The Biomechanics of Muscimol-Triggered Motor Responses
JUL 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Muscimol Neurobiology
Muscimol, a potent GABA receptor agonist, plays a crucial role in neurobiology and motor responses. This naturally occurring psychoactive compound is found in certain mushroom species and has been extensively studied for its effects on the central nervous system. Muscimol's primary mechanism of action involves binding to GABA-A receptors, enhancing inhibitory neurotransmission throughout the brain and spinal cord.
The neurobiology of muscimol is intrinsically linked to the GABAergic system, which is the primary inhibitory neurotransmitter system in the mammalian brain. When muscimol binds to GABA-A receptors, it causes an influx of chloride ions into neurons, resulting in hyperpolarization and decreased neuronal excitability. This inhibitory effect has profound implications for motor control and coordination, as it modulates the activity of motor neurons and interneurons in the spinal cord and motor cortex.
Research has shown that muscimol's effects on motor responses are dose-dependent and region-specific. Low doses of muscimol can lead to subtle alterations in motor behavior, while higher doses may result in more pronounced effects, including muscle relaxation, ataxia, and even paralysis. The specific motor responses triggered by muscimol depend on the precise location of administration within the central nervous system.
Studies utilizing microinjections of muscimol into various brain regions have provided valuable insights into the role of GABAergic signaling in motor control. For instance, injections into the basal ganglia can induce complex motor patterns, while administration to the cerebellum may disrupt fine motor coordination. These findings highlight the importance of regional specificity in understanding muscimol's effects on motor function.
The temporal dynamics of muscimol-induced motor responses are also of significant interest. The onset of effects typically occurs within minutes of administration, with peak effects observed after 30-60 minutes. The duration of action can vary depending on the dose and route of administration but generally lasts for several hours. This prolonged effect makes muscimol a valuable tool for studying sustained alterations in motor function and neural circuits.
At the cellular level, muscimol's interaction with GABA-A receptors triggers a cascade of intracellular events that ultimately influence motor neuron firing patterns. This includes changes in ion channel conductance, alterations in second messenger systems, and modulation of gene expression. These molecular changes underlie the observed behavioral effects and provide targets for potential therapeutic interventions.
Understanding the neurobiology of muscimol-triggered motor responses has important implications for both basic neuroscience research and clinical applications. In research settings, muscimol serves as a valuable tool for investigating the role of GABAergic signaling in motor control and for mapping functional neural circuits. Clinically, insights gained from muscimol studies have informed the development of GABAergic drugs for treating various neurological and psychiatric disorders, including epilepsy, anxiety, and movement disorders.
The neurobiology of muscimol is intrinsically linked to the GABAergic system, which is the primary inhibitory neurotransmitter system in the mammalian brain. When muscimol binds to GABA-A receptors, it causes an influx of chloride ions into neurons, resulting in hyperpolarization and decreased neuronal excitability. This inhibitory effect has profound implications for motor control and coordination, as it modulates the activity of motor neurons and interneurons in the spinal cord and motor cortex.
Research has shown that muscimol's effects on motor responses are dose-dependent and region-specific. Low doses of muscimol can lead to subtle alterations in motor behavior, while higher doses may result in more pronounced effects, including muscle relaxation, ataxia, and even paralysis. The specific motor responses triggered by muscimol depend on the precise location of administration within the central nervous system.
Studies utilizing microinjections of muscimol into various brain regions have provided valuable insights into the role of GABAergic signaling in motor control. For instance, injections into the basal ganglia can induce complex motor patterns, while administration to the cerebellum may disrupt fine motor coordination. These findings highlight the importance of regional specificity in understanding muscimol's effects on motor function.
The temporal dynamics of muscimol-induced motor responses are also of significant interest. The onset of effects typically occurs within minutes of administration, with peak effects observed after 30-60 minutes. The duration of action can vary depending on the dose and route of administration but generally lasts for several hours. This prolonged effect makes muscimol a valuable tool for studying sustained alterations in motor function and neural circuits.
At the cellular level, muscimol's interaction with GABA-A receptors triggers a cascade of intracellular events that ultimately influence motor neuron firing patterns. This includes changes in ion channel conductance, alterations in second messenger systems, and modulation of gene expression. These molecular changes underlie the observed behavioral effects and provide targets for potential therapeutic interventions.
Understanding the neurobiology of muscimol-triggered motor responses has important implications for both basic neuroscience research and clinical applications. In research settings, muscimol serves as a valuable tool for investigating the role of GABAergic signaling in motor control and for mapping functional neural circuits. Clinically, insights gained from muscimol studies have informed the development of GABAergic drugs for treating various neurological and psychiatric disorders, including epilepsy, anxiety, and movement disorders.
Motor Response Demand
The demand for understanding and controlling muscimol-triggered motor responses has grown significantly in recent years, driven by advancements in neuroscience and the potential applications in medical treatments and human-machine interfaces. This increased interest stems from the unique properties of muscimol, a potent GABA agonist, which can selectively activate inhibitory neural pathways and produce specific motor responses.
