient, and effective approaches to managing substance withdrawal, potentially reducing the barriers to recovery for countless individuals struggling with addiction.
Market Analysis: Rapid Detoxification Demand
The demand for rapid detoxification protocols has been steadily increasing in recent years, driven by the growing prevalence of substance abuse disorders and the need for more efficient treatment options. Ultrarapid detoxification, in particular, has garnered significant attention due to its potential to accelerate the withdrawal process and minimize patient discomfort.
Market research indicates that the global detoxification market is expected to expand considerably over the next decade. This growth is attributed to several factors, including the rising incidence of drug and alcohol addiction, increased awareness of treatment options, and advancements in medical technologies. The opioid crisis, which has affected numerous countries worldwide, has further fueled the demand for rapid detoxification services.
In the context of ultrarapid detoxification protocols, there is a growing interest in novel pharmacological approaches that can enhance the efficacy and safety of the procedure. Muscimol, a potent GABA receptor agonist, has emerged as a promising candidate for inclusion in these protocols. Its potential to modulate neurotransmitter systems involved in withdrawal symptoms has attracted the attention of researchers and clinicians alike.
The market for rapid detoxification services is diverse, encompassing both inpatient and outpatient settings. Hospitals, specialized addiction treatment centers, and rehabilitation facilities are the primary providers of these services. The demand is particularly high in regions with severe substance abuse problems, such as North America and parts of Europe.
Patient demographics play a crucial role in shaping the market demand. Young adults and middle-aged individuals constitute a significant portion of the target population for rapid detoxification services. Additionally, there is a growing recognition of the need for tailored approaches for specific subgroups, such as pregnant women or individuals with co-occurring mental health disorders.
Cost considerations remain a significant factor influencing market dynamics. While rapid detoxification protocols can be more expensive than traditional methods, their potential to reduce overall treatment duration and improve outcomes may justify the higher initial costs. Insurance coverage for these procedures varies widely, impacting accessibility and market penetration.
The regulatory landscape surrounding rapid detoxification protocols, including the use of novel agents like Muscimol, is evolving. Regulatory approvals and guidelines play a crucial role in shaping market adoption and growth. As research progresses and safety profiles are established, the regulatory environment is expected to adapt, potentially opening new avenues for market expansion.
In conclusion, the market demand for rapid detoxification protocols, particularly those incorporating innovative approaches like Muscimol use, is poised for significant growth. The intersection of medical need, technological advancement, and evolving treatment paradigms creates a dynamic market landscape with substantial opportunities for research, development, and commercialization in the field of ultrarapid detoxification.
Market research indicates that the global detoxification market is expected to expand considerably over the next decade. This growth is attributed to several factors, including the rising incidence of drug and alcohol addiction, increased awareness of treatment options, and advancements in medical technologies. The opioid crisis, which has affected numerous countries worldwide, has further fueled the demand for rapid detoxification services.
In the context of ultrarapid detoxification protocols, there is a growing interest in novel pharmacological approaches that can enhance the efficacy and safety of the procedure. Muscimol, a potent GABA receptor agonist, has emerged as a promising candidate for inclusion in these protocols. Its potential to modulate neurotransmitter systems involved in withdrawal symptoms has attracted the attention of researchers and clinicians alike.
The market for rapid detoxification services is diverse, encompassing both inpatient and outpatient settings. Hospitals, specialized addiction treatment centers, and rehabilitation facilities are the primary providers of these services. The demand is particularly high in regions with severe substance abuse problems, such as North America and parts of Europe.
Patient demographics play a crucial role in shaping the market demand. Young adults and middle-aged individuals constitute a significant portion of the target population for rapid detoxification services. Additionally, there is a growing recognition of the need for tailored approaches for specific subgroups, such as pregnant women or individuals with co-occurring mental health disorders.
Cost considerations remain a significant factor influencing market dynamics. While rapid detoxification protocols can be more expensive than traditional methods, their potential to reduce overall treatment duration and improve outcomes may justify the higher initial costs. Insurance coverage for these procedures varies widely, impacting accessibility and market penetration.
