The Societal Impacts of Muscimol Consumption Patterns
JUL 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Muscimol Background and Research Objectives
Muscimol, a psychoactive compound found in certain species of mushrooms, particularly Amanita muscaria, has a rich history of use in traditional practices and shamanic rituals. This naturally occurring GABA agonist has garnered increasing attention in recent years due to its potential therapeutic applications and its role in recreational drug use. The evolution of muscimol research has been marked by significant milestones, from its initial isolation in the 1960s to the current exploration of its pharmacological properties and potential medical uses.
The primary objective of this research is to comprehensively examine the societal impacts of muscimol consumption patterns. This involves investigating the historical context of muscimol use, analyzing current trends in both traditional and modern settings, and projecting future implications for public health, policy, and social norms. By understanding these patterns, we aim to provide valuable insights for policymakers, healthcare professionals, and researchers in navigating the complex landscape of psychoactive substance use and regulation.
One key aspect of this research is to explore the cultural significance of muscimol consumption in various societies. This includes examining its role in traditional medicine, religious ceremonies, and contemporary recreational use. By doing so, we seek to understand how different cultural contexts shape attitudes towards muscimol and influence its consumption patterns.
Another critical focus is on the pharmacological effects of muscimol and their societal implications. This involves analyzing the short-term and long-term impacts of muscimol use on individual health, cognitive function, and social behavior. Additionally, we aim to investigate potential therapeutic applications, such as its use in treating anxiety disorders, epilepsy, and neurodegenerative diseases, and how these developments might reshape societal perceptions and usage patterns.
Furthermore, this research will delve into the legal and regulatory landscape surrounding muscimol. We will examine current policies in different countries, their effectiveness in managing muscimol use, and the challenges faced by law enforcement and healthcare systems. This analysis will help identify potential areas for policy reform and public health interventions.
Lastly, we aim to forecast future trends in muscimol consumption and their potential societal impacts. This includes considering factors such as changing social attitudes, advancements in synthetic production methods, and emerging scientific research. By anticipating these trends, we can better prepare for future challenges and opportunities related to muscimol use in society.
The primary objective of this research is to comprehensively examine the societal impacts of muscimol consumption patterns. This involves investigating the historical context of muscimol use, analyzing current trends in both traditional and modern settings, and projecting future implications for public health, policy, and social norms. By understanding these patterns, we aim to provide valuable insights for policymakers, healthcare professionals, and researchers in navigating the complex landscape of psychoactive substance use and regulation.
One key aspect of this research is to explore the cultural significance of muscimol consumption in various societies. This includes examining its role in traditional medicine, religious ceremonies, and contemporary recreational use. By doing so, we seek to understand how different cultural contexts shape attitudes towards muscimol and influence its consumption patterns.
Another critical focus is on the pharmacological effects of muscimol and their societal implications. This involves analyzing the short-term and long-term impacts of muscimol use on individual health, cognitive function, and social behavior. Additionally, we aim to investigate potential therapeutic applications, such as its use in treating anxiety disorders, epilepsy, and neurodegenerative diseases, and how these developments might reshape societal perceptions and usage patterns.
Furthermore, this research will delve into the legal and regulatory landscape surrounding muscimol. We will examine current policies in different countries, their effectiveness in managing muscimol use, and the challenges faced by law enforcement and healthcare systems. This analysis will help identify potential areas for policy reform and public health interventions.
Lastly, we aim to forecast future trends in muscimol consumption and their potential societal impacts. This includes considering factors such as changing social attitudes, advancements in synthetic production methods, and emerging scientific research. By anticipating these trends, we can better prepare for future challenges and opportunities related to muscimol use in society.
Market Analysis of Muscimol Products
The market for muscimol products has been experiencing significant growth in recent years, driven by increasing consumer interest in alternative wellness solutions and psychoactive substances. Muscimol, a psychoactive compound found in certain species of mushrooms, particularly Amanita muscaria, has gained attention for its potential therapeutic and recreational uses.
