The Science Behind Antifreeze's Corrosion Inhibitors
JUL 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Antifreeze Corrosion Inhibition: Background and Objectives
Antifreeze, a crucial component in automotive and industrial cooling systems, has been a subject of continuous technological evolution since its inception. The primary function of antifreeze is to lower the freezing point of water-based coolants, preventing damage to engines and machinery in cold temperatures. However, the corrosive nature of these solutions posed significant challenges to the longevity and efficiency of cooling systems.
The development of corrosion inhibitors for antifreeze solutions marks a pivotal advancement in this field. These inhibitors are designed to protect metal components from degradation caused by the aggressive chemical environment within cooling systems. The science behind antifreeze corrosion inhibitors has its roots in electrochemistry and materials science, with early research dating back to the mid-20th century.
As automotive and industrial technologies progressed, the demand for more effective and environmentally friendly antifreeze formulations grew. This led to intensive research efforts focused on understanding the mechanisms of corrosion in cooling systems and developing innovative inhibitor compounds. The objectives of these studies were multifaceted, aiming to enhance the protective capabilities of antifreeze while addressing concerns related to toxicity, biodegradability, and overall system performance.
The evolution of antifreeze corrosion inhibitors has been driven by several key factors. These include the need for compatibility with a wide range of metals used in modern engines, the desire for extended service intervals, and increasingly stringent environmental regulations. Researchers have explored various classes of organic and inorganic compounds, each offering unique protective properties and presenting distinct challenges in formulation and application.
One of the primary goals in the development of antifreeze corrosion inhibitors has been to create synergistic blends that provide comprehensive protection against different types of corrosion. This includes inhibition of uniform corrosion, pitting, crevice corrosion, and galvanic corrosion. Additionally, these inhibitors must maintain their effectiveness over a broad range of temperatures and pH levels, ensuring reliable performance under diverse operating conditions.
The scientific pursuit of advanced antifreeze corrosion inhibitors continues to evolve, with current research focusing on nanotechnology-based solutions, smart inhibitors that respond to environmental changes, and bio-inspired compounds that mimic natural anti-corrosion mechanisms. These cutting-edge approaches aim to push the boundaries of corrosion protection, potentially revolutionizing the field of coolant technology.
The development of corrosion inhibitors for antifreeze solutions marks a pivotal advancement in this field. These inhibitors are designed to protect metal components from degradation caused by the aggressive chemical environment within cooling systems. The science behind antifreeze corrosion inhibitors has its roots in electrochemistry and materials science, with early research dating back to the mid-20th century.
As automotive and industrial technologies progressed, the demand for more effective and environmentally friendly antifreeze formulations grew. This led to intensive research efforts focused on understanding the mechanisms of corrosion in cooling systems and developing innovative inhibitor compounds. The objectives of these studies were multifaceted, aiming to enhance the protective capabilities of antifreeze while addressing concerns related to toxicity, biodegradability, and overall system performance.
The evolution of antifreeze corrosion inhibitors has been driven by several key factors. These include the need for compatibility with a wide range of metals used in modern engines, the desire for extended service intervals, and increasingly stringent environmental regulations. Researchers have explored various classes of organic and inorganic compounds, each offering unique protective properties and presenting distinct challenges in formulation and application.
One of the primary goals in the development of antifreeze corrosion inhibitors has been to create synergistic blends that provide comprehensive protection against different types of corrosion. This includes inhibition of uniform corrosion, pitting, crevice corrosion, and galvanic corrosion. Additionally, these inhibitors must maintain their effectiveness over a broad range of temperatures and pH levels, ensuring reliable performance under diverse operating conditions.
The scientific pursuit of advanced antifreeze corrosion inhibitors continues to evolve, with current research focusing on nanotechnology-based solutions, smart inhibitors that respond to environmental changes, and bio-inspired compounds that mimic natural anti-corrosion mechanisms. These cutting-edge approaches aim to push the boundaries of corrosion protection, potentially revolutionizing the field of coolant technology.
Market Analysis of Corrosion-Resistant Antifreeze
The market for corrosion-resistant antifreeze has experienced significant growth in recent years, driven by increasing awareness of the importance of engine protection and the rising demand for high-performance vehicles. The global antifreeze market, which includes corrosion-resistant formulations, was valued at approximately $5.5 billion in 2020 and is projected to reach $7.9 billion by 2027, growing at a CAGR of 5.2% during the forecast period.