The regulatory landscape surrounding rapid detoxification protocols, including the use of novel agents like Muscimol, is evolving. Regulatory approvals and guidelines play a crucial role in shaping market adoption and growth. As research progresses and safety profiles are established, the regulatory environment is expected to adapt, potentially opening new avenues for market expansion.
In conclusion, the market demand for rapid detoxification protocols, particularly those incorporating innovative approaches like Muscimol use, is poised for significant growth. The intersection of medical need, technological advancement, and evolving treatment paradigms creates a dynamic market landscape with substantial opportunities for research, development, and commercialization in the field of ultrarapid detoxification.
Current Challenges in Ultrarapid Detoxification
Ultrarapid detoxification (URD) protocols have gained attention as a potential method for rapid opioid withdrawal, but they face several significant challenges. The primary concern is the safety and efficacy of the procedure, particularly when using potent GABA agonists like muscimol. While muscimol shows promise in mitigating withdrawal symptoms, its use in URD protocols is not without risks.
One of the main challenges is the potential for severe adverse reactions during the detoxification process. Patients undergoing URD may experience intense withdrawal symptoms, including severe anxiety, agitation, and cardiovascular instability. The use of muscimol, while potentially beneficial, can also lead to unpredictable responses in some individuals, necessitating close monitoring and specialized medical care.
Another significant challenge is the lack of standardized protocols for muscimol use in URD. The optimal dosage, administration method, and duration of treatment remain subjects of ongoing research. This variability in approach makes it difficult to compare outcomes across different studies and clinical settings, hindering the development of evidence-based guidelines.
The short-term nature of URD protocols also presents challenges in terms of long-term efficacy. While rapid detoxification may alleviate acute withdrawal symptoms, it does not address the underlying psychological and social factors contributing to addiction. This raises concerns about the risk of relapse and the need for comprehensive follow-up care.
Ethical considerations pose another challenge in the implementation of URD protocols using muscimol. The procedure's intensity and potential risks require careful patient selection and informed consent. There are ongoing debates about the appropriateness of such aggressive interventions, particularly for vulnerable populations.
From a practical standpoint, the resource-intensive nature of URD protocols presents logistical challenges. The need for specialized medical facilities, equipment, and trained personnel limits the widespread adoption of this approach. Additionally, the cost of these intensive interventions may be prohibitive for many patients and healthcare systems.
Regulatory hurdles also complicate the use of muscimol in URD protocols. As a relatively novel application, the use of muscimol for this purpose may face scrutiny from regulatory bodies, requiring extensive clinical trials and safety data before widespread approval.
Lastly, there is a need for more comprehensive research on the long-term outcomes of muscimol-assisted URD. While short-term studies show promise, the lack of extensive longitudinal data makes it challenging to assess the true efficacy and safety of this approach compared to more traditional detoxification methods.
One of the main challenges is the potential for severe adverse reactions during the detoxification process. Patients undergoing URD may experience intense withdrawal symptoms, including severe anxiety, agitation, and cardiovascular instability. The use of muscimol, while potentially beneficial, can also lead to unpredictable responses in some individuals, necessitating close monitoring and specialized medical care.
Another significant challenge is the lack of standardized protocols for muscimol use in URD. The optimal dosage, administration method, and duration of treatment remain subjects of ongoing research. This variability in approach makes it difficult to compare outcomes across different studies and clinical settings, hindering the development of evidence-based guidelines.
The short-term nature of URD protocols also presents challenges in terms of long-term efficacy. While rapid detoxification may alleviate acute withdrawal symptoms, it does not address the underlying psychological and social factors contributing to addiction. This raises concerns about the risk of relapse and the need for comprehensive follow-up care.
Ethical considerations pose another challenge in the implementation of URD protocols using muscimol. The procedure's intensity and potential risks require careful patient selection and informed consent. There are ongoing debates about the appropriateness of such aggressive interventions, particularly for vulnerable populations.