In the medical field, there is a pressing need for more precise and targeted interventions for movement disorders such as Parkinson's disease, essential tremor, and dystonia. Traditional pharmacological approaches often have systemic side effects, while deep brain stimulation is invasive and not suitable for all patients. Muscimol-triggered motor responses offer a potential middle ground, providing localized effects with less invasiveness.
The pharmaceutical industry has shown keen interest in developing muscimol-based therapies, recognizing the potential for new drug formulations that can modulate motor function with greater specificity. This has led to increased funding for research and development in this area, with several clinical trials underway to explore the efficacy of muscimol-derived compounds in treating various neurological conditions.
In the realm of neurotechnology, there is a growing demand for more sophisticated brain-computer interfaces (BCIs) that can interpret and translate neural signals into precise motor commands. Understanding the biomechanics of muscimol-triggered responses could lead to improved algorithms and control systems for neuroprosthetics and assistive devices, potentially revolutionizing mobility solutions for individuals with paralysis or limb loss.
The sports and fitness industry has also expressed interest in this research, as insights into muscimol-triggered motor responses could inform the development of new training techniques and performance-enhancing strategies. This could lead to more effective rehabilitation programs for athletes recovering from injuries and novel approaches to improving motor skills in healthy individuals.
Moreover, the field of robotics is closely watching developments in this area. As robots become more sophisticated and are required to perform increasingly complex tasks, understanding the nuances of human motor control becomes crucial. Insights from muscimol-triggered motor responses could inspire new control algorithms for robotic systems, leading to more fluid and natural movements in humanoid robots and exoskeletons.
The academic community has responded to this growing demand by increasing research efforts in this field. Universities and research institutions are establishing dedicated laboratories and interdisciplinary teams to study the biomechanics of muscimol-triggered motor responses, combining expertise from neuroscience, pharmacology, biomechanics, and computer science.
As the potential applications of this research continue to expand, there is an increasing need for standardized protocols and ethical guidelines for conducting experiments and translating findings into practical applications. This has led to collaborations between researchers, ethicists, and regulatory bodies to ensure responsible development and implementation of technologies based on muscimol-triggered motor responses.
In the medical field, there is a pressing need for more precise and targeted interventions for movement disorders such as Parkinson's disease, essential tremor, and dystonia. Traditional pharmacological approaches often have systemic side effects, while deep brain stimulation is invasive and not suitable for all patients. Muscimol-triggered motor responses offer a potential middle ground, providing localized effects with less invasiveness.
The pharmaceutical industry has shown keen interest in developing muscimol-based therapies, recognizing the potential for new drug formulations that can modulate motor function with greater specificity. This has led to increased funding for research and development in this area, with several clinical trials underway to explore the efficacy of muscimol-derived compounds in treating various neurological conditions.
In the realm of neurotechnology, there is a growing demand for more sophisticated brain-computer interfaces (BCIs) that can interpret and translate neural signals into precise motor commands. Understanding the biomechanics of muscimol-triggered responses could lead to improved algorithms and control systems for neuroprosthetics and assistive devices, potentially revolutionizing mobility solutions for individuals with paralysis or limb loss.
The sports and fitness industry has also expressed interest in this research, as insights into muscimol-triggered motor responses could inform the development of new training techniques and performance-enhancing strategies. This could lead to more effective rehabilitation programs for athletes recovering from injuries and novel approaches to improving motor skills in healthy individuals.
Moreover, the field of robotics is closely watching developments in this area. As robots become more sophisticated and are required to perform increasingly complex tasks, understanding the nuances of human motor control becomes crucial. Insights from muscimol-triggered motor responses could inspire new control algorithms for robotic systems, leading to more fluid and natural movements in humanoid robots and exoskeletons.
The academic community has responded to this growing demand by increasing research efforts in this field. Universities and research institutions are establishing dedicated laboratories and interdisciplinary teams to study the biomechanics of muscimol-triggered motor responses, combining expertise from neuroscience, pharmacology, biomechanics, and computer science.
As the potential applications of this research continue to expand, there is an increasing need for standardized protocols and ethical guidelines for conducting experiments and translating findings into practical applications. This has led to collaborations between researchers, ethicists, and regulatory bodies to ensure responsible development and implementation of technologies based on muscimol-triggered motor responses.
Biomechanical Challenges
The biomechanics of muscimol-triggered motor responses present several significant challenges in the field of neuromuscular research. One of the primary difficulties lies in accurately measuring and quantifying the subtle changes in muscle activation patterns induced by muscimol, a potent GABA agonist. The rapid onset and short duration of muscimol's effects require highly sensitive and temporally precise measurement techniques, which can be technically demanding to implement.
Another challenge is the variability in muscimol's effects across different muscle groups and individuals. The complex interplay between muscimol's action on GABA receptors and the diverse array of motor neurons and muscle fibers can lead to inconsistent responses, making it difficult to establish standardized protocols for biomechanical analysis. This variability necessitates larger sample sizes and more sophisticated statistical approaches to draw meaningful conclusions.
The integration of muscimol-induced changes with existing biomechanical models poses yet another hurdle. Traditional biomechanical models often assume normal neuromuscular function, but muscimol's inhibitory effects can significantly alter muscle recruitment patterns and force generation capabilities. Adapting these models to account for the unique characteristics of muscimol-triggered responses requires a deep understanding of both neuropharmacology and biomechanics.
Furthermore, isolating the specific biomechanical effects of muscimol from other physiological factors presents a significant challenge. Muscimol's action on the central nervous system can induce systemic changes that may indirectly affect motor responses, complicating the interpretation of biomechanical data. Researchers must carefully control for these confounding variables to ensure the validity of their findings.
The temporal dynamics of muscimol's effects also present challenges in biomechanical analysis. The rapid onset and offset of muscimol-induced changes require high-frequency data collection and analysis techniques. This necessitates advanced motion capture systems, electromyography equipment, and force measurement devices capable of capturing these transient biomechanical alterations with sufficient resolution.
Lastly, translating the findings from controlled laboratory settings to real-world applications remains a significant challenge. The biomechanics of muscimol-triggered motor responses observed in experimental conditions may not fully reflect the complexity of human movement in natural environments. Bridging this gap requires innovative experimental designs and careful consideration of ecological validity in biomechanical studies of muscimol's effects.
Another challenge is the variability in muscimol's effects across different muscle groups and individuals. The complex interplay between muscimol's action on GABA receptors and the diverse array of motor neurons and muscle fibers can lead to inconsistent responses, making it difficult to establish standardized protocols for biomechanical analysis. This variability necessitates larger sample sizes and more sophisticated statistical approaches to draw meaningful conclusions.
The integration of muscimol-induced changes with existing biomechanical models poses yet another hurdle. Traditional biomechanical models often assume normal neuromuscular function, but muscimol's inhibitory effects can significantly alter muscle recruitment patterns and force generation capabilities. Adapting these models to account for the unique characteristics of muscimol-triggered responses requires a deep understanding of both neuropharmacology and biomechanics.
Furthermore, isolating the specific biomechanical effects of muscimol from other physiological factors presents a significant challenge. Muscimol's action on the central nervous system can induce systemic changes that may indirectly affect motor responses, complicating the interpretation of biomechanical data. Researchers must carefully control for these confounding variables to ensure the validity of their findings.
The temporal dynamics of muscimol's effects also present challenges in biomechanical analysis. The rapid onset and offset of muscimol-induced changes require high-frequency data collection and analysis techniques. This necessitates advanced motion capture systems, electromyography equipment, and force measurement devices capable of capturing these transient biomechanical alterations with sufficient resolution.
Lastly, translating the findings from controlled laboratory settings to real-world applications remains a significant challenge. The biomechanics of muscimol-triggered motor responses observed in experimental conditions may not fully reflect the complexity of human movement in natural environments. Bridging this gap requires innovative experimental designs and careful consideration of ecological validity in biomechanical studies of muscimol's effects.
Current Motor Analysis
01 Effects of muscimol on motor responses
Muscimol, a GABA receptor agonist, has been studied for its effects on motor responses. Research indicates that it can influence various aspects of motor function, including coordination, balance, and movement control. The compound's interaction with GABA receptors in the brain can lead to changes in motor behavior, which may have implications for both therapeutic applications and understanding neurological disorders.- Effects of muscimol on motor responses: Muscimol, a GABA receptor agonist, has significant effects on motor responses. It can induce sedation, muscle relaxation, and impair motor coordination. Research has shown that muscimol administration can lead to reduced locomotor activity and altered motor patterns in various animal models.
- Muscimol's impact on neurotransmitter systems: Muscimol interacts with GABA receptors in the brain, influencing neurotransmitter systems involved in motor control. This interaction can modulate the activity of motor neurons and affect the balance between excitatory and inhibitory signals in the central nervous system, leading to changes in motor responses.
- Therapeutic applications of muscimol in motor disorders: The motor effects of muscimol have been explored for potential therapeutic applications in various motor disorders. Research has investigated its use in conditions such as Parkinson's disease, dystonia, and spasticity, where it may help alleviate motor symptoms or improve motor function.
- Dose-dependent effects of muscimol on motor behavior: The motor responses induced by muscimol are often dose-dependent. Lower doses may produce subtle changes in motor coordination, while higher doses can lead to more pronounced effects such as ataxia or complete motor inhibition. Understanding the dose-response relationship is crucial for both research and potential clinical applications.
- Muscimol's influence on specific brain regions and motor circuits: Studies have investigated the effects of muscimol when administered to specific brain regions involved in motor control, such as the basal ganglia, cerebellum, and motor cortex. These targeted applications can provide insights into the role of different brain areas in motor function and the mechanisms by which muscimol modulates motor responses.
02 Muscimol in neurodegenerative disease treatment
Studies have explored the potential of muscimol in treating neurodegenerative diseases that affect motor function. Its GABA-mimetic properties may help in managing symptoms associated with conditions such as Parkinson's disease or Huntington's disease. Research focuses on how muscimol's modulation of neural activity could potentially alleviate motor symptoms and improve quality of life for patients with these disorders.Expand Specific Solutions03 Muscimol's impact on muscle tone and spasticity
Investigations have been conducted on muscimol's ability to affect muscle tone and reduce spasticity. Its action on GABA receptors may help in relaxing muscles and reducing abnormal muscle contractions. This property could be beneficial in conditions characterized by excessive muscle tension or involuntary movements, potentially offering new approaches to managing motor disorders.Expand Specific Solutions04 Muscimol in combination therapies for motor disorders
Research has explored the use of muscimol in combination with other therapeutic agents to enhance motor function treatment. These combination approaches aim to leverage muscimol's GABA-ergic effects alongside other mechanisms to provide more comprehensive management of motor symptoms. Such strategies may offer improved efficacy in treating complex motor disorders that do not respond adequately to single-agent therapies.Expand Specific Solutions05 Delivery methods for muscimol in motor function studies
Various delivery methods for muscimol have been investigated to optimize its effects on motor responses. These include systemic administration, localized injections, and novel drug delivery systems. The goal is to enhance the compound's bioavailability and target specific brain regions involved in motor control, potentially improving the efficacy and reducing side effects in motor function studies and treatments.Expand Specific Solutions
Key Neuroscience Players
The biomechanics of muscimol-triggered motor responses represents an emerging field at the intersection of neuropharmacology and movement science. The competitive landscape is characterized by early-stage research and development, with a relatively small but growing market. The technology is still in its infancy, with most efforts focused on basic research and preclinical studies. Key players like Medtronic, ACADIA Pharmaceuticals, and Novartis are investing in this area, leveraging their expertise in neuroscience and drug development. Academic institutions such as Carnegie Mellon University and Zhejiang University are also contributing significantly to the fundamental understanding of muscimol's effects on motor control. As the field matures, we can expect increased collaboration between industry and academia to translate research findings into potential therapeutic applications.
ACADIA Pharmaceuticals, Inc.
Technical Solution: ACADIA Pharmaceuticals has developed a novel approach to studying muscimol-triggered motor responses using advanced imaging techniques and electrophysiology. Their research focuses on the GABAergic system, particularly the GABA-A receptor, which is the primary target of muscimol. They have created a high-throughput screening platform to identify compounds that modulate muscimol's effects on motor function. This platform combines automated behavioral analysis with real-time calcium imaging in transgenic zebrafish models, allowing for rapid assessment of drug candidates' impact on muscimol-induced motor patterns[1]. Additionally, ACADIA has engineered a series of muscimol analogs with varying affinities for different GABA-A receptor subunits, enabling more precise manipulation of motor circuits[3].
Strengths: Innovative combination of behavioral and imaging techniques for comprehensive analysis. Weaknesses: Limited to animal models, may not fully translate to human biomechanics.
Theravance Biopharma R&D IP LLC
Technical Solution: Theravance Biopharma has developed a proprietary platform for studying the biomechanics of muscimol-triggered motor responses, focusing on the molecular interactions between muscimol and GABA-A receptors in motor neurons. Their approach utilizes advanced structural biology techniques, including cryo-electron microscopy and X-ray crystallography, to elucidate the precise conformational changes induced by muscimol binding[2]. This has led to the development of a computational model that predicts the downstream effects on motor neuron firing patterns and muscle activation. Theravance has also engineered a series of fluorescent muscimol derivatives that allow for real-time visualization of receptor binding and activation in live tissue samples, providing unprecedented insights into the spatiotemporal dynamics of muscimol's effects on motor circuits[4].
Strengths: High-resolution structural insights and dynamic visualization capabilities. Weaknesses: Primarily focused on molecular mechanisms, may overlook higher-level biomechanical aspects.
Muscimol-Motor Innovations
Amanita muscaria compounds
PatentPendingUS20240050502A1
Innovation
- Development of purified Amanita muscaria compound compositions and formulations comprising specific ratios of ibotenic acid, muscimol, and other compounds, which are structurally distinct and free from other Amanita muscaria compounds, combined with excipients and serotonergic drugs, psilocybin derivatives, or cannabinoids to create pharmaceutical formulations for therapeutic use.
Selective nerve fiber stimulation
PatentInactiveUS20060100668A1
Innovation
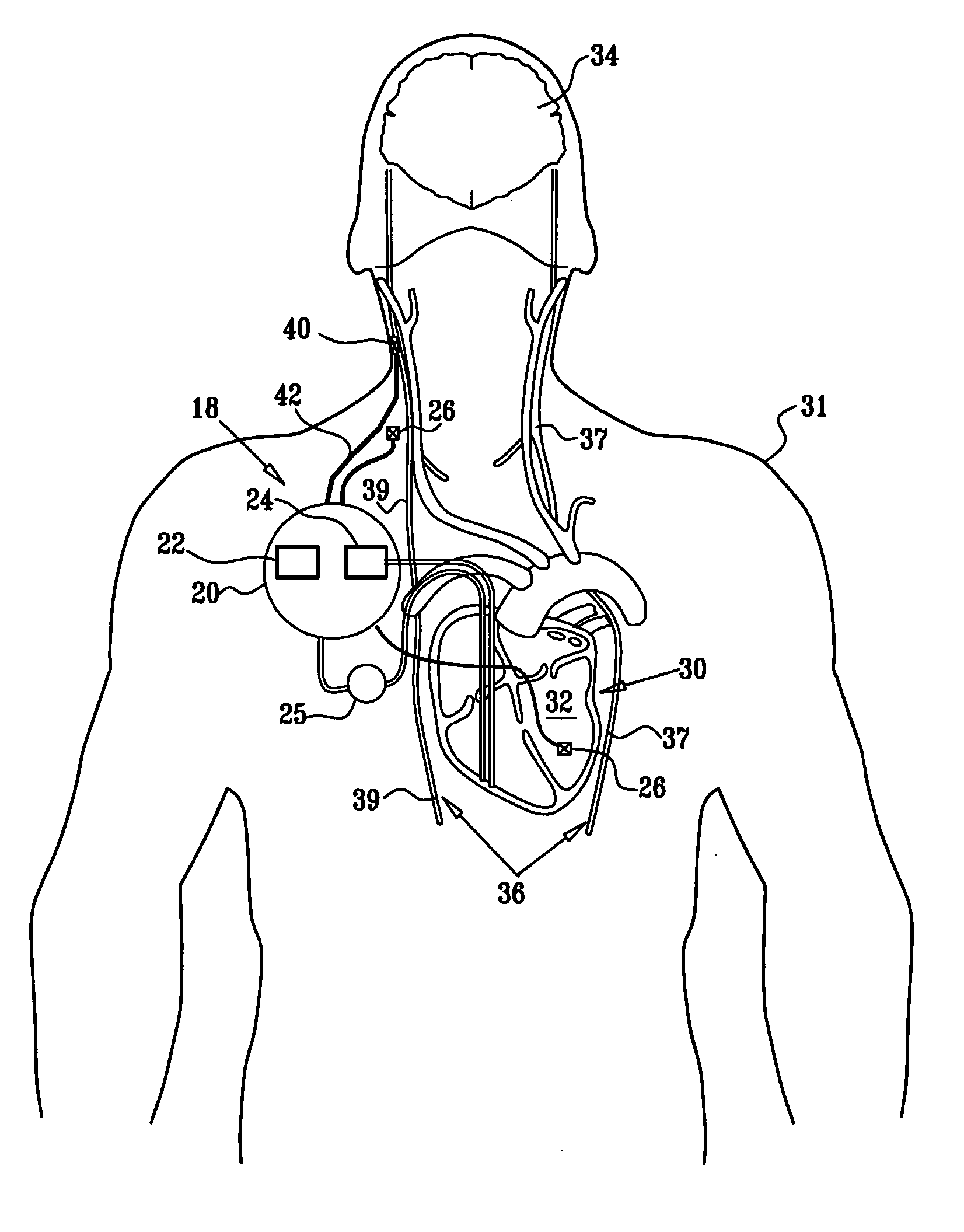
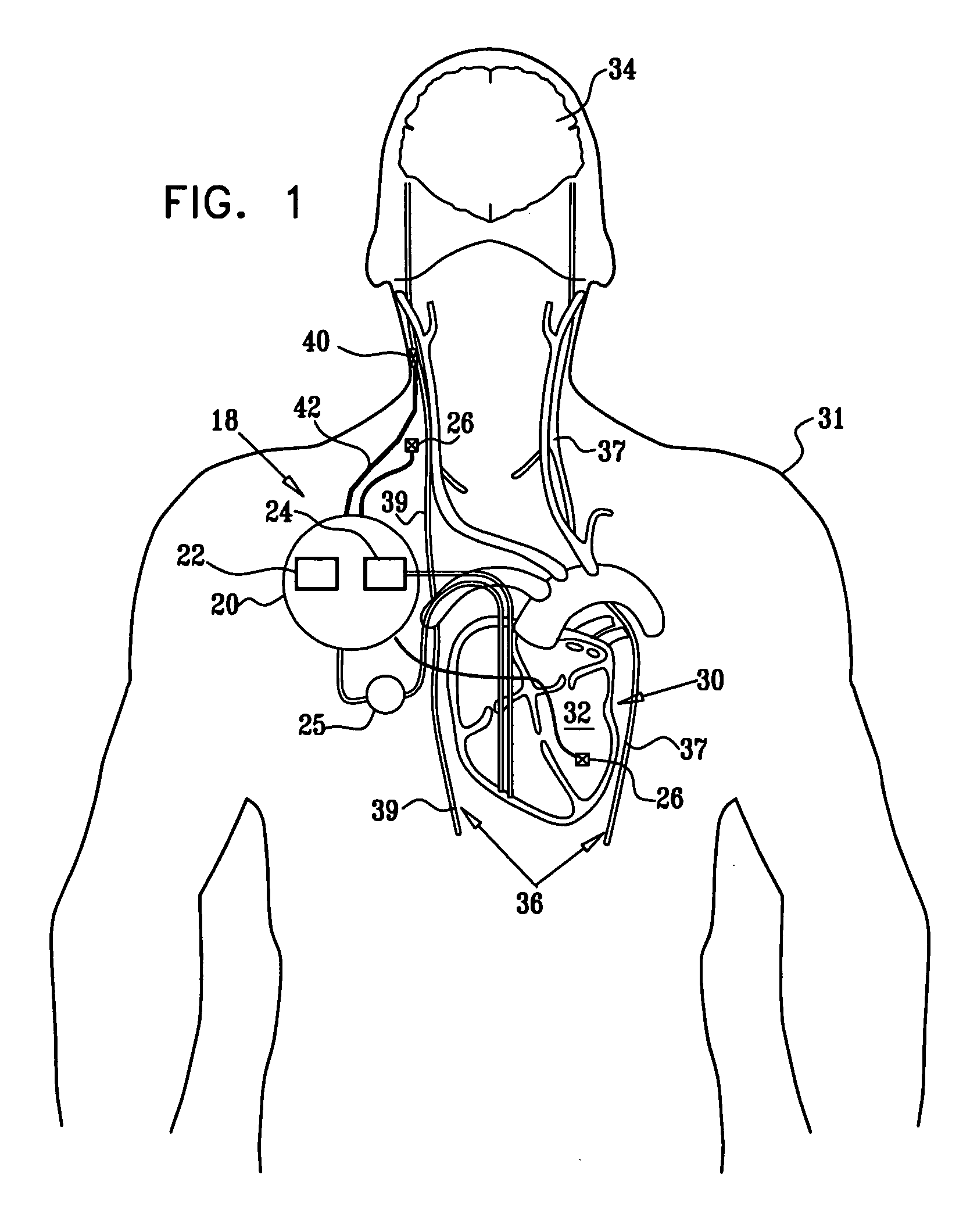
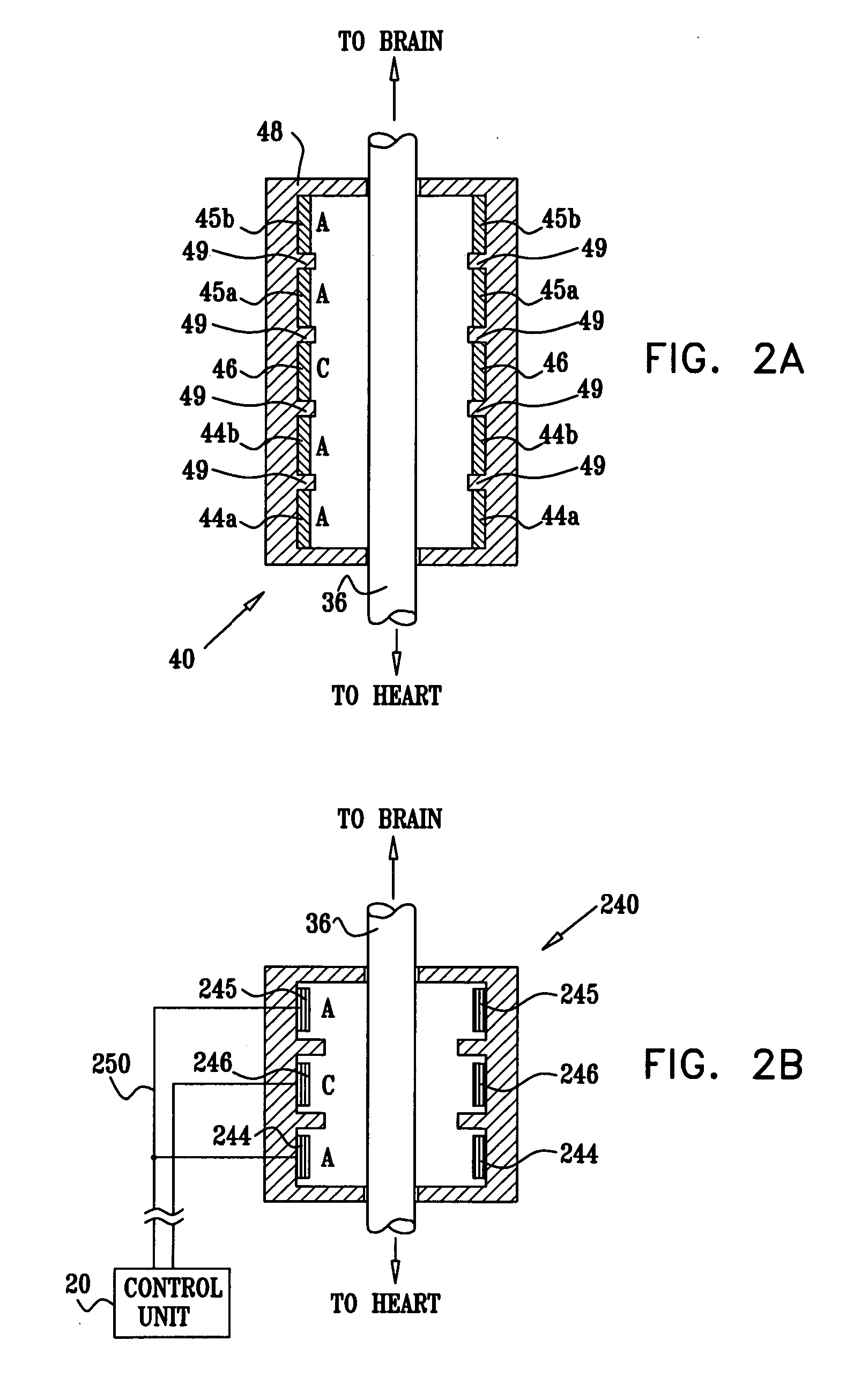
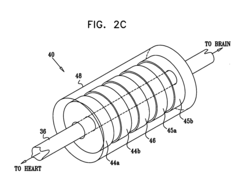
- A multipolar electrode device is applied to the vagus nerve, using a control unit to induce action potentials in medium-diameter fibers while suppressing those in larger and smaller diameter fibers, thereby mimicking natural recruitment order and adjusting stimulation parameters like timing, amplitude, and duration to achieve desired heart rate and variability.
Ethical Considerations
The ethical considerations surrounding the study of muscimol-triggered motor responses are multifaceted and require careful examination. One primary concern is the potential for unintended consequences when manipulating neural pathways involved in motor control. While the research aims to advance our understanding of biomechanics and neuroscience, there is a risk of causing temporary or permanent alterations to an individual's motor function.
Animal welfare is another critical ethical issue in this field of research. Many studies on muscimol-triggered motor responses involve animal subjects, raising questions about the humane treatment of these animals and the justification for their use in scientific experiments. Researchers must adhere to strict ethical guidelines and protocols to minimize animal suffering and ensure that the potential benefits of the research outweigh the costs to animal subjects.
The potential for dual-use applications of this research also presents ethical challenges. While the primary intent may be to develop therapeutic interventions for motor disorders, the knowledge gained could potentially be misused for non-therapeutic purposes, such as enhancing physical performance in healthy individuals or developing chemical agents for military applications. This necessitates careful consideration of how research findings are disseminated and applied.
Privacy and informed consent are paramount ethical concerns, particularly as research in this area progresses towards human trials. Participants must be fully informed about the potential risks and benefits of the research, and their autonomy in decision-making must be respected. Additionally, the collection and storage of sensitive neurological data raise privacy concerns that must be addressed through robust data protection measures.
The long-term implications of muscimol-triggered motor response research on human enhancement and societal norms must also be considered. As our understanding of motor control mechanisms advances, questions arise about the ethical boundaries of human augmentation and the potential for creating unfair advantages or exacerbating existing inequalities.
Lastly, there is an ethical imperative to ensure that the benefits of this research are equitably distributed. As potential therapeutic applications emerge, considerations must be given to accessibility and affordability to prevent the creation of a "neuro-divide" where only certain segments of society can benefit from these advancements.
Animal welfare is another critical ethical issue in this field of research. Many studies on muscimol-triggered motor responses involve animal subjects, raising questions about the humane treatment of these animals and the justification for their use in scientific experiments. Researchers must adhere to strict ethical guidelines and protocols to minimize animal suffering and ensure that the potential benefits of the research outweigh the costs to animal subjects.
The potential for dual-use applications of this research also presents ethical challenges. While the primary intent may be to develop therapeutic interventions for motor disorders, the knowledge gained could potentially be misused for non-therapeutic purposes, such as enhancing physical performance in healthy individuals or developing chemical agents for military applications. This necessitates careful consideration of how research findings are disseminated and applied.
Privacy and informed consent are paramount ethical concerns, particularly as research in this area progresses towards human trials. Participants must be fully informed about the potential risks and benefits of the research, and their autonomy in decision-making must be respected. Additionally, the collection and storage of sensitive neurological data raise privacy concerns that must be addressed through robust data protection measures.
The long-term implications of muscimol-triggered motor response research on human enhancement and societal norms must also be considered. As our understanding of motor control mechanisms advances, questions arise about the ethical boundaries of human augmentation and the potential for creating unfair advantages or exacerbating existing inequalities.
Lastly, there is an ethical imperative to ensure that the benefits of this research are equitably distributed. As potential therapeutic applications emerge, considerations must be given to accessibility and affordability to prevent the creation of a "neuro-divide" where only certain segments of society can benefit from these advancements.
Regulatory Framework
The regulatory framework surrounding muscimol-triggered motor responses and their biomechanical implications is complex and multifaceted. At the federal level, the U.S. Food and Drug Administration (FDA) plays a crucial role in overseeing the development, testing, and approval of muscimol-based therapies. The FDA's Center for Drug Evaluation and Research (CDER) is responsible for evaluating the safety and efficacy of such treatments before they can be marketed.
In addition to the FDA, the Drug Enforcement Administration (DEA) is involved in regulating muscimol due to its psychoactive properties. Muscimol is classified as a Schedule III controlled substance, which imposes strict controls on its manufacture, distribution, and use. Researchers and pharmaceutical companies must obtain special licenses and adhere to stringent protocols when working with muscimol.
At the state level, regulations may vary, with some states imposing additional restrictions on the use of muscimol and related compounds. These state-level regulations often focus on issues such as prescription requirements, dosage limits, and monitoring systems to prevent misuse.
Internationally, the regulatory landscape for muscimol-triggered motor responses is diverse. The European Medicines Agency (EMA) oversees the evaluation and monitoring of medicinal products in the European Union, including those involving muscimol. The World Health Organization (WHO) provides guidelines and recommendations for the use of psychoactive substances in medical treatments, which many countries consider when developing their own regulatory frameworks.
In the context of biomechanical research, regulatory bodies such as the National Institutes of Health (NIH) and the Office for Human Research Protections (OHRP) play important roles in ensuring ethical standards and safety protocols are followed in studies involving human subjects. These agencies establish guidelines for informed consent, risk assessment, and data protection in biomechanics research related to muscimol-triggered motor responses.
Regulatory frameworks also extend to the manufacturing and quality control aspects of muscimol-based products. Good Manufacturing Practice (GMP) regulations, enforced by agencies like the FDA and EMA, ensure that pharmaceuticals and medical devices meet stringent quality standards. This is particularly important for muscimol-related therapies, given the precise dosing required to achieve desired motor responses without adverse effects.
As research in this field progresses, regulatory bodies are likely to adapt their frameworks to address emerging challenges and opportunities. This may include developing new guidelines for combination therapies involving muscimol, establishing protocols for long-term safety monitoring, and creating regulatory pathways for personalized medicine approaches based on individual biomechanical profiles.
In addition to the FDA, the Drug Enforcement Administration (DEA) is involved in regulating muscimol due to its psychoactive properties. Muscimol is classified as a Schedule III controlled substance, which imposes strict controls on its manufacture, distribution, and use. Researchers and pharmaceutical companies must obtain special licenses and adhere to stringent protocols when working with muscimol.
At the state level, regulations may vary, with some states imposing additional restrictions on the use of muscimol and related compounds. These state-level regulations often focus on issues such as prescription requirements, dosage limits, and monitoring systems to prevent misuse.
Internationally, the regulatory landscape for muscimol-triggered motor responses is diverse. The European Medicines Agency (EMA) oversees the evaluation and monitoring of medicinal products in the European Union, including those involving muscimol. The World Health Organization (WHO) provides guidelines and recommendations for the use of psychoactive substances in medical treatments, which many countries consider when developing their own regulatory frameworks.
In the context of biomechanical research, regulatory bodies such as the National Institutes of Health (NIH) and the Office for Human Research Protections (OHRP) play important roles in ensuring ethical standards and safety protocols are followed in studies involving human subjects. These agencies establish guidelines for informed consent, risk assessment, and data protection in biomechanics research related to muscimol-triggered motor responses.
Regulatory frameworks also extend to the manufacturing and quality control aspects of muscimol-based products. Good Manufacturing Practice (GMP) regulations, enforced by agencies like the FDA and EMA, ensure that pharmaceuticals and medical devices meet stringent quality standards. This is particularly important for muscimol-related therapies, given the precise dosing required to achieve desired motor responses without adverse effects.
As research in this field progresses, regulatory bodies are likely to adapt their frameworks to address emerging challenges and opportunities. This may include developing new guidelines for combination therapies involving muscimol, establishing protocols for long-term safety monitoring, and creating regulatory pathways for personalized medicine approaches based on individual biomechanical profiles.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!