The Pharmacological Dissection of Muscimol in Toxicology
JUL 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Muscimol Toxicology Background and Objectives
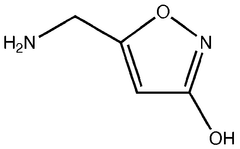
Muscimol, a potent psychoactive compound found in various species of mushrooms, particularly Amanita muscaria, has been a subject of fascination and study in the field of toxicology for decades. This naturally occurring GABA receptor agonist has played a significant role in shaping our understanding of neurotransmitter systems and their impact on human physiology and behavior.
The exploration of muscimol's toxicological profile dates back to the mid-20th century, with early studies focusing on its psychoactive effects and potential therapeutic applications. As research progressed, the compound's mechanism of action became clearer, revealing its strong affinity for GABA-A receptors in the central nervous system. This discovery opened new avenues for investigating the GABAergic system and its role in various neurological and psychiatric disorders.
In recent years, the pharmacological dissection of muscimol has gained renewed interest due to advancements in neuroscience and drug discovery technologies. Researchers are now able to examine the compound's effects at a molecular level, providing insights into its structure-activity relationships and potential for developing novel therapeutic agents.
The primary objective of current muscimol toxicology research is to comprehensively understand its pharmacokinetics, pharmacodynamics, and toxicological profile. This includes elucidating the compound's absorption, distribution, metabolism, and excretion (ADME) properties, as well as its acute and chronic effects on various organ systems. Additionally, researchers aim to identify potential drug interactions and assess the risk of dependence or addiction associated with muscimol use.
Another crucial goal is to explore the therapeutic potential of muscimol and its derivatives. By leveraging its GABA-A receptor agonist properties, scientists are investigating its possible applications in treating anxiety disorders, epilepsy, and sleep disturbances. However, the development of safe and effective muscimol-based therapies requires a thorough understanding of its toxicological profile and potential side effects.
Furthermore, the study of muscimol's toxicology has implications beyond human health. Ecological research aims to understand the compound's role in fungal defense mechanisms and its impact on wildlife that may consume mushrooms containing muscimol. This broader perspective contributes to our understanding of natural toxins and their evolutionary significance.
As technology continues to advance, new tools such as high-throughput screening, in silico modeling, and advanced imaging techniques are being employed to further dissect muscimol's pharmacological properties. These approaches promise to reveal new insights into the compound's molecular interactions and potential off-target effects, ultimately contributing to a more comprehensive toxicological profile.
The exploration of muscimol's toxicological profile dates back to the mid-20th century, with early studies focusing on its psychoactive effects and potential therapeutic applications. As research progressed, the compound's mechanism of action became clearer, revealing its strong affinity for GABA-A receptors in the central nervous system. This discovery opened new avenues for investigating the GABAergic system and its role in various neurological and psychiatric disorders.
In recent years, the pharmacological dissection of muscimol has gained renewed interest due to advancements in neuroscience and drug discovery technologies. Researchers are now able to examine the compound's effects at a molecular level, providing insights into its structure-activity relationships and potential for developing novel therapeutic agents.
The primary objective of current muscimol toxicology research is to comprehensively understand its pharmacokinetics, pharmacodynamics, and toxicological profile. This includes elucidating the compound's absorption, distribution, metabolism, and excretion (ADME) properties, as well as its acute and chronic effects on various organ systems. Additionally, researchers aim to identify potential drug interactions and assess the risk of dependence or addiction associated with muscimol use.
Another crucial goal is to explore the therapeutic potential of muscimol and its derivatives. By leveraging its GABA-A receptor agonist properties, scientists are investigating its possible applications in treating anxiety disorders, epilepsy, and sleep disturbances. However, the development of safe and effective muscimol-based therapies requires a thorough understanding of its toxicological profile and potential side effects.
Furthermore, the study of muscimol's toxicology has implications beyond human health. Ecological research aims to understand the compound's role in fungal defense mechanisms and its impact on wildlife that may consume mushrooms containing muscimol. This broader perspective contributes to our understanding of natural toxins and their evolutionary significance.
As technology continues to advance, new tools such as high-throughput screening, in silico modeling, and advanced imaging techniques are being employed to further dissect muscimol's pharmacological properties. These approaches promise to reveal new insights into the compound's molecular interactions and potential off-target effects, ultimately contributing to a more comprehensive toxicological profile.
Market Analysis for Muscimol-Based Research
The market for muscimol-based research is experiencing significant growth, driven by increasing interest in its potential therapeutic applications and the expanding field of neuropharmacology. Muscimol, a potent GABA receptor agonist derived from the Amanita muscaria mushroom, has garnered attention for its unique pharmacological properties and potential in treating various neurological disorders.
The global neuroscience market, which encompasses muscimol research, is projected to expand substantially in the coming years. This growth is fueled by the rising prevalence of neurological disorders, increased funding for neuroscience research, and advancements in brain imaging technologies. Within this broader context, the specific market for GABA receptor modulators, including muscimol, is showing promising trends.
Pharmaceutical companies and research institutions are increasingly investing in the development of novel GABA-targeting compounds, with muscimol serving as a valuable research tool and potential therapeutic agent. The market for muscimol and related compounds is particularly robust in regions with strong biotechnology and pharmaceutical sectors, such as North America, Europe, and parts of Asia.
One of the key drivers of market demand for muscimol-based research is its potential application in treating anxiety disorders, epilepsy, and sleep disturbances. As mental health awareness grows globally, there is an increasing need for innovative treatments that can address these conditions more effectively and with fewer side effects than existing medications.
The toxicology sector also represents a significant market for muscimol research. As regulatory bodies worldwide tighten safety standards for pharmaceuticals and environmental contaminants, there is a growing demand for advanced toxicological screening methods. Muscimol's well-defined pharmacological profile makes it an excellent candidate for developing new toxicity assessment tools and models.
Academic research institutions constitute another major market segment for muscimol-based studies. The compound's unique properties make it valuable for investigating GABAergic neurotransmission, neuroplasticity, and cognitive function. This academic interest translates into a steady demand for high-purity muscimol and related research tools.
The market for muscimol research is also benefiting from technological advancements in drug delivery systems and synthetic chemistry. These developments are enabling more precise and controlled administration of muscimol in experimental settings, expanding its research applications and potential therapeutic uses.
While the market shows promising growth, it faces challenges such as regulatory hurdles and the need for extensive clinical trials to establish safety and efficacy profiles for muscimol-based therapies. However, the increasing collaboration between academic institutions and pharmaceutical companies is expected to accelerate research progress and market development in this field.
The global neuroscience market, which encompasses muscimol research, is projected to expand substantially in the coming years. This growth is fueled by the rising prevalence of neurological disorders, increased funding for neuroscience research, and advancements in brain imaging technologies. Within this broader context, the specific market for GABA receptor modulators, including muscimol, is showing promising trends.
Pharmaceutical companies and research institutions are increasingly investing in the development of novel GABA-targeting compounds, with muscimol serving as a valuable research tool and potential therapeutic agent. The market for muscimol and related compounds is particularly robust in regions with strong biotechnology and pharmaceutical sectors, such as North America, Europe, and parts of Asia.
One of the key drivers of market demand for muscimol-based research is its potential application in treating anxiety disorders, epilepsy, and sleep disturbances. As mental health awareness grows globally, there is an increasing need for innovative treatments that can address these conditions more effectively and with fewer side effects than existing medications.
The toxicology sector also represents a significant market for muscimol research. As regulatory bodies worldwide tighten safety standards for pharmaceuticals and environmental contaminants, there is a growing demand for advanced toxicological screening methods. Muscimol's well-defined pharmacological profile makes it an excellent candidate for developing new toxicity assessment tools and models.
Academic research institutions constitute another major market segment for muscimol-based studies. The compound's unique properties make it valuable for investigating GABAergic neurotransmission, neuroplasticity, and cognitive function. This academic interest translates into a steady demand for high-purity muscimol and related research tools.
The market for muscimol research is also benefiting from technological advancements in drug delivery systems and synthetic chemistry. These developments are enabling more precise and controlled administration of muscimol in experimental settings, expanding its research applications and potential therapeutic uses.
While the market shows promising growth, it faces challenges such as regulatory hurdles and the need for extensive clinical trials to establish safety and efficacy profiles for muscimol-based therapies. However, the increasing collaboration between academic institutions and pharmaceutical companies is expected to accelerate research progress and market development in this field.
Current Challenges in Muscimol Toxicology
The field of muscimol toxicology currently faces several significant challenges that hinder comprehensive understanding and effective management of its pharmacological effects. One primary obstacle is the limited availability of standardized analytical methods for detecting and quantifying muscimol in biological samples. This lack of standardization makes it difficult to compare results across different studies and laboratories, potentially leading to inconsistencies in toxicological assessments.
Another pressing challenge is the incomplete understanding of muscimol's complex pharmacokinetics and pharmacodynamics. While it is known that muscimol primarily acts as a potent GABA-A receptor agonist, the full spectrum of its interactions with other neurotransmitter systems and cellular pathways remains unclear. This knowledge gap impedes the development of targeted interventions and antidotes for muscimol intoxication.
The variability in individual responses to muscimol exposure presents a significant hurdle in toxicology. Factors such as genetic polymorphisms, age, and pre-existing medical conditions can greatly influence the severity and manifestation of muscimol toxicity. This heterogeneity complicates the establishment of universal guidelines for diagnosis and treatment.
Furthermore, the potential long-term effects of chronic low-dose muscimol exposure are not well-characterized. This is particularly concerning given the increasing interest in microdosing practices and the use of muscimol-containing mushrooms in traditional medicine. The lack of longitudinal studies on the cumulative effects of muscimol poses challenges for risk assessment and public health recommendations.
The emergence of novel synthetic analogues of muscimol introduces additional complexities to toxicological evaluations. These designer compounds may exhibit altered pharmacological profiles, potentially leading to unexpected toxicities that are not easily detected by conventional screening methods. This evolving landscape necessitates continuous adaptation of analytical techniques and toxicological assessments.
Lastly, the ethical and regulatory challenges surrounding research on muscimol and its derivatives impede progress in toxicological studies. Restrictions on the use of psychoactive substances in research settings limit the scope and depth of investigations, particularly in human subjects. This constraint hampers the development of evidence-based protocols for managing muscimol intoxication and understanding its broader pharmacological impacts.
Addressing these challenges requires a multidisciplinary approach, combining advances in analytical chemistry, neuropharmacology, and clinical toxicology. Collaborative efforts between academic institutions, regulatory bodies, and pharmaceutical industries are essential to overcome these hurdles and enhance our understanding of muscimol toxicology.
Another pressing challenge is the incomplete understanding of muscimol's complex pharmacokinetics and pharmacodynamics. While it is known that muscimol primarily acts as a potent GABA-A receptor agonist, the full spectrum of its interactions with other neurotransmitter systems and cellular pathways remains unclear. This knowledge gap impedes the development of targeted interventions and antidotes for muscimol intoxication.
The variability in individual responses to muscimol exposure presents a significant hurdle in toxicology. Factors such as genetic polymorphisms, age, and pre-existing medical conditions can greatly influence the severity and manifestation of muscimol toxicity. This heterogeneity complicates the establishment of universal guidelines for diagnosis and treatment.
Furthermore, the potential long-term effects of chronic low-dose muscimol exposure are not well-characterized. This is particularly concerning given the increasing interest in microdosing practices and the use of muscimol-containing mushrooms in traditional medicine. The lack of longitudinal studies on the cumulative effects of muscimol poses challenges for risk assessment and public health recommendations.
The emergence of novel synthetic analogues of muscimol introduces additional complexities to toxicological evaluations. These designer compounds may exhibit altered pharmacological profiles, potentially leading to unexpected toxicities that are not easily detected by conventional screening methods. This evolving landscape necessitates continuous adaptation of analytical techniques and toxicological assessments.
Lastly, the ethical and regulatory challenges surrounding research on muscimol and its derivatives impede progress in toxicological studies. Restrictions on the use of psychoactive substances in research settings limit the scope and depth of investigations, particularly in human subjects. This constraint hampers the development of evidence-based protocols for managing muscimol intoxication and understanding its broader pharmacological impacts.
Addressing these challenges requires a multidisciplinary approach, combining advances in analytical chemistry, neuropharmacology, and clinical toxicology. Collaborative efforts between academic institutions, regulatory bodies, and pharmaceutical industries are essential to overcome these hurdles and enhance our understanding of muscimol toxicology.
Existing Muscimol Toxicology Methodologies
01 Pharmaceutical compositions containing muscimol
Muscimol is used in pharmaceutical compositions for various therapeutic applications. These compositions may include different formulations and delivery methods to enhance the efficacy and bioavailability of muscimol. The compositions can be designed for treating neurological disorders, anxiety, or other conditions affected by GABA receptor modulation.- Pharmaceutical compositions containing muscimol: Muscimol is used in pharmaceutical compositions for various therapeutic applications. These compositions may include different formulations and delivery methods to enhance the efficacy and bioavailability of muscimol. The compositions can be designed for treating neurological disorders, anxiety, or other conditions affected by GABA receptor modulation.
- Muscimol analogs and derivatives: Research focuses on developing and synthesizing muscimol analogs and derivatives. These modified compounds aim to improve upon the properties of muscimol, such as increased potency, selectivity, or reduced side effects. The analogs may have different chemical structures but retain or enhance the pharmacological activity of muscimol.
- Methods of administering muscimol: Various methods for administering muscimol are explored to optimize its therapeutic effects. These may include novel drug delivery systems, controlled release formulations, or targeted delivery to specific areas of the body. The administration methods aim to improve the drug's efficacy, reduce side effects, and enhance patient compliance.
- Muscimol in combination therapies: Muscimol is studied in combination with other therapeutic agents to create synergistic effects or address multiple aspects of a condition. These combination therapies may involve using muscimol alongside other GABA modulators, antidepressants, or compounds that target different neurotransmitter systems to enhance overall treatment efficacy.
- Muscimol for specific medical indications: Research investigates the use of muscimol for specific medical indications beyond its traditional applications. This includes exploring its potential in treating conditions such as epilepsy, sleep disorders, or certain types of pain. Studies focus on determining optimal dosages, treatment durations, and patient populations for these new indications.
02 Muscimol derivatives and analogs
Research focuses on developing muscimol derivatives and analogs with improved pharmacological properties. These modified compounds aim to enhance therapeutic effects, reduce side effects, or improve drug delivery. Synthetic methods for creating these derivatives are explored to optimize their potential as pharmaceutical agents.Expand Specific Solutions03 Use of muscimol in neurostimulation therapies
Muscimol is investigated for its potential in neurostimulation therapies. It may be used in combination with electrical or other forms of stimulation to modulate neural activity. This approach is explored for treating various neurological and psychiatric conditions, potentially offering new therapeutic strategies.Expand Specific Solutions04 Muscimol in combination therapies
Muscimol is studied in combination with other active ingredients to create synergistic therapeutic effects. These combinations may target multiple pathways or receptors simultaneously, potentially enhancing treatment efficacy for complex disorders. The combinations are explored for various indications, including neurological and psychiatric conditions.Expand Specific Solutions05 Novel delivery systems for muscimol
Innovative delivery systems are developed to improve the administration and efficacy of muscimol. These may include nanoparticle formulations, transdermal patches, or other advanced drug delivery technologies. The goal is to enhance bioavailability, control release rates, or target specific areas of the body for optimal therapeutic effect.Expand Specific Solutions
Key Players in Muscimol Research
The pharmacological dissection of muscimol in toxicology represents a niche yet evolving field within the broader pharmaceutical and biotechnology landscape. The market is in its early stages, with limited commercial applications but growing research interest. Key players like Vertex Pharmaceuticals, ACADIA Pharmaceuticals, and CaaMTech are exploring muscimol's potential in neuroscience and mental health applications. The technology is still in the research and development phase, with most activities focused on preclinical studies and early-stage clinical trials. As the understanding of muscimol's mechanisms and potential therapeutic applications deepens, we can expect increased investment and market growth in this specialized area of pharmacology.
Psyched Wellness Ltd.
Technical Solution: Psyched Wellness is pioneering the use of Amanita muscaria mushroom extract, which contains muscimol, for potential therapeutic applications. Their approach involves developing a proprietary extraction and purification process to create a standardized extract of muscimol[1]. The company is conducting preclinical studies to evaluate the safety and efficacy of their AME-1 formulation, which is derived from Amanita muscaria. They are exploring its potential for stress reduction, promoting restful sleep, and supporting cognitive function[2]. Psyched Wellness is also investigating the pharmacokinetics and pharmacodynamics of muscimol to better understand its mechanisms of action and potential therapeutic applications[3].
Strengths: Focused research on muscimol, innovative extraction techniques, potential for novel therapeutic applications. Weaknesses: Early-stage research, regulatory challenges, limited clinical data on muscimol's long-term effects.
CaaMTech LLC
Technical Solution: CaaMTech is at the forefront of muscimol research, focusing on the development of novel prodrug formulations to enhance its therapeutic potential. Their approach involves synthesizing muscimol derivatives with improved pharmacokinetic profiles, aiming to optimize bioavailability and reduce potential side effects[4]. The company has developed a series of patented muscimol prodrugs, including water-soluble variants, which could potentially address challenges in drug delivery and absorption[5]. CaaMTech is also exploring the synergistic effects of muscimol with other compounds found in Amanita muscaria, aiming to create more effective and targeted therapeutic formulations[6].
Strengths: Advanced chemical synthesis capabilities, focus on improving muscimol's pharmacological properties, potential for novel drug formulations. Weaknesses: Early-stage research, complex regulatory pathway for novel compounds, potential competition from established pharmaceuticals.
Core Innovations in Muscimol Pharmacology
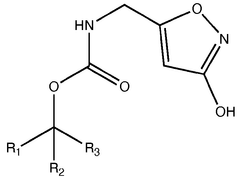
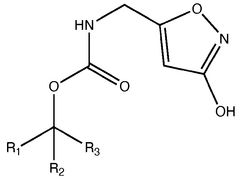
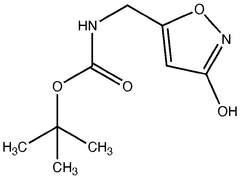
Pharmaceutical intermediates and methods for preparing the same in the synthesis of muscimol and congeners and derivatives thereof
PatentWO2025128106A1
Innovation
- A novel method for preparing muscimol mono-BOC and muscimol hydrochloride that avoids the use of ion exchange chromatography by modifying the original synthetic route to include a flow reactor for the cyclization step and using BOC anhydride to purify the muscimol, thereby stabilizing the product and improving yields.
Processes for extracting muscimol from amanita muscaria
PatentWO2022187974A1
Innovation
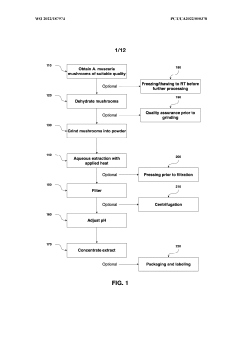
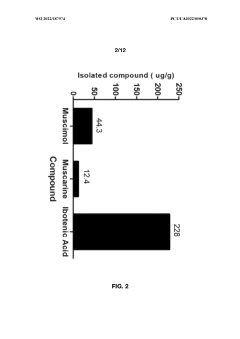
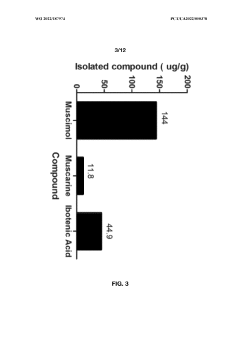
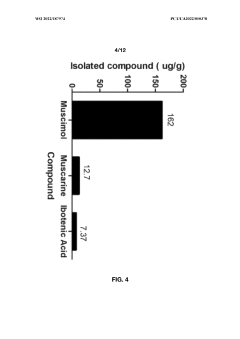
- Aqueous extraction methods involving heat, pH reduction, and concentration techniques such as distillation and refluxing are employed to decrease ibotenic acid content and increase muscimol content in the extract, including steps like grinding the mushroom biomass, filtering, and acidification to facilitate decarboxylation of ibotenic acid to muscimol.
Regulatory Framework for Muscimol Studies
The regulatory framework for muscimol studies is a complex and evolving landscape that requires careful navigation by researchers and pharmaceutical companies. At the international level, the World Health Organization (WHO) provides guidelines for the classification and control of psychoactive substances, including muscimol. These guidelines inform national policies and regulations across different countries.
In the United States, the Food and Drug Administration (FDA) plays a crucial role in overseeing muscimol research. The FDA's Center for Drug Evaluation and Research (CDER) is responsible for evaluating the safety and efficacy of new drugs, including those derived from or related to muscimol. Researchers must comply with the FDA's Investigational New Drug (IND) application process before conducting clinical trials involving muscimol or its derivatives.
The Drug Enforcement Administration (DEA) also has a significant role in regulating muscimol studies. As a psychoactive compound, muscimol is subject to the Controlled Substances Act. Currently, muscimol itself is not specifically scheduled, but its source, Amanita muscaria mushrooms, is listed as a poisonous plant. This creates a regulatory gray area that researchers must carefully navigate.
In Europe, the European Medicines Agency (EMA) provides guidance on the development and testing of new pharmaceutical compounds. Researchers working with muscimol must adhere to the EMA's guidelines on non-clinical and clinical development, as well as specific regulations related to psychoactive substances.
Ethical considerations are paramount in muscimol research. Institutional Review Boards (IRBs) or Ethics Committees play a crucial role in ensuring that studies involving human subjects are conducted safely and ethically. These bodies review research protocols, informed consent procedures, and potential risks to participants.
Safety monitoring is a critical aspect of the regulatory framework. Researchers must implement robust pharmacovigilance systems to detect and report adverse events related to muscimol use. This includes both short-term effects observed during clinical trials and potential long-term consequences that may emerge through post-market surveillance.
Data protection and privacy regulations also impact muscimol studies. Researchers must comply with laws such as the General Data Protection Regulation (GDPR) in Europe or the Health Insurance Portability and Accountability Act (HIPAA) in the United States when handling participant data.
As research into muscimol and related compounds progresses, regulatory frameworks are likely to evolve. Researchers and pharmaceutical companies must stay informed about changes in regulations and guidelines to ensure compliance and facilitate the development of potential therapeutic applications of muscimol in toxicology.
In the United States, the Food and Drug Administration (FDA) plays a crucial role in overseeing muscimol research. The FDA's Center for Drug Evaluation and Research (CDER) is responsible for evaluating the safety and efficacy of new drugs, including those derived from or related to muscimol. Researchers must comply with the FDA's Investigational New Drug (IND) application process before conducting clinical trials involving muscimol or its derivatives.
The Drug Enforcement Administration (DEA) also has a significant role in regulating muscimol studies. As a psychoactive compound, muscimol is subject to the Controlled Substances Act. Currently, muscimol itself is not specifically scheduled, but its source, Amanita muscaria mushrooms, is listed as a poisonous plant. This creates a regulatory gray area that researchers must carefully navigate.
In Europe, the European Medicines Agency (EMA) provides guidance on the development and testing of new pharmaceutical compounds. Researchers working with muscimol must adhere to the EMA's guidelines on non-clinical and clinical development, as well as specific regulations related to psychoactive substances.
Ethical considerations are paramount in muscimol research. Institutional Review Boards (IRBs) or Ethics Committees play a crucial role in ensuring that studies involving human subjects are conducted safely and ethically. These bodies review research protocols, informed consent procedures, and potential risks to participants.
Safety monitoring is a critical aspect of the regulatory framework. Researchers must implement robust pharmacovigilance systems to detect and report adverse events related to muscimol use. This includes both short-term effects observed during clinical trials and potential long-term consequences that may emerge through post-market surveillance.
Data protection and privacy regulations also impact muscimol studies. Researchers must comply with laws such as the General Data Protection Regulation (GDPR) in Europe or the Health Insurance Portability and Accountability Act (HIPAA) in the United States when handling participant data.
As research into muscimol and related compounds progresses, regulatory frameworks are likely to evolve. Researchers and pharmaceutical companies must stay informed about changes in regulations and guidelines to ensure compliance and facilitate the development of potential therapeutic applications of muscimol in toxicology.
Ethical Considerations in Muscimol Research
The ethical considerations in muscimol research are multifaceted and require careful attention from researchers, regulatory bodies, and the scientific community at large. One primary concern is the potential for abuse and misuse of muscimol, given its psychoactive properties. As a GABA receptor agonist, muscimol can produce sedative, hypnotic, and hallucinogenic effects, which raises questions about its controlled substance status and the need for strict regulations in its handling and distribution for research purposes.
Another critical ethical issue is the use of animal models in muscimol toxicology studies. While animal research has been instrumental in understanding the pharmacological properties of muscimol, it is essential to adhere to the principles of the 3Rs (Replacement, Reduction, and Refinement) to minimize animal suffering and maximize the scientific value of experiments. Researchers must justify the use of animal models and explore alternative methods where possible.
The potential therapeutic applications of muscimol, particularly in neurological disorders, bring forth ethical considerations regarding clinical trials and human subject research. Ensuring informed consent, minimizing risks to participants, and maintaining transparency in reporting both positive and negative results are paramount. Additionally, the long-term effects of muscimol exposure, especially on brain function and development, must be thoroughly investigated before considering any therapeutic applications.
Ethical sourcing of muscimol is another important consideration. As it is naturally found in certain mushroom species, there are concerns about the environmental impact of harvesting these fungi and the potential exploitation of indigenous knowledge related to their use. Researchers should prioritize sustainable and responsible sourcing practices.
Data integrity and reproducibility in muscimol research also present ethical challenges. Given the complex nature of its effects on the central nervous system, researchers must be vigilant in their methodologies and data reporting to prevent misinterpretation or misuse of findings. This includes addressing potential conflicts of interest and ensuring transparency in funding sources.
Lastly, the ethical implications of muscimol research extend to public health and safety. As knowledge about its pharmacological properties advances, there is a responsibility to communicate findings accurately to the public and policymakers. This includes addressing potential risks associated with recreational use and the importance of maintaining a balanced perspective on both the potential benefits and dangers of muscimol-related compounds.
Another critical ethical issue is the use of animal models in muscimol toxicology studies. While animal research has been instrumental in understanding the pharmacological properties of muscimol, it is essential to adhere to the principles of the 3Rs (Replacement, Reduction, and Refinement) to minimize animal suffering and maximize the scientific value of experiments. Researchers must justify the use of animal models and explore alternative methods where possible.
The potential therapeutic applications of muscimol, particularly in neurological disorders, bring forth ethical considerations regarding clinical trials and human subject research. Ensuring informed consent, minimizing risks to participants, and maintaining transparency in reporting both positive and negative results are paramount. Additionally, the long-term effects of muscimol exposure, especially on brain function and development, must be thoroughly investigated before considering any therapeutic applications.
Ethical sourcing of muscimol is another important consideration. As it is naturally found in certain mushroom species, there are concerns about the environmental impact of harvesting these fungi and the potential exploitation of indigenous knowledge related to their use. Researchers should prioritize sustainable and responsible sourcing practices.
Data integrity and reproducibility in muscimol research also present ethical challenges. Given the complex nature of its effects on the central nervous system, researchers must be vigilant in their methodologies and data reporting to prevent misinterpretation or misuse of findings. This includes addressing potential conflicts of interest and ensuring transparency in funding sources.
Lastly, the ethical implications of muscimol research extend to public health and safety. As knowledge about its pharmacological properties advances, there is a responsibility to communicate findings accurately to the public and policymakers. This includes addressing potential risks associated with recreational use and the importance of maintaining a balanced perspective on both the potential benefits and dangers of muscimol-related compounds.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!