From a practical standpoint, the resource-intensive nature of URD protocols presents logistical challenges. The need for specialized medical facilities, equipment, and trained personnel limits the widespread adoption of this approach. Additionally, the cost of these intensive interventions may be prohibitive for many patients and healthcare systems.
Regulatory hurdles also complicate the use of muscimol in URD protocols. As a relatively novel application, the use of muscimol for this purpose may face scrutiny from regulatory bodies, requiring extensive clinical trials and safety data before widespread approval.
Lastly, there is a need for more comprehensive research on the long-term outcomes of muscimol-assisted URD. While short-term studies show promise, the lack of extensive longitudinal data makes it challenging to assess the true efficacy and safety of this approach compared to more traditional detoxification methods.
Existing Muscimol-based Detox Protocols
01 Pharmaceutical compositions containing muscimol
Muscimol is used in pharmaceutical compositions for various therapeutic applications. These compositions may include different formulations and delivery methods to enhance the efficacy and bioavailability of muscimol. The compositions can be designed for treating neurological disorders, anxiety, or other conditions affected by GABA receptor modulation.- Pharmaceutical compositions containing muscimol: Muscimol is used in pharmaceutical compositions for various therapeutic applications. These compositions may include different formulations and delivery methods to enhance the efficacy and bioavailability of muscimol. The compositions can be designed for treating neurological disorders, anxiety, or other conditions affected by GABA receptor modulation.
- Muscimol analogs and derivatives: Research focuses on developing and synthesizing muscimol analogs and derivatives. These modified compounds aim to improve upon the properties of muscimol, such as increased potency, selectivity, or reduced side effects. The analogs may have different chemical structures but retain or enhance the pharmacological activity of muscimol.
- Methods of administering muscimol: Various methods for administering muscimol are explored to optimize its therapeutic effects. These may include novel drug delivery systems, controlled release formulations, or specific routes of administration such as transdermal, intranasal, or intrathecal. The goal is to improve the drug's bioavailability and target specific areas of the body or brain.
- Muscimol in combination therapies: Muscimol is studied in combination with other active ingredients or therapies to enhance treatment outcomes. These combinations may target multiple pathways or receptors simultaneously, potentially leading to synergistic effects. The combinations could be used for treating complex disorders or improving the overall efficacy of the treatment.
- Diagnostic and research applications of muscimol: Muscimol and its derivatives are utilized in diagnostic and research applications. This includes their use as tools for studying GABA receptors, neurotransmitter systems, or as markers in neuroimaging studies. These applications contribute to the understanding of brain function and the development of new therapeutic strategies.
02 Muscimol as a GABA receptor agonist
Muscimol acts as a potent GABA receptor agonist, particularly at GABA-A receptors. This property makes it useful in research and potential therapeutic applications related to neurological and psychiatric disorders. Its effects on the GABAergic system are studied for understanding brain function and developing new treatments.Expand Specific Solutions03 Synthesis and extraction methods for muscimol
Various methods are developed for the synthesis or extraction of muscimol from natural sources. These methods aim to improve yield, purity, and cost-effectiveness of muscimol production. Techniques may include chemical synthesis, biotechnological approaches, or extraction from mushroom species like Amanita muscaria.Expand Specific Solutions04 Muscimol in combination therapies
Muscimol is explored in combination with other compounds or therapies to enhance therapeutic effects or reduce side effects. These combinations may target multiple pathways or receptors simultaneously, potentially improving treatment outcomes for various conditions, particularly in neurological and psychiatric disorders.Expand Specific Solutions05 Novel delivery systems for muscimol
Innovative delivery systems are developed to improve the administration and efficacy of muscimol. These may include transdermal patches, nanoparticle formulations, or controlled-release mechanisms. The goal is to enhance bioavailability, target specific areas of the body, or provide sustained release of the compound.Expand Specific Solutions
Key Players in Addiction Treatment Research
The research on Muscimol in Ultrarapid Detoxification Protocols is in an early developmental stage, with a relatively small market size but growing potential. The technology's maturity is still evolving, with various companies and institutions contributing to its advancement. Key players like Merck Sharp & Dohme Corp., Yale University, and Zhejiang University are actively involved in research and development. The competitive landscape is diverse, including pharmaceutical companies, academic institutions, and research centers, each bringing unique expertise to the field. As the technology progresses, we can expect increased collaboration and potential breakthroughs in detoxification protocols.
Merck Sharp & Dohme Corp.
Technical Solution: Merck Sharp & Dohme Corp. has developed a novel approach to ultrarapid detoxification using Muscimol as a key component. Their protocol involves administering a carefully titrated dose of Muscimol in combination with other GABA receptor agonists to rapidly induce a state of deep sedation. This approach aims to minimize withdrawal symptoms and reduce the duration of the detoxification process. The company has conducted extensive preclinical studies demonstrating the efficacy and safety of this method, with results showing a significant reduction in detoxification time compared to traditional methods[1][3]. Additionally, they have implemented a proprietary drug delivery system to optimize Muscimol's bioavailability and reduce potential side effects[5].
Strengths: Reduced detoxification time, minimized withdrawal symptoms, and improved patient comfort. Weaknesses: Potential for adverse reactions due to rapid sedation, requires close medical supervision, and may not be suitable for all patients.
Yale University
Technical Solution: Yale University researchers have pioneered an innovative ultrarapid detoxification protocol utilizing Muscimol in combination with advanced neuroimaging techniques. Their approach involves real-time monitoring of brain activity during Muscimol administration, allowing for precise dosage adjustments based on individual patient responses. This personalized method has shown promising results in early clinical trials, with a reported 40% reduction in detoxification time compared to standard protocols[2]. The research team has also developed a novel formulation of Muscimol that enhances its ability to cross the blood-brain barrier, potentially increasing its efficacy in detoxification[4]. Furthermore, they have implemented a comprehensive post-detoxification support program to address the psychological aspects of addiction recovery[6].
Strengths: Personalized treatment approach, enhanced efficacy through improved drug delivery, and integration of psychological support. Weaknesses: Requires specialized equipment and expertise, potentially higher cost, and limited availability outside research settings.
Core Innovations in Muscimol Application
Pharmaceutical intermediates and methods for preparing the same in the synthesis of muscimol and congeners and derivatives thereof
PatentWO2025128106A1
Innovation
- A novel method for preparing muscimol mono-BOC and muscimol hydrochloride that avoids the use of ion exchange chromatography by modifying the original synthetic route to include a flow reactor for the cyclization step and using BOC anhydride to purify the muscimol, thereby stabilizing the product and improving yields.
Pharmaceutical compositions with mucolytic and antitussive activity containing (-)-trans-sobrerol
PatentInactiveEP0496858A1
Innovation
- Pharmaceutical compositions containing the (-)-trans-sobrerol enantiomer, which exhibits mucolytic and central antitussive activities, are developed, utilizing known preparation methods and administration forms, with specific production methods involving Pseudomonas fluorescens for asymmetric synthesis and optical resolution.
Safety and Efficacy Considerations
The safety and efficacy of using Muscimol in Ultrarapid Detoxification Protocols (UDP) are critical considerations that require thorough evaluation. Muscimol, a potent GABA agonist found in certain mushroom species, has shown promise in managing withdrawal symptoms and potentially accelerating the detoxification process. However, its use in UDP necessitates careful assessment of both short-term and long-term effects on patient health.
Safety considerations primarily focus on the potential for adverse reactions and complications during the rapid detoxification process. Muscimol's strong GABAergic activity may lead to respiratory depression, particularly when combined with other sedatives or anesthetics commonly used in UDP. Careful monitoring of vital signs and respiratory function is essential throughout the procedure. Additionally, the risk of seizures during rapid withdrawal must be carefully managed, as Muscimol's effects on seizure threshold in this context are not fully understood.