The automotive sector remains the primary consumer of corrosion-resistant antifreeze, accounting for over 70% of the market share. This dominance is attributed to the growing automotive industry, particularly in emerging economies, and the increasing average lifespan of vehicles. The commercial vehicle segment, including trucks and buses, has shown the fastest growth rate due to the need for enhanced engine protection in heavy-duty applications.
Geographically, North America and Europe have traditionally been the largest markets for corrosion-resistant antifreeze, owing to their mature automotive industries and stringent environmental regulations. However, the Asia-Pacific region is emerging as the fastest-growing market, with China and India leading the charge. This growth is fueled by rapid industrialization, increasing vehicle ownership, and a growing focus on vehicle maintenance.
The market is characterized by intense competition among key players such as Shell, ExxonMobil, Total, and BASF. These companies are investing heavily in research and development to create more effective and environmentally friendly corrosion inhibitors. There is a notable trend towards the development of organic acid technology (OAT) based antifreeze, which offers superior corrosion protection and longer service life compared to traditional formulations.
Environmental concerns and regulatory pressures are shaping the market landscape. There is a growing demand for eco-friendly antifreeze products that are biodegradable and less toxic. This has led to the development of propylene glycol-based antifreeze as an alternative to the more toxic ethylene glycol-based products. The shift towards electric vehicles (EVs) is also influencing the market, as EVs require specialized cooling systems with different corrosion inhibition needs.
The aftermarket segment for corrosion-resistant antifreeze is witnessing steady growth, driven by increasing consumer awareness about the importance of regular coolant replacement. This trend is particularly strong in developed markets where vehicle owners are more likely to invest in premium, long-lasting antifreeze products.
The automotive sector remains the primary consumer of corrosion-resistant antifreeze, accounting for over 70% of the market share. This dominance is attributed to the growing automotive industry, particularly in emerging economies, and the increasing average lifespan of vehicles. The commercial vehicle segment, including trucks and buses, has shown the fastest growth rate due to the need for enhanced engine protection in heavy-duty applications.
Geographically, North America and Europe have traditionally been the largest markets for corrosion-resistant antifreeze, owing to their mature automotive industries and stringent environmental regulations. However, the Asia-Pacific region is emerging as the fastest-growing market, with China and India leading the charge. This growth is fueled by rapid industrialization, increasing vehicle ownership, and a growing focus on vehicle maintenance.
The market is characterized by intense competition among key players such as Shell, ExxonMobil, Total, and BASF. These companies are investing heavily in research and development to create more effective and environmentally friendly corrosion inhibitors. There is a notable trend towards the development of organic acid technology (OAT) based antifreeze, which offers superior corrosion protection and longer service life compared to traditional formulations.
Environmental concerns and regulatory pressures are shaping the market landscape. There is a growing demand for eco-friendly antifreeze products that are biodegradable and less toxic. This has led to the development of propylene glycol-based antifreeze as an alternative to the more toxic ethylene glycol-based products. The shift towards electric vehicles (EVs) is also influencing the market, as EVs require specialized cooling systems with different corrosion inhibition needs.
The aftermarket segment for corrosion-resistant antifreeze is witnessing steady growth, driven by increasing consumer awareness about the importance of regular coolant replacement. This trend is particularly strong in developed markets where vehicle owners are more likely to invest in premium, long-lasting antifreeze products.
Current Challenges in Antifreeze Corrosion Protection
Despite significant advancements in antifreeze technology, corrosion protection remains a persistent challenge in the automotive and industrial sectors. The primary issue stems from the inherent corrosive nature of ethylene glycol and propylene glycol, the base components of most antifreeze formulations. These chemicals, while effective at lowering the freezing point of water, can be highly aggressive towards metal surfaces, particularly in high-temperature environments.
One of the main challenges is the development of corrosion inhibitors that can effectively protect a wide range of metals simultaneously. Modern engines and cooling systems often incorporate various metals, including aluminum, steel, cast iron, copper, and brass. Each of these metals exhibits different electrochemical properties and corrosion mechanisms, making it difficult to formulate a single inhibitor package that provides comprehensive protection.
The longevity of corrosion inhibitors is another significant concern. As antifreeze circulates through a system, the inhibitors can become depleted over time, reducing their effectiveness. This depletion is accelerated by factors such as high temperatures, oxidation, and contamination from external sources. Developing inhibitors that maintain their protective properties over extended periods, without frequent replacement or replenishment, remains a key challenge for researchers and manufacturers.
Environmental considerations also pose challenges in antifreeze corrosion protection. Traditional corrosion inhibitors often contained toxic substances like nitrites, phosphates, and borates. However, increasing environmental regulations and concerns about ecological impact have necessitated the development of more environmentally friendly alternatives. Balancing effective corrosion protection with environmental safety is an ongoing challenge in the industry.
The complexity of modern cooling systems further complicates corrosion protection efforts. Advanced engine designs, including those for electric and hybrid vehicles, often feature more intricate cooling circuits with varying temperature zones. These systems may also incorporate new materials or alloys that require specialized protection. Developing inhibitors that can perform effectively across these diverse conditions and materials presents a significant technical hurdle.
Lastly, the interaction between corrosion inhibitors and other additives in antifreeze formulations poses another challenge. Antifreeze often contains multiple additives for various purposes, such as anti-foaming agents, pH buffers, and dye markers. Ensuring that corrosion inhibitors remain effective and do not interfere with or degrade other additives is crucial for maintaining overall antifreeze performance and system integrity.
One of the main challenges is the development of corrosion inhibitors that can effectively protect a wide range of metals simultaneously. Modern engines and cooling systems often incorporate various metals, including aluminum, steel, cast iron, copper, and brass. Each of these metals exhibits different electrochemical properties and corrosion mechanisms, making it difficult to formulate a single inhibitor package that provides comprehensive protection.
The longevity of corrosion inhibitors is another significant concern. As antifreeze circulates through a system, the inhibitors can become depleted over time, reducing their effectiveness. This depletion is accelerated by factors such as high temperatures, oxidation, and contamination from external sources. Developing inhibitors that maintain their protective properties over extended periods, without frequent replacement or replenishment, remains a key challenge for researchers and manufacturers.
Environmental considerations also pose challenges in antifreeze corrosion protection. Traditional corrosion inhibitors often contained toxic substances like nitrites, phosphates, and borates. However, increasing environmental regulations and concerns about ecological impact have necessitated the development of more environmentally friendly alternatives. Balancing effective corrosion protection with environmental safety is an ongoing challenge in the industry.
The complexity of modern cooling systems further complicates corrosion protection efforts. Advanced engine designs, including those for electric and hybrid vehicles, often feature more intricate cooling circuits with varying temperature zones. These systems may also incorporate new materials or alloys that require specialized protection. Developing inhibitors that can perform effectively across these diverse conditions and materials presents a significant technical hurdle.
Lastly, the interaction between corrosion inhibitors and other additives in antifreeze formulations poses another challenge. Antifreeze often contains multiple additives for various purposes, such as anti-foaming agents, pH buffers, and dye markers. Ensuring that corrosion inhibitors remain effective and do not interfere with or degrade other additives is crucial for maintaining overall antifreeze performance and system integrity.
Existing Corrosion Inhibition Mechanisms in Antifreeze
01 Use of organic compounds as corrosion inhibitors
Various organic compounds are utilized as corrosion inhibitors in antifreeze formulations. These compounds form protective films on metal surfaces, preventing direct contact between the metal and corrosive elements in the antifreeze solution. Examples include azoles, amines, and carboxylic acids, which can effectively reduce corrosion rates in cooling systems.- Use of organic compounds as corrosion inhibitors: Various organic compounds are utilized as effective corrosion inhibitors in antifreeze formulations. These compounds form protective films on metal surfaces, preventing direct contact between the metal and corrosive elements in the antifreeze solution. Examples include azoles, carboxylic acids, and their derivatives, which can significantly reduce corrosion rates in cooling systems.
- Inorganic corrosion inhibitors in antifreeze solutions: Inorganic compounds play a crucial role in corrosion inhibition for antifreeze formulations. These inhibitors, such as silicates, phosphates, and nitrites, form protective layers on metal surfaces or alter the chemistry of the solution to reduce corrosion. They are often used in combination with organic inhibitors for enhanced protection in various cooling system applications.
- Synergistic combinations of corrosion inhibitors: Antifreeze formulations often employ synergistic combinations of different corrosion inhibitors to achieve superior protection. These combinations can include mixtures of organic and inorganic inhibitors, or multiple organic compounds that work together to provide comprehensive corrosion protection for various metals present in cooling systems.
- pH control for corrosion inhibition in antifreeze: Maintaining an optimal pH range is crucial for effective corrosion inhibition in antifreeze solutions. pH control agents are incorporated into formulations to ensure that the antifreeze remains within a specific pH range, typically slightly alkaline, to minimize corrosion of metal components in the cooling system.
- Environmentally friendly corrosion inhibitors for antifreeze: Development of eco-friendly corrosion inhibitors for antifreeze applications has gained importance due to environmental concerns. These inhibitors are designed to provide effective corrosion protection while minimizing environmental impact. They often include biodegradable compounds or naturally derived substances that offer comparable performance to traditional inhibitors.
02 Inorganic corrosion inhibitors in antifreeze solutions
Inorganic compounds are employed as corrosion inhibitors in antifreeze formulations. These substances, such as molybdates, nitrites, and silicates, work by forming protective oxide layers on metal surfaces or by altering the pH of the solution to reduce corrosion. They are often used in combination with organic inhibitors for enhanced protection.Expand Specific Solutions03 Synergistic combinations of corrosion inhibitors
Antifreeze formulations often incorporate synergistic combinations of different corrosion inhibitors to provide comprehensive protection against various types of corrosion. These combinations may include both organic and inorganic inhibitors, as well as pH buffers and stabilizers, to create a more effective and long-lasting corrosion inhibition system.Expand Specific Solutions04 Environmentally friendly corrosion inhibitors
Development of eco-friendly corrosion inhibitors for antifreeze applications is a growing trend. These inhibitors are designed to be biodegradable, non-toxic, and sustainable while maintaining effective corrosion protection. Plant extracts, amino acids, and other naturally derived compounds are being explored as alternatives to traditional synthetic inhibitors.Expand Specific Solutions05 Corrosion inhibition testing methods
Various testing methods are employed to evaluate the effectiveness of corrosion inhibitors in antifreeze formulations. These include electrochemical techniques, weight loss measurements, and simulated service tests. Advanced analytical tools and long-term performance studies are used to assess the durability and efficiency of corrosion inhibition systems under different operating conditions.Expand Specific Solutions
Key Players in Antifreeze and Corrosion Inhibitor Industry
The antifreeze corrosion inhibitor market is in a mature stage, with established players and ongoing research for improved formulations. The global market size is estimated to be over $5 billion, driven by the automotive and industrial sectors. Technologically, the field is moderately mature but continues to evolve, with companies like BASF, Prestone, and Chevron leading innovation. Emerging players such as Dober Chemical and HAERTOL Chemie are also contributing to advancements. The focus is on developing more environmentally friendly and efficient inhibitors, with a trend towards organic and hybrid formulations.
BASF Corp.
Technical Solution: BASF Corp. has developed advanced corrosion inhibitors for antifreeze applications, utilizing a combination of organic and inorganic compounds. Their Glysantin® line of products incorporates silicate-organic acid technology (Si-OAT), which forms a protective layer on metal surfaces[1]. This technology combines the rapid action of silicates with the long-lasting protection of organic acids. BASF's corrosion inhibitors are designed to be compatible with various coolant base fluids, including ethylene glycol and propylene glycol, ensuring broad applicability across different antifreeze formulations[2]. The company has also invested in research to develop bio-based corrosion inhibitors, aiming to improve sustainability in antifreeze production[3].
Strengths: Comprehensive protection for multiple metals, long-lasting effectiveness, and environmentally friendly options. Weaknesses: Potentially higher cost compared to traditional inhibitors, and may require specific handling procedures.
Clariant Produkte (Deutschland) GmbH
Technical Solution: Clariant has developed a range of corrosion inhibitors for antifreeze applications under their Corroguard® brand. Their technology focuses on synergistic blends of organic and inorganic inhibitors, tailored for different cooling system materials. Clariant's approach includes the use of carboxylate-based inhibitors, which form a thin, protective film on metal surfaces[4]. This film is self-healing, providing continuous protection against corrosion. Additionally, Clariant has introduced nitrite-free formulations to address environmental concerns and comply with stricter regulations[5]. Their research also extends to developing inhibitors that are compatible with new materials used in electric vehicle cooling systems[6].
Strengths: Adaptable to various cooling system materials, environmentally friendly options, and focus on emerging technologies. Weaknesses: May require more frequent monitoring of coolant condition, and potentially higher initial cost.
Innovative Corrosion Inhibitor Compounds: Analysis
Antifreeze
PatentInactiveEP3476903A1
Innovation
- A combination of succinic acid, benzotriazole, and potassium hydroxide (KOH) with a pH range of 10.4 to 10.8, or succinic acid, cinnamic acid, benzotriazole, and KOH with a pH range of 8.5 to 10.8, which synergistically provides excellent frost protection and corrosion protection for all common metals, including solder, while reducing the need for high benzotriazole concentrations.
Glycerin-containing antifreezing agent concentrates with corrosion protection
PatentWO2006092376A1
Innovation
- An antifreeze/corrosion protection concentrate containing 10 to 50% glycerol by weight, with a preferred range of 15 to 35%, along with additional alcohols like ethylene glycol, and specific corrosion inhibitors such as aliphatic and aromatic acids, amines, and silicates, which are mixed with a superconcentrate to create a balanced composition for enhanced performance.
Environmental Impact of Corrosion Inhibitors
The environmental impact of corrosion inhibitors used in antifreeze formulations is a critical consideration in the automotive and industrial sectors. These inhibitors, while essential for protecting metal components from corrosion, can have significant ecological consequences when released into the environment. Many traditional corrosion inhibitors contain heavy metals, such as chromates, which are highly toxic to aquatic life and can persist in ecosystems for extended periods.
As antifreeze solutions are periodically replaced or leak from vehicles, these inhibitors can find their way into soil and water systems. Once in the environment, they can accumulate in sediments, be absorbed by plants, and enter the food chain, potentially causing long-term ecological damage. The bioaccumulation of these substances in organisms can lead to reduced biodiversity and disrupted ecosystem functions.
Recent studies have shown that some corrosion inhibitors can interfere with the natural processes of wastewater treatment plants, reducing their efficiency in removing pollutants. This interference can result in the release of partially treated water into natural water bodies, further exacerbating environmental concerns. Additionally, the production and disposal of these inhibitors contribute to industrial waste and can lead to air and water pollution if not properly managed.
In response to these environmental challenges, there has been a growing trend towards developing more eco-friendly corrosion inhibitors. Green chemistry initiatives have led to the exploration of plant-based inhibitors, such as those derived from agricultural by-products or natural extracts. These alternatives often demonstrate lower toxicity and improved biodegradability compared to their synthetic counterparts.
However, the transition to environmentally benign inhibitors is not without challenges. Ensuring the same level of corrosion protection while minimizing environmental impact requires extensive research and testing. Balancing performance, cost-effectiveness, and environmental safety remains a key focus for researchers and manufacturers in the antifreeze industry.
Regulatory bodies worldwide have begun to implement stricter guidelines on the use and disposal of corrosion inhibitors. This has prompted industries to invest in more sustainable practices, including closed-loop recycling systems for antifreeze and improved containment measures to prevent environmental contamination. The development of advanced treatment technologies for removing these inhibitors from wastewater has also gained momentum, aiming to mitigate their release into natural ecosystems.
As antifreeze solutions are periodically replaced or leak from vehicles, these inhibitors can find their way into soil and water systems. Once in the environment, they can accumulate in sediments, be absorbed by plants, and enter the food chain, potentially causing long-term ecological damage. The bioaccumulation of these substances in organisms can lead to reduced biodiversity and disrupted ecosystem functions.
Recent studies have shown that some corrosion inhibitors can interfere with the natural processes of wastewater treatment plants, reducing their efficiency in removing pollutants. This interference can result in the release of partially treated water into natural water bodies, further exacerbating environmental concerns. Additionally, the production and disposal of these inhibitors contribute to industrial waste and can lead to air and water pollution if not properly managed.
In response to these environmental challenges, there has been a growing trend towards developing more eco-friendly corrosion inhibitors. Green chemistry initiatives have led to the exploration of plant-based inhibitors, such as those derived from agricultural by-products or natural extracts. These alternatives often demonstrate lower toxicity and improved biodegradability compared to their synthetic counterparts.
However, the transition to environmentally benign inhibitors is not without challenges. Ensuring the same level of corrosion protection while minimizing environmental impact requires extensive research and testing. Balancing performance, cost-effectiveness, and environmental safety remains a key focus for researchers and manufacturers in the antifreeze industry.
Regulatory bodies worldwide have begun to implement stricter guidelines on the use and disposal of corrosion inhibitors. This has prompted industries to invest in more sustainable practices, including closed-loop recycling systems for antifreeze and improved containment measures to prevent environmental contamination. The development of advanced treatment technologies for removing these inhibitors from wastewater has also gained momentum, aiming to mitigate their release into natural ecosystems.
Performance Testing and Quality Standards
Performance testing and quality standards play a crucial role in ensuring the effectiveness and reliability of antifreeze corrosion inhibitors. These evaluations are essential for manufacturers to validate their products and for consumers to make informed decisions about the antifreeze they use in their vehicles or industrial applications.
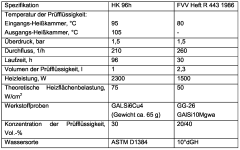
One of the primary performance tests for antifreeze corrosion inhibitors is the ASTM D1384 standard. This test simulates the corrosive environment found in cooling systems by exposing metal specimens to antifreeze solutions under controlled conditions. The test evaluates the weight loss of various metals, including copper, solder, brass, steel, cast iron, and aluminum, over a specified period. The results provide valuable insights into the inhibitor's ability to protect different metal components within the cooling system.
Another important test is the ASTM D2570, also known as the Simulated Service Test. This more rigorous evaluation subjects metal specimens to antifreeze solutions under dynamic conditions, including temperature cycling and aeration. The test better replicates real-world conditions and assesses the long-term performance of corrosion inhibitors.
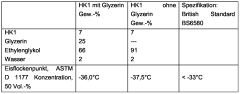
Quality standards for antifreeze corrosion inhibitors often include specifications for pH levels, reserve alkalinity, and specific gravity. These parameters are critical in maintaining the overall effectiveness of the antifreeze solution. For instance, the pH level must be carefully controlled to ensure optimal corrosion protection without causing damage to non-metallic components in the cooling system.
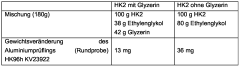
Manufacturers also conduct stability tests to evaluate the shelf life and long-term effectiveness of their corrosion inhibitors. These tests assess factors such as precipitation, phase separation, and color stability under various storage conditions and temperatures. Such evaluations help ensure that the antifreeze maintains its protective properties throughout its intended lifespan.
Environmental impact and toxicity testing have become increasingly important in recent years. Regulatory bodies often require manufacturers to demonstrate that their corrosion inhibitors meet specific environmental standards and do not pose significant risks to ecosystems when disposed of or accidentally released.
Compatibility testing is another crucial aspect of quality assurance. This involves evaluating how the antifreeze corrosion inhibitors interact with other fluids and materials commonly found in cooling systems, such as engine oils, gaskets, and hoses. Ensuring compatibility helps prevent unintended chemical reactions or degradation of system components.
As technology advances, new performance tests and quality standards continue to emerge. For example, some manufacturers are developing tests to evaluate the effectiveness of corrosion inhibitors in hybrid and electric vehicle cooling systems, which may have different requirements compared to traditional internal combustion engines.
One of the primary performance tests for antifreeze corrosion inhibitors is the ASTM D1384 standard. This test simulates the corrosive environment found in cooling systems by exposing metal specimens to antifreeze solutions under controlled conditions. The test evaluates the weight loss of various metals, including copper, solder, brass, steel, cast iron, and aluminum, over a specified period. The results provide valuable insights into the inhibitor's ability to protect different metal components within the cooling system.
Another important test is the ASTM D2570, also known as the Simulated Service Test. This more rigorous evaluation subjects metal specimens to antifreeze solutions under dynamic conditions, including temperature cycling and aeration. The test better replicates real-world conditions and assesses the long-term performance of corrosion inhibitors.
Quality standards for antifreeze corrosion inhibitors often include specifications for pH levels, reserve alkalinity, and specific gravity. These parameters are critical in maintaining the overall effectiveness of the antifreeze solution. For instance, the pH level must be carefully controlled to ensure optimal corrosion protection without causing damage to non-metallic components in the cooling system.
Manufacturers also conduct stability tests to evaluate the shelf life and long-term effectiveness of their corrosion inhibitors. These tests assess factors such as precipitation, phase separation, and color stability under various storage conditions and temperatures. Such evaluations help ensure that the antifreeze maintains its protective properties throughout its intended lifespan.
Environmental impact and toxicity testing have become increasingly important in recent years. Regulatory bodies often require manufacturers to demonstrate that their corrosion inhibitors meet specific environmental standards and do not pose significant risks to ecosystems when disposed of or accidentally released.
Compatibility testing is another crucial aspect of quality assurance. This involves evaluating how the antifreeze corrosion inhibitors interact with other fluids and materials commonly found in cooling systems, such as engine oils, gaskets, and hoses. Ensuring compatibility helps prevent unintended chemical reactions or degradation of system components.
As technology advances, new performance tests and quality standards continue to emerge. For example, some manufacturers are developing tests to evaluate the effectiveness of corrosion inhibitors in hybrid and electric vehicle cooling systems, which may have different requirements compared to traditional internal combustion engines.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!