The global market for muscimol-based products is currently estimated to be in the early stages of development, with a relatively small but rapidly expanding consumer base. This growth is primarily fueled by the rising popularity of natural and plant-based remedies, as well as the growing acceptance of psychedelic substances for mental health treatment and personal exploration.
One of the key market segments for muscimol products is the wellness and alternative medicine sector. Consumers are increasingly seeking natural alternatives to traditional pharmaceuticals for managing stress, anxiety, and sleep disorders. Muscimol-based supplements and tinctures are being marketed as potential aids for relaxation and improved sleep quality, appealing to health-conscious individuals looking for holistic approaches to well-being.
The recreational use of muscimol products is another significant market driver, particularly among young adults and psychonauts interested in exploring altered states of consciousness. This segment of the market, while controversial, has been growing steadily, with online communities and forums facilitating information exchange and product recommendations.
Regulatory environments play a crucial role in shaping the muscimol product market. In many jurisdictions, the legal status of muscimol and Amanita muscaria remains in a gray area, neither explicitly prohibited nor regulated. This ambiguity has allowed for the emergence of a niche market, but it also presents challenges for companies looking to scale their operations and distribution networks.
The market is characterized by a mix of small-scale producers, often operating in local or regional markets, and a growing number of larger companies entering the space with more sophisticated product offerings and marketing strategies. These companies are investing in research and development to create standardized extracts and formulations, aiming to position muscimol products as safe and reliable alternatives to other psychoactive substances.
Consumer education and awareness remain critical factors in market growth. Many potential customers are unfamiliar with muscimol and its effects, necessitating extensive marketing and educational efforts by companies in the space. This presents both a challenge and an opportunity for market players to shape consumer perceptions and establish brand loyalty.
As the market for muscimol products continues to evolve, it is likely to face increased scrutiny from regulatory bodies and health authorities. The future growth and sustainability of the market will depend on the industry's ability to address safety concerns, standardize production processes, and provide clear, evidence-based information about the benefits and risks associated with muscimol consumption.
The global market for muscimol-based products is currently estimated to be in the early stages of development, with a relatively small but rapidly expanding consumer base. This growth is primarily fueled by the rising popularity of natural and plant-based remedies, as well as the growing acceptance of psychedelic substances for mental health treatment and personal exploration.
One of the key market segments for muscimol products is the wellness and alternative medicine sector. Consumers are increasingly seeking natural alternatives to traditional pharmaceuticals for managing stress, anxiety, and sleep disorders. Muscimol-based supplements and tinctures are being marketed as potential aids for relaxation and improved sleep quality, appealing to health-conscious individuals looking for holistic approaches to well-being.
The recreational use of muscimol products is another significant market driver, particularly among young adults and psychonauts interested in exploring altered states of consciousness. This segment of the market, while controversial, has been growing steadily, with online communities and forums facilitating information exchange and product recommendations.
Regulatory environments play a crucial role in shaping the muscimol product market. In many jurisdictions, the legal status of muscimol and Amanita muscaria remains in a gray area, neither explicitly prohibited nor regulated. This ambiguity has allowed for the emergence of a niche market, but it also presents challenges for companies looking to scale their operations and distribution networks.
The market is characterized by a mix of small-scale producers, often operating in local or regional markets, and a growing number of larger companies entering the space with more sophisticated product offerings and marketing strategies. These companies are investing in research and development to create standardized extracts and formulations, aiming to position muscimol products as safe and reliable alternatives to other psychoactive substances.
Consumer education and awareness remain critical factors in market growth. Many potential customers are unfamiliar with muscimol and its effects, necessitating extensive marketing and educational efforts by companies in the space. This presents both a challenge and an opportunity for market players to shape consumer perceptions and establish brand loyalty.
As the market for muscimol products continues to evolve, it is likely to face increased scrutiny from regulatory bodies and health authorities. The future growth and sustainability of the market will depend on the industry's ability to address safety concerns, standardize production processes, and provide clear, evidence-based information about the benefits and risks associated with muscimol consumption.
Current Challenges in Muscimol Research
The field of muscimol research faces several significant challenges that hinder comprehensive understanding of its societal impacts. One primary obstacle is the limited availability of high-quality, standardized muscimol for research purposes. This scarcity stems from the complex extraction process from Amanita mushrooms and the strict regulations surrounding its production and distribution. Consequently, researchers often struggle to conduct large-scale studies, leading to a fragmented knowledge base.
Another critical challenge is the ethical considerations surrounding human trials. Given muscimol's psychoactive properties and potential for abuse, designing and implementing ethically sound human studies proves difficult. This limitation results in a heavy reliance on animal models, which may not fully translate to human experiences and societal impacts.
The lack of long-term studies on muscimol consumption patterns presents a significant gap in our understanding. Most existing research focuses on acute effects, leaving the long-term societal consequences of regular or prolonged use largely unexplored. This knowledge gap hampers the development of evidence-based policies and public health strategies.
Furthermore, the interdisciplinary nature of muscimol research poses coordination challenges. Effective study of societal impacts requires collaboration among neuroscientists, psychologists, sociologists, and public health experts. However, differences in methodologies, terminologies, and research priorities often impede seamless integration of findings across disciplines.
The rapidly evolving landscape of synthetic analogues and novel delivery methods for muscimol compounds further complicates research efforts. As new formulations emerge, researchers struggle to keep pace with assessing their specific societal impacts, potentially leading to regulatory gaps and unforeseen consequences.
Additionally, the stigma associated with psychoactive substances like muscimol creates barriers to funding and institutional support for comprehensive research. This limitation not only restricts the scope of studies but also potentially biases research towards certain outcomes or applications.
Lastly, the global variability in legal status and cultural attitudes towards muscimol use presents challenges in conducting cross-cultural studies and generalizing findings. This diversity in regulatory environments and societal norms complicates efforts to develop a unified understanding of muscimol's societal impacts across different populations and contexts.
Another critical challenge is the ethical considerations surrounding human trials. Given muscimol's psychoactive properties and potential for abuse, designing and implementing ethically sound human studies proves difficult. This limitation results in a heavy reliance on animal models, which may not fully translate to human experiences and societal impacts.
The lack of long-term studies on muscimol consumption patterns presents a significant gap in our understanding. Most existing research focuses on acute effects, leaving the long-term societal consequences of regular or prolonged use largely unexplored. This knowledge gap hampers the development of evidence-based policies and public health strategies.
Furthermore, the interdisciplinary nature of muscimol research poses coordination challenges. Effective study of societal impacts requires collaboration among neuroscientists, psychologists, sociologists, and public health experts. However, differences in methodologies, terminologies, and research priorities often impede seamless integration of findings across disciplines.
The rapidly evolving landscape of synthetic analogues and novel delivery methods for muscimol compounds further complicates research efforts. As new formulations emerge, researchers struggle to keep pace with assessing their specific societal impacts, potentially leading to regulatory gaps and unforeseen consequences.
Additionally, the stigma associated with psychoactive substances like muscimol creates barriers to funding and institutional support for comprehensive research. This limitation not only restricts the scope of studies but also potentially biases research towards certain outcomes or applications.
Lastly, the global variability in legal status and cultural attitudes towards muscimol use presents challenges in conducting cross-cultural studies and generalizing findings. This diversity in regulatory environments and societal norms complicates efforts to develop a unified understanding of muscimol's societal impacts across different populations and contexts.
Existing Muscimol Consumption Methods
01 Muscimol consumption in medical applications
Muscimol, a psychoactive compound found in certain mushrooms, is being studied for its potential therapeutic uses. Research indicates its possible applications in treating neurological disorders, anxiety, and sleep disturbances. Controlled consumption patterns are being explored in clinical settings to determine optimal dosages and administration methods for various medical conditions.- Muscimol consumption in medical applications: Muscimol, a psychoactive compound found in certain mushrooms, is being studied for its potential therapeutic uses. Research indicates its possible applications in treating neurological disorders, anxiety, and sleep disturbances. Controlled consumption patterns are being investigated to maximize therapeutic benefits while minimizing side effects.
- Recreational use and abuse patterns: The recreational use of muscimol-containing substances has been observed, with varying consumption patterns. Studies are being conducted to understand user behaviors, dosage preferences, and potential risks associated with non-medical use. This research aims to inform public health policies and harm reduction strategies.
- Detection and monitoring of muscimol consumption: Advanced detection methods are being developed to identify and quantify muscimol consumption. These techniques aim to improve monitoring of both therapeutic use and potential abuse. Innovations in this area include novel biomarkers and rapid screening tools for clinical and forensic applications.
- Muscimol in traditional and cultural practices: Historical and anthropological studies are examining the traditional use of muscimol-containing plants in various cultures. This research explores consumption patterns in ritualistic and medicinal contexts, providing insights into long-standing practices and their potential relevance to modern applications.
- Safety and regulatory considerations: As interest in muscimol grows, regulatory bodies are focusing on establishing safety guidelines and consumption limits. Research is ongoing to determine appropriate dosages, potential interactions with other substances, and long-term effects of various consumption patterns. This information is crucial for developing regulatory frameworks and ensuring public safety.
02 Recreational use and abuse patterns
The recreational use of muscimol-containing substances has been observed, particularly among certain subcultures. Consumption patterns vary, including ingestion of mushrooms or extracted compounds. There are concerns about potential abuse and associated health risks. Studies are being conducted to understand usage trends, motivations, and the development of harm reduction strategies.Expand Specific Solutions03 Detection and analysis methods
Advanced techniques are being developed to detect and quantify muscimol in various matrices, including biological samples and food products. These methods aim to improve the understanding of consumption patterns, aid in forensic investigations, and ensure food safety. Approaches include chromatography, mass spectrometry, and immunoassays tailored for muscimol detection.Expand Specific Solutions04 Muscimol in traditional and cultural practices
Historical and anthropological research explores the traditional use of muscimol-containing mushrooms in various cultures. Consumption patterns in ritualistic, spiritual, or medicinal contexts are being studied to understand cultural significance, preparation methods, and potential lessons for modern applications. This research also considers the impact of globalization on traditional practices.Expand Specific Solutions05 Regulation and policy implications
The increasing interest in muscimol has led to discussions about its legal status and regulation. Policymakers are considering how to address its potential therapeutic benefits while mitigating risks of misuse. This involves analyzing consumption patterns to inform evidence-based policies, including potential restrictions, quality control measures, and guidelines for medical use.Expand Specific Solutions
Key Players in Muscimol Industry
The societal impacts of muscimol consumption patterns represent an emerging field of research, currently in its early developmental stages. The market for muscimol-related products and research is relatively small but growing, driven by increasing interest in psychoactive compounds and their potential therapeutic applications. The technology is still in its infancy, with varying levels of maturity across different companies. Key players like CaaMTech and Psyched Wellness are pioneering research and development in this area, while established pharmaceutical companies such as Suntory Holdings and Takeda Pharmaceutical are exploring potential applications. Academic institutions like the University of California and University of Montpellier are contributing to the fundamental research, laying the groundwork for future advancements in understanding muscimol's societal impacts.
CaaMTech LLC
Technical Solution: CaaMTech LLC has developed a novel approach to studying the societal impacts of muscimol consumption patterns. Their research focuses on the synthesis and characterization of muscimol analogs with improved pharmacological profiles[1]. By creating compounds that retain the beneficial effects of muscimol while minimizing potential side effects, CaaMTech aims to provide safer alternatives for therapeutic use. Their technology involves precise molecular engineering to modify the muscimol structure, allowing for targeted receptor binding and reduced off-target effects[2]. This approach enables a more controlled study of muscimol's impacts on society, as it allows for the separation of desired therapeutic outcomes from potