The Interaction of Perchloric Acid with Biological Membranes
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perchloric Acid Membrane Interaction Background
Perchloric acid, a strong oxidizing agent and one of the most potent mineral acids, has been the subject of extensive research due to its unique properties and potential applications in various fields. The interaction between perchloric acid and biological membranes has garnered significant attention in recent years, as it presents both challenges and opportunities in biochemistry, pharmacology, and medical research.
The study of perchloric acid's interaction with biological membranes dates back to the mid-20th century when researchers began investigating the effects of strong acids on cellular structures. Initially, the focus was primarily on the destructive nature of perchloric acid on biological tissues. However, as analytical techniques advanced, scientists discovered that controlled exposure to perchloric acid could provide valuable insights into membrane structure and function.
In the 1970s and 1980s, researchers made significant strides in understanding the mechanisms by which perchloric acid interacts with lipid bilayers, the fundamental structure of biological membranes. These studies revealed that perchloric acid could induce changes in membrane permeability, fluidity, and overall integrity. The acid's strong oxidizing properties were found to cause lipid peroxidation, leading to the breakdown of membrane structures.
As the field progressed, scientists began to explore the potential applications of perchloric acid-membrane interactions. In the 1990s, researchers discovered that controlled exposure to perchloric acid could be used to selectively permeabilize cell membranes, allowing for the introduction of various molecules into cells. This finding opened up new avenues for drug delivery and gene therapy research.
The turn of the millennium saw a shift towards more sophisticated analytical techniques, such as advanced spectroscopy and microscopy methods, which allowed for real-time observation of perchloric acid-membrane interactions at the molecular level. These advancements led to a deeper understanding of the kinetics and thermodynamics involved in the process, paving the way for more targeted applications in biotechnology and medicine.
In recent years, the focus has expanded to include the environmental and health implications of perchloric acid exposure. Researchers have been investigating the long-term effects of low-level perchlorate contamination on biological systems, particularly in the context of water pollution and food chain contamination. This has led to increased interest in developing methods for detecting and mitigating perchlorate in the environment.
The ongoing research into perchloric acid-membrane interactions continues to evolve, with current efforts aimed at harnessing the unique properties of this interaction for novel applications in drug delivery, biosensors, and membrane technology. As our understanding of these complex interactions deepens, it is expected that new breakthroughs will emerge, potentially revolutionizing various fields of science and technology.
The study of perchloric acid's interaction with biological membranes dates back to the mid-20th century when researchers began investigating the effects of strong acids on cellular structures. Initially, the focus was primarily on the destructive nature of perchloric acid on biological tissues. However, as analytical techniques advanced, scientists discovered that controlled exposure to perchloric acid could provide valuable insights into membrane structure and function.
In the 1970s and 1980s, researchers made significant strides in understanding the mechanisms by which perchloric acid interacts with lipid bilayers, the fundamental structure of biological membranes. These studies revealed that perchloric acid could induce changes in membrane permeability, fluidity, and overall integrity. The acid's strong oxidizing properties were found to cause lipid peroxidation, leading to the breakdown of membrane structures.
As the field progressed, scientists began to explore the potential applications of perchloric acid-membrane interactions. In the 1990s, researchers discovered that controlled exposure to perchloric acid could be used to selectively permeabilize cell membranes, allowing for the introduction of various molecules into cells. This finding opened up new avenues for drug delivery and gene therapy research.
The turn of the millennium saw a shift towards more sophisticated analytical techniques, such as advanced spectroscopy and microscopy methods, which allowed for real-time observation of perchloric acid-membrane interactions at the molecular level. These advancements led to a deeper understanding of the kinetics and thermodynamics involved in the process, paving the way for more targeted applications in biotechnology and medicine.
In recent years, the focus has expanded to include the environmental and health implications of perchloric acid exposure. Researchers have been investigating the long-term effects of low-level perchlorate contamination on biological systems, particularly in the context of water pollution and food chain contamination. This has led to increased interest in developing methods for detecting and mitigating perchlorate in the environment.
The ongoing research into perchloric acid-membrane interactions continues to evolve, with current efforts aimed at harnessing the unique properties of this interaction for novel applications in drug delivery, biosensors, and membrane technology. As our understanding of these complex interactions deepens, it is expected that new breakthroughs will emerge, potentially revolutionizing various fields of science and technology.
Market Analysis for Perchloric Acid Applications
The market for perchloric acid applications has shown significant growth in recent years, driven by its unique properties and diverse uses across multiple industries. The global perchloric acid market size was valued at approximately $250 million in 2020 and is projected to reach $350 million by 2025, growing at a CAGR of 6.8% during the forecast period.
The pharmaceutical and healthcare sectors represent the largest end-use segment for perchloric acid, accounting for nearly 40% of the total market share. In these industries, perchloric acid is primarily used as a reagent in analytical chemistry and as a catalyst in various pharmaceutical processes. The increasing demand for high-quality drugs and the growing emphasis on research and development activities in the pharmaceutical sector are expected to fuel the market growth further.
The electronics industry is another significant consumer of perchloric acid, particularly in the manufacturing of printed circuit boards and semiconductors. With the rapid expansion of the electronics sector and the increasing adoption of advanced technologies such as 5G and IoT, the demand for perchloric acid in this segment is anticipated to grow substantially over the coming years.
In the aerospace and defense sectors, perchloric acid finds applications in rocket propellants and explosives. The growing investments in space exploration programs and defense modernization initiatives across various countries are likely to create new opportunities for perchloric acid suppliers in this market segment.
The Asia-Pacific region dominates the global perchloric acid market, accounting for approximately 45% of the total market share. This can be attributed to the rapid industrialization, growing pharmaceutical and electronics industries, and increasing research and development activities in countries like China, India, and Japan. North America and Europe follow closely, with significant demand coming from the pharmaceutical and aerospace sectors.
However, the market faces certain challenges, including stringent regulations regarding the handling and transportation of perchloric acid due to its highly corrosive and potentially explosive nature. These safety concerns have led to the development of alternative chemicals in some applications, which could potentially limit market growth to some extent.
Despite these challenges, the overall market outlook for perchloric acid remains positive. The increasing focus on renewable energy sources and the growing demand for electric vehicles are expected to create new opportunities for perchloric acid in battery technologies. Additionally, ongoing research into the interaction of perchloric acid with biological membranes could potentially open up new applications in the field of drug delivery and biomedical research, further expanding the market potential in the coming years.
The pharmaceutical and healthcare sectors represent the largest end-use segment for perchloric acid, accounting for nearly 40% of the total market share. In these industries, perchloric acid is primarily used as a reagent in analytical chemistry and as a catalyst in various pharmaceutical processes. The increasing demand for high-quality drugs and the growing emphasis on research and development activities in the pharmaceutical sector are expected to fuel the market growth further.
The electronics industry is another significant consumer of perchloric acid, particularly in the manufacturing of printed circuit boards and semiconductors. With the rapid expansion of the electronics sector and the increasing adoption of advanced technologies such as 5G and IoT, the demand for perchloric acid in this segment is anticipated to grow substantially over the coming years.
In the aerospace and defense sectors, perchloric acid finds applications in rocket propellants and explosives. The growing investments in space exploration programs and defense modernization initiatives across various countries are likely to create new opportunities for perchloric acid suppliers in this market segment.
The Asia-Pacific region dominates the global perchloric acid market, accounting for approximately 45% of the total market share. This can be attributed to the rapid industrialization, growing pharmaceutical and electronics industries, and increasing research and development activities in countries like China, India, and Japan. North America and Europe follow closely, with significant demand coming from the pharmaceutical and aerospace sectors.
However, the market faces certain challenges, including stringent regulations regarding the handling and transportation of perchloric acid due to its highly corrosive and potentially explosive nature. These safety concerns have led to the development of alternative chemicals in some applications, which could potentially limit market growth to some extent.
Despite these challenges, the overall market outlook for perchloric acid remains positive. The increasing focus on renewable energy sources and the growing demand for electric vehicles are expected to create new opportunities for perchloric acid in battery technologies. Additionally, ongoing research into the interaction of perchloric acid with biological membranes could potentially open up new applications in the field of drug delivery and biomedical research, further expanding the market potential in the coming years.
Current Challenges in Perchloric Acid-Membrane Studies
The study of perchloric acid's interaction with biological membranes faces several significant challenges that hinder comprehensive understanding and practical applications. One primary obstacle is the highly reactive and oxidizing nature of perchloric acid, which can rapidly degrade biological samples and interfere with experimental protocols. This reactivity makes it difficult to isolate and observe the specific interactions between perchloric acid and membrane components without compromising the integrity of the biological system.
Another challenge lies in the complexity of biological membranes themselves. These structures consist of diverse lipids, proteins, and carbohydrates, each potentially interacting differently with perchloric acid. Elucidating the precise mechanisms of interaction for each component requires sophisticated analytical techniques and careful experimental design to avoid confounding factors.
The concentration-dependent effects of perchloric acid pose an additional challenge. At low concentrations, perchloric acid may induce subtle changes in membrane properties that are difficult to detect and quantify. Conversely, at higher concentrations, it can cause rapid and extensive damage, making it challenging to study the progression of interactions over time.
Researchers also face difficulties in developing appropriate model systems that accurately represent the complexity of biological membranes while allowing for controlled experimentation. Simplified membrane models may not fully capture the nuances of perchloric acid interactions in living systems, while studies on intact cells or tissues introduce variables that can complicate data interpretation.
The potential for perchloric acid to induce oxidative stress and generate reactive oxygen species further complicates research efforts. Distinguishing between direct effects of perchloric acid on membranes and secondary effects mediated by oxidative damage requires careful experimental design and the use of appropriate controls and antioxidants.
Lastly, the lack of standardized protocols for studying perchloric acid-membrane interactions hampers the comparison of results across different studies. Variations in experimental conditions, membrane compositions, and analytical methods can lead to conflicting or inconsistent findings, making it challenging to build a coherent understanding of the underlying mechanisms.
Addressing these challenges will require interdisciplinary approaches, combining expertise from chemistry, biology, and biophysics. Advanced imaging techniques, such as high-resolution microscopy and spectroscopy, may offer new insights into the spatial and temporal dynamics of perchloric acid-membrane interactions. Additionally, the development of novel membrane mimetics and in vitro models that better replicate the complexity of biological membranes could provide more relevant and translatable results for understanding these interactions in living systems.
Another challenge lies in the complexity of biological membranes themselves. These structures consist of diverse lipids, proteins, and carbohydrates, each potentially interacting differently with perchloric acid. Elucidating the precise mechanisms of interaction for each component requires sophisticated analytical techniques and careful experimental design to avoid confounding factors.
The concentration-dependent effects of perchloric acid pose an additional challenge. At low concentrations, perchloric acid may induce subtle changes in membrane properties that are difficult to detect and quantify. Conversely, at higher concentrations, it can cause rapid and extensive damage, making it challenging to study the progression of interactions over time.
Researchers also face difficulties in developing appropriate model systems that accurately represent the complexity of biological membranes while allowing for controlled experimentation. Simplified membrane models may not fully capture the nuances of perchloric acid interactions in living systems, while studies on intact cells or tissues introduce variables that can complicate data interpretation.
The potential for perchloric acid to induce oxidative stress and generate reactive oxygen species further complicates research efforts. Distinguishing between direct effects of perchloric acid on membranes and secondary effects mediated by oxidative damage requires careful experimental design and the use of appropriate controls and antioxidants.
Lastly, the lack of standardized protocols for studying perchloric acid-membrane interactions hampers the comparison of results across different studies. Variations in experimental conditions, membrane compositions, and analytical methods can lead to conflicting or inconsistent findings, making it challenging to build a coherent understanding of the underlying mechanisms.
Addressing these challenges will require interdisciplinary approaches, combining expertise from chemistry, biology, and biophysics. Advanced imaging techniques, such as high-resolution microscopy and spectroscopy, may offer new insights into the spatial and temporal dynamics of perchloric acid-membrane interactions. Additionally, the development of novel membrane mimetics and in vitro models that better replicate the complexity of biological membranes could provide more relevant and translatable results for understanding these interactions in living systems.
Existing Methodologies for Studying Acid-Membrane Interactions
01 Chemical reactions and synthesis
Perchloric acid is used in various chemical reactions and synthesis processes. It serves as a strong oxidizing agent and can participate in the production of different compounds. The acid's interaction with other substances is crucial in these processes, often leading to the formation of new materials or products.- Perchloric acid in chemical reactions: Perchloric acid is used as a strong oxidizing agent in various chemical reactions. It plays a crucial role in oxidation processes and can interact with different compounds to facilitate chemical transformations. The acid's strong oxidizing properties make it useful in analytical chemistry and industrial applications.
- Safety measures for handling perchloric acid: Due to its highly reactive nature, special safety precautions are necessary when handling perchloric acid. This includes using appropriate personal protective equipment, proper storage facilities, and specialized handling techniques to prevent accidents and ensure worker safety. Proper ventilation and containment systems are crucial to manage potential hazards.
- Perchloric acid in material analysis: Perchloric acid is utilized in various analytical techniques for material characterization and analysis. It can be used in sample preparation, digestion processes, and as a component in analytical reagents. The acid's strong oxidizing properties make it effective in breaking down complex materials for further analysis.
- Perchloric acid in battery technology: Perchloric acid and its salts find applications in battery technology, particularly in the development of high-performance batteries. It can be used as an electrolyte component or in the preparation of electrode materials. The acid's properties contribute to improved battery performance and efficiency.
- Environmental impact and waste management: The use of perchloric acid raises environmental concerns due to its potential impact on ecosystems. Proper waste management and disposal techniques are essential to minimize environmental contamination. Research focuses on developing methods for the safe neutralization and disposal of perchloric acid waste, as well as strategies for reducing its environmental footprint in industrial processes.
02 Safety and handling procedures
Due to its highly reactive nature, special safety measures and handling procedures are required when working with perchloric acid. This includes the use of specialized equipment, protective gear, and storage facilities to prevent accidents and ensure safe handling of the acid in laboratory and industrial settings.Expand Specific Solutions03 Analytical applications
Perchloric acid finds extensive use in analytical chemistry. It is employed in various analytical techniques, including sample preparation, digestion processes, and as a component in mobile phases for chromatography. The acid's strong oxidizing properties make it valuable for breaking down complex organic compounds in analytical procedures.Expand Specific Solutions04 Material compatibility and corrosion
The interaction of perchloric acid with different materials is a critical consideration in its use and storage. It can cause severe corrosion in many metals and other materials. Understanding these interactions is essential for selecting appropriate containment materials and designing equipment that can withstand the acid's corrosive effects.Expand Specific Solutions05 Environmental and waste management
The use of perchloric acid has environmental implications, necessitating proper waste management and disposal procedures. This includes neutralization techniques, treatment methods, and specialized disposal systems to minimize environmental impact and ensure compliance with regulations governing hazardous chemical waste.Expand Specific Solutions
Key Players in Perchloric Acid Research
The interaction of perchloric acid with biological membranes represents a niche area of research within the broader field of membrane biology and chemical interactions. The competitive landscape is characterized by a limited number of specialized players, primarily academic institutions and research-focused companies. The market is in its early stages, with a relatively small but growing interest from both scientific and industrial sectors. Key players include universities like Université Catholique de Louvain and University of Delaware, as well as companies such as Ecolab USA, Inc. and Kurita Water Industries Ltd. The technology is still in the developmental phase, with ongoing research to fully understand the mechanisms and potential applications. As the field matures, we may see increased collaboration between academic institutions and industry partners to translate findings into practical applications.
Université Catholique de Louvain
Technical Solution: The Université Catholique de Louvain has conducted extensive research on the interaction of perchloric acid with biological membranes. Their approach involves using advanced spectroscopic techniques, such as Fourier-transform infrared spectroscopy (FTIR) and nuclear magnetic resonance (NMR), to study the molecular-level interactions between perchloric acid and lipid bilayers[1]. They have developed a model that describes the penetration of perchloric acid into the membrane and its effects on membrane structure and fluidity. Their research has shown that perchloric acid can cause significant disruption to membrane integrity, leading to increased permeability and potential cell death[2]. The university has also investigated the use of protective agents to mitigate the damaging effects of perchloric acid on biological membranes[3].
Strengths: Advanced analytical techniques, comprehensive molecular-level understanding. Weaknesses: Limited focus on practical applications, primarily academic research.
Beijing University of Chemical Technology
Technical Solution: Beijing University of Chemical Technology has developed a novel approach to studying the interaction of perchloric acid with biological membranes using artificial membrane systems. Their research utilizes liposome models and supported lipid bilayers to simulate biological membranes under controlled conditions[1]. They have employed a combination of techniques, including dynamic light scattering, zeta potential measurements, and atomic force microscopy, to characterize the effects of perchloric acid on membrane properties[2]. Their findings suggest that perchloric acid induces significant changes in membrane surface charge and morphology, leading to potential disruption of cellular functions[3]. The university has also explored the development of protective coatings and materials that can resist perchloric acid-induced damage to biological membranes, with potential applications in protective equipment and medical devices[4].
Strengths: Innovative use of artificial membrane systems, multidisciplinary approach combining chemistry and materials science. Weaknesses: May not fully capture the complexity of real biological systems.
Innovative Approaches in Perchloric Acid-Membrane Research
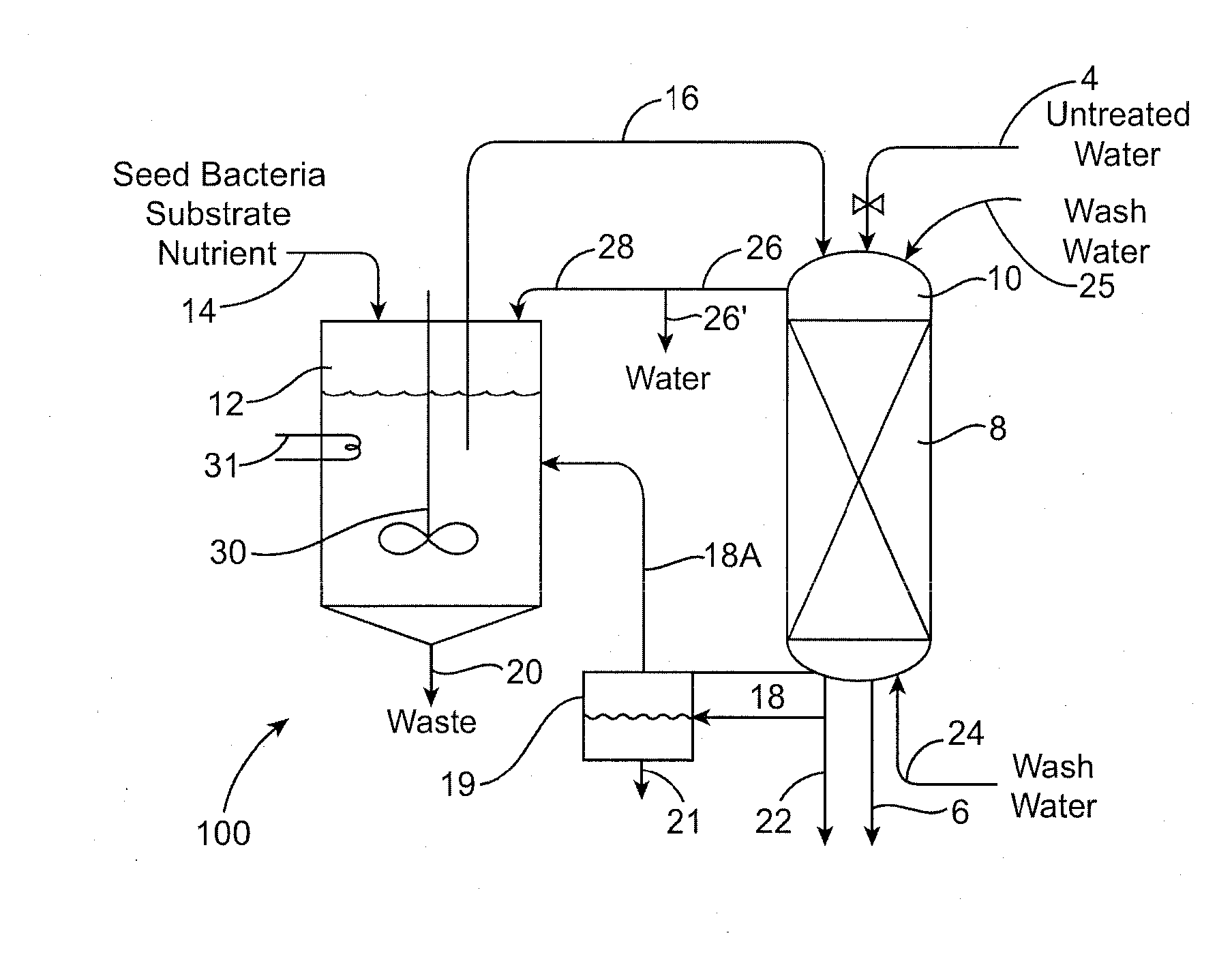
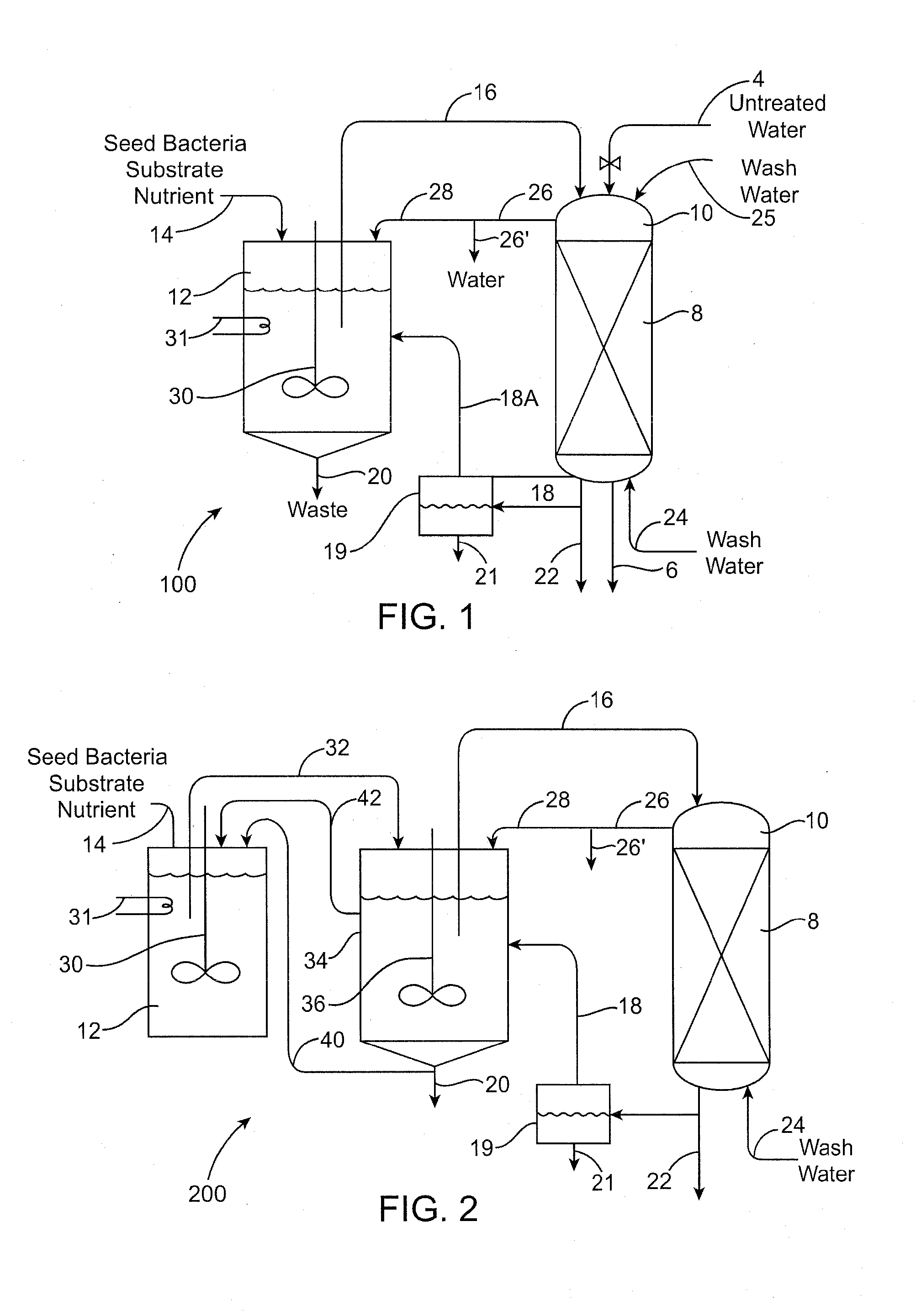
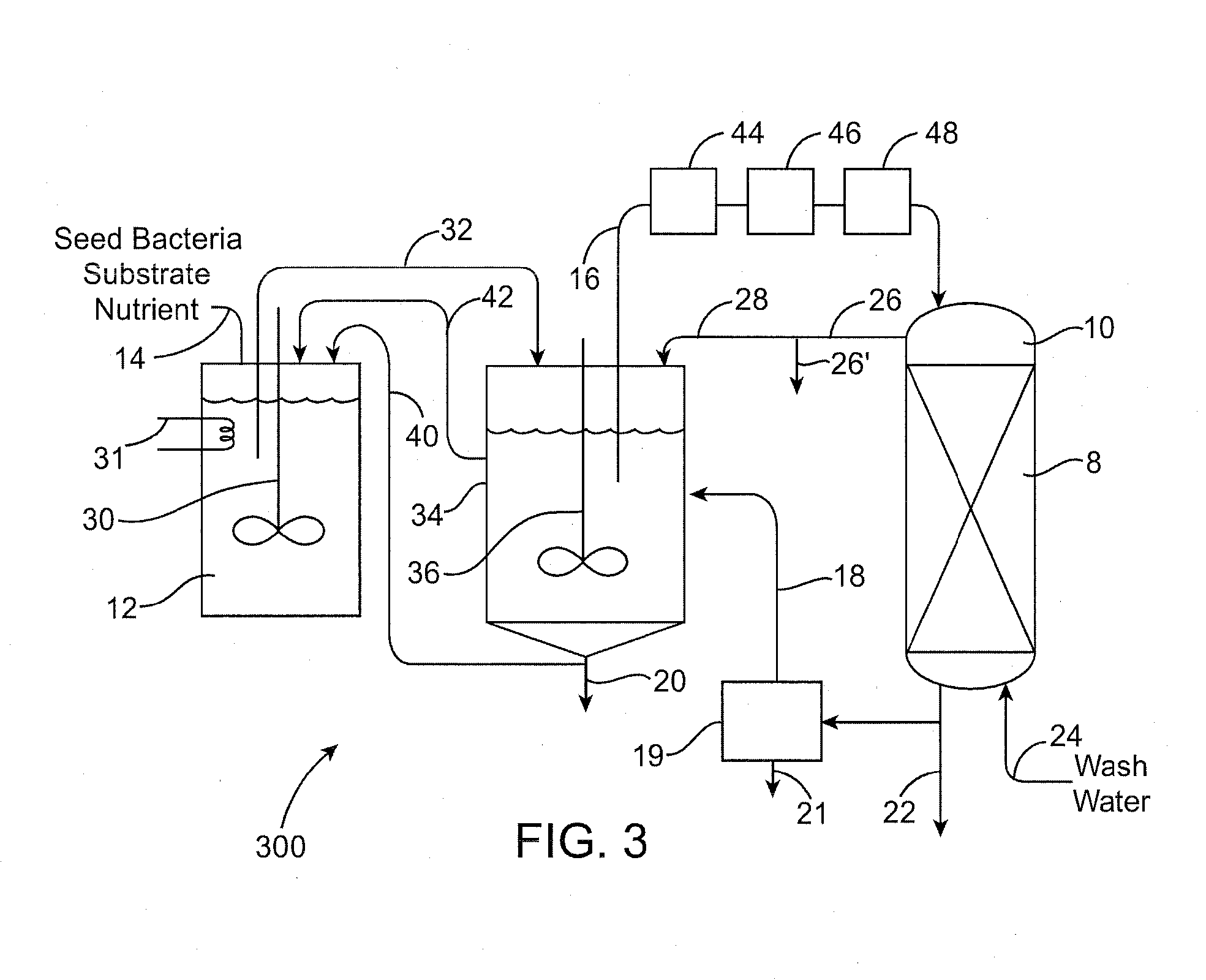
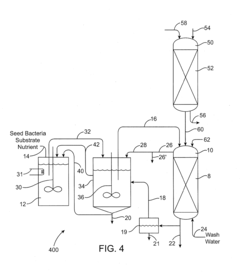
Biodegradation of oxyanions such as perchlorate on ion exchange resins
PatentInactiveUS20090047732A1
Innovation
- A perchlorate-destroying microbial fluid product, enriched with microorganisms such as Dechloromonas and Pseudomonas, is used to contact perchlorate-loaded ion exchange resins, reducing perchlorate loads through anaerobic/anoxic conditions, converting perchlorate to less tightly bound nonperchlorate species like chloride, allowing for resin regeneration and safe disposal or reuse.
Controlled transport through multiple reversible interaction point membranes
PatentInactiveUS20070259452A1
Innovation
- The method involves using a polyelectrolyte complex film with a positively-charged and negatively-charged polyelectrolyte, where a second chemical species selectively interacts with the membrane to create sites for the passage of a first chemical species, enhancing the membrane's permeability and selectivity through reversible interactions.
Safety Regulations for Perchloric Acid Handling
The handling of perchloric acid requires strict adherence to safety regulations due to its highly reactive and potentially explosive nature. Proper safety measures are essential to protect personnel and prevent accidents in laboratory and industrial settings.
Personal protective equipment (PPE) is mandatory when working with perchloric acid. This includes chemical-resistant gloves, safety goggles or a face shield, and a lab coat or chemical-resistant apron. In cases where vapor exposure is possible, respiratory protection may also be necessary.
Storage of perchloric acid must be carefully managed. It should be kept in a cool, well-ventilated area, away from combustible materials and other chemicals. Glass or other inert containers should be used, and secondary containment is recommended to prevent spills.
Dedicated fume hoods with wash-down systems are required for operations involving heated perchloric acid. These specialized hoods prevent the accumulation of explosive perchlorates in the ventilation system.
Spill response procedures must be in place, including the availability of appropriate neutralizing agents and absorbents. Personnel should be trained in proper spill cleanup techniques and the use of emergency equipment.
Regular inspections of storage areas and equipment used with perchloric acid are crucial. This includes checking for signs of corrosion, leaks, or the formation of perchlorate crystals, which can be shock-sensitive.
Proper disposal of perchloric acid waste is essential. It should never be disposed of down the drain or mixed with organic waste. Specialized waste disposal services are typically required for the safe handling of perchloric acid waste.
Training programs for all personnel working with perchloric acid are mandatory. These should cover safe handling procedures, emergency response, and the specific hazards associated with perchloric acid.
Documentation and record-keeping are important aspects of safety regulations. This includes maintaining safety data sheets (SDS), documenting training sessions, and keeping logs of perchloric acid usage and disposal.
Compliance with local, state, and federal regulations regarding the use and storage of perchloric acid is necessary. This may include obtaining permits, reporting requirements, and adhering to specific storage quantity limits.
Personal protective equipment (PPE) is mandatory when working with perchloric acid. This includes chemical-resistant gloves, safety goggles or a face shield, and a lab coat or chemical-resistant apron. In cases where vapor exposure is possible, respiratory protection may also be necessary.
Storage of perchloric acid must be carefully managed. It should be kept in a cool, well-ventilated area, away from combustible materials and other chemicals. Glass or other inert containers should be used, and secondary containment is recommended to prevent spills.
Dedicated fume hoods with wash-down systems are required for operations involving heated perchloric acid. These specialized hoods prevent the accumulation of explosive perchlorates in the ventilation system.
Spill response procedures must be in place, including the availability of appropriate neutralizing agents and absorbents. Personnel should be trained in proper spill cleanup techniques and the use of emergency equipment.
Regular inspections of storage areas and equipment used with perchloric acid are crucial. This includes checking for signs of corrosion, leaks, or the formation of perchlorate crystals, which can be shock-sensitive.
Proper disposal of perchloric acid waste is essential. It should never be disposed of down the drain or mixed with organic waste. Specialized waste disposal services are typically required for the safe handling of perchloric acid waste.
Training programs for all personnel working with perchloric acid are mandatory. These should cover safe handling procedures, emergency response, and the specific hazards associated with perchloric acid.
Documentation and record-keeping are important aspects of safety regulations. This includes maintaining safety data sheets (SDS), documenting training sessions, and keeping logs of perchloric acid usage and disposal.
Compliance with local, state, and federal regulations regarding the use and storage of perchloric acid is necessary. This may include obtaining permits, reporting requirements, and adhering to specific storage quantity limits.
Environmental Impact of Perchloric Acid Use
The use of perchloric acid in various industrial and research applications has raised significant concerns regarding its environmental impact. As a strong oxidizing agent, perchloric acid can have detrimental effects on ecosystems when released into the environment. One of the primary concerns is its potential to contaminate water sources, including groundwater and surface water bodies.
When perchloric acid enters aquatic environments, it can disrupt the natural pH balance, leading to acidification of water bodies. This change in pH can have far-reaching consequences for aquatic life, affecting the survival and reproduction of various species. Fish, amphibians, and aquatic plants are particularly vulnerable to these changes, potentially resulting in population declines and altered ecosystem dynamics.
Furthermore, perchloric acid can persist in the environment for extended periods, especially in soil and sediments. This persistence increases the risk of long-term environmental contamination and bioaccumulation in food chains. As perchlorate ions, a byproduct of perchloric acid, move through ecosystems, they can be taken up by plants and subsequently transferred to higher trophic levels, potentially impacting terrestrial wildlife and human health.
The release of perchloric acid into the atmosphere, often through industrial processes or improper handling, can contribute to air pollution. When perchloric acid vapors combine with moisture in the air, they can form acid rain, exacerbating the environmental impact on both terrestrial and aquatic ecosystems. This can lead to soil degradation, damage to vegetation, and further acidification of water bodies.
In terms of soil contamination, perchloric acid can alter soil chemistry, affecting nutrient availability and microbial communities. This can have cascading effects on plant growth and soil fertility, potentially impacting agricultural productivity in affected areas. Additionally, the mobility of perchlorate ions in soil can lead to widespread contamination of groundwater resources, posing challenges for water treatment and management.
The environmental impact of perchloric acid use extends beyond immediate ecosystems to broader environmental concerns. Its production and use contribute to industrial waste streams, which require specialized treatment and disposal methods to prevent environmental contamination. Improper disposal or accidental releases can lead to localized environmental disasters, necessitating costly cleanup efforts and long-term monitoring programs.
Given these environmental risks, there is a growing emphasis on developing alternative technologies and implementing stricter regulations for the use and handling of perchloric acid. Research into more environmentally friendly substitutes and improved containment and treatment methods is ongoing, aiming to mitigate the environmental impact of this powerful chemical compound.
When perchloric acid enters aquatic environments, it can disrupt the natural pH balance, leading to acidification of water bodies. This change in pH can have far-reaching consequences for aquatic life, affecting the survival and reproduction of various species. Fish, amphibians, and aquatic plants are particularly vulnerable to these changes, potentially resulting in population declines and altered ecosystem dynamics.
Furthermore, perchloric acid can persist in the environment for extended periods, especially in soil and sediments. This persistence increases the risk of long-term environmental contamination and bioaccumulation in food chains. As perchlorate ions, a byproduct of perchloric acid, move through ecosystems, they can be taken up by plants and subsequently transferred to higher trophic levels, potentially impacting terrestrial wildlife and human health.
The release of perchloric acid into the atmosphere, often through industrial processes or improper handling, can contribute to air pollution. When perchloric acid vapors combine with moisture in the air, they can form acid rain, exacerbating the environmental impact on both terrestrial and aquatic ecosystems. This can lead to soil degradation, damage to vegetation, and further acidification of water bodies.
In terms of soil contamination, perchloric acid can alter soil chemistry, affecting nutrient availability and microbial communities. This can have cascading effects on plant growth and soil fertility, potentially impacting agricultural productivity in affected areas. Additionally, the mobility of perchlorate ions in soil can lead to widespread contamination of groundwater resources, posing challenges for water treatment and management.
The environmental impact of perchloric acid use extends beyond immediate ecosystems to broader environmental concerns. Its production and use contribute to industrial waste streams, which require specialized treatment and disposal methods to prevent environmental contamination. Improper disposal or accidental releases can lead to localized environmental disasters, necessitating costly cleanup efforts and long-term monitoring programs.
Given these environmental risks, there is a growing emphasis on developing alternative technologies and implementing stricter regulations for the use and handling of perchloric acid. Research into more environmentally friendly substitutes and improved containment and treatment methods is ongoing, aiming to mitigate the environmental impact of this powerful chemical compound.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!