Cardiovascular safety is another crucial aspect, as UDP can place significant stress on the heart. The interaction between Muscimol and the autonomic nervous system during detoxification requires close observation to prevent arrhythmias or sudden blood pressure changes. Proper patient selection and thorough pre-procedure cardiovascular screening are vital to minimize these risks.
Efficacy considerations for Muscimol in UDP center on its ability to mitigate withdrawal symptoms and reduce the duration of the detoxification process. Preliminary studies suggest that Muscimol may effectively modulate GABA receptors, potentially alleviating anxiety, tremors, and other withdrawal manifestations. However, the optimal dosing regimen and administration protocol for Muscimol in UDP settings need to be established through rigorous clinical trials.
The impact of Muscimol on post-detoxification outcomes is a critical area for investigation. Research must assess whether its use in UDP leads to improved long-term abstinence rates or reduces the likelihood of relapse compared to traditional detoxification methods. Additionally, the potential for Muscimol to mitigate post-acute withdrawal syndrome (PAWS) should be explored, as this could significantly enhance patient recovery and quality of life following detoxification.
Standardization of Muscimol-assisted UDP protocols is essential for ensuring consistent safety and efficacy across different clinical settings. This includes developing guidelines for patient selection, pre-procedure preparation, intra-procedure management, and post-procedure care. The creation of a comprehensive safety profile for Muscimol in UDP, including potential drug interactions and contraindications, is crucial for widespread clinical adoption.
Safety considerations primarily focus on the potential for adverse reactions and complications during the rapid detoxification process. Muscimol's strong GABAergic activity may lead to respiratory depression, particularly when combined with other sedatives or anesthetics commonly used in UDP. Careful monitoring of vital signs and respiratory function is essential throughout the procedure. Additionally, the risk of seizures during rapid withdrawal must be carefully managed, as Muscimol's effects on seizure threshold in this context are not fully understood.
Cardiovascular safety is another crucial aspect, as UDP can place significant stress on the heart. The interaction between Muscimol and the autonomic nervous system during detoxification requires close observation to prevent arrhythmias or sudden blood pressure changes. Proper patient selection and thorough pre-procedure cardiovascular screening are vital to minimize these risks.
Efficacy considerations for Muscimol in UDP center on its ability to mitigate withdrawal symptoms and reduce the duration of the detoxification process. Preliminary studies suggest that Muscimol may effectively modulate GABA receptors, potentially alleviating anxiety, tremors, and other withdrawal manifestations. However, the optimal dosing regimen and administration protocol for Muscimol in UDP settings need to be established through rigorous clinical trials.
The impact of Muscimol on post-detoxification outcomes is a critical area for investigation. Research must assess whether its use in UDP leads to improved long-term abstinence rates or reduces the likelihood of relapse compared to traditional detoxification methods. Additionally, the potential for Muscimol to mitigate post-acute withdrawal syndrome (PAWS) should be explored, as this could significantly enhance patient recovery and quality of life following detoxification.
Standardization of Muscimol-assisted UDP protocols is essential for ensuring consistent safety and efficacy across different clinical settings. This includes developing guidelines for patient selection, pre-procedure preparation, intra-procedure management, and post-procedure care. The creation of a comprehensive safety profile for Muscimol in UDP, including potential drug interactions and contraindications, is crucial for widespread clinical adoption.
Regulatory Landscape for Novel Detox Methods
The regulatory landscape for novel detoxification methods, particularly those involving the use of Muscimol in ultrarapid detoxification protocols, is complex and evolving. Regulatory bodies worldwide are grappling with the challenges posed by innovative approaches to substance abuse treatment.
In the United States, the Food and Drug Administration (FDA) plays a crucial role in overseeing the development and approval of new detoxification methods. The FDA's Center for Drug Evaluation and Research (CDER) is responsible for evaluating the safety and efficacy of novel pharmacological interventions, including those utilizing Muscimol. The agency requires rigorous clinical trials and extensive documentation before granting approval for new treatment protocols.
The European Medicines Agency (EMA) maintains similar stringent standards for the European Union. The EMA's Committee for Medicinal Products for Human Use (CHMP) is tasked with assessing novel detoxification methods and providing recommendations for market authorization. Both the FDA and EMA have established expedited review processes for treatments addressing urgent medical needs, which could potentially apply to innovative detoxification protocols.
Regulatory frameworks in other countries vary, with some nations adopting more permissive approaches to experimental treatments. This has led to the emergence of "medical tourism" for detoxification procedures, raising concerns about patient safety and ethical considerations in cross-border healthcare.
The use of Muscimol, a psychoactive compound found in certain mushroom species, in ultrarapid detoxification protocols presents unique regulatory challenges. As a naturally occurring substance with potential for abuse, Muscimol falls under the purview of controlled substance regulations in many jurisdictions. This adds an additional layer of complexity to the approval process for Muscimol-based detoxification methods.
Regulatory bodies are also grappling with the ethical implications of ultrarapid detoxification protocols. Concerns about patient safety, informed consent, and long-term efficacy have prompted calls for more stringent oversight of these procedures. Some regulatory agencies have implemented specific guidelines for rapid detoxification methods, addressing issues such as patient selection criteria, monitoring requirements, and follow-up care protocols.
The international nature of drug development and substance abuse treatment has necessitated increased cooperation between regulatory agencies. Initiatives such as the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) aim to standardize regulatory requirements across different regions, potentially streamlining the approval process for novel detoxification methods.
As research on Muscimol and other innovative approaches to detoxification continues to advance, regulatory frameworks are likely to evolve. Policymakers and regulatory bodies face the challenge of balancing the need for rigorous safety standards with the urgency of addressing the global substance abuse crisis. This dynamic regulatory landscape will play a crucial role in shaping the future of detoxification treatments and their accessibility to patients worldwide.
In the United States, the Food and Drug Administration (FDA) plays a crucial role in overseeing the development and approval of new detoxification methods. The FDA's Center for Drug Evaluation and Research (CDER) is responsible for evaluating the safety and efficacy of novel pharmacological interventions, including those utilizing Muscimol. The agency requires rigorous clinical trials and extensive documentation before granting approval for new treatment protocols.
The European Medicines Agency (EMA) maintains similar stringent standards for the European Union. The EMA's Committee for Medicinal Products for Human Use (CHMP) is tasked with assessing novel detoxification methods and providing recommendations for market authorization. Both the FDA and EMA have established expedited review processes for treatments addressing urgent medical needs, which could potentially apply to innovative detoxification protocols.
Regulatory frameworks in other countries vary, with some nations adopting more permissive approaches to experimental treatments. This has led to the emergence of "medical tourism" for detoxification procedures, raising concerns about patient safety and ethical considerations in cross-border healthcare.
The use of Muscimol, a psychoactive compound found in certain mushroom species, in ultrarapid detoxification protocols presents unique regulatory challenges. As a naturally occurring substance with potential for abuse, Muscimol falls under the purview of controlled substance regulations in many jurisdictions. This adds an additional layer of complexity to the approval process for Muscimol-based detoxification methods.
Regulatory bodies are also grappling with the ethical implications of ultrarapid detoxification protocols. Concerns about patient safety, informed consent, and long-term efficacy have prompted calls for more stringent oversight of these procedures. Some regulatory agencies have implemented specific guidelines for rapid detoxification methods, addressing issues such as patient selection criteria, monitoring requirements, and follow-up care protocols.
The international nature of drug development and substance abuse treatment has necessitated increased cooperation between regulatory agencies. Initiatives such as the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) aim to standardize regulatory requirements across different regions, potentially streamlining the approval process for novel detoxification methods.
As research on Muscimol and other innovative approaches to detoxification continues to advance, regulatory frameworks are likely to evolve. Policymakers and regulatory bodies face the challenge of balancing the need for rigorous safety standards with the urgency of addressing the global substance abuse crisis. This dynamic regulatory landscape will play a crucial role in shaping the future of detoxification treatments and their accessibility to patients worldwide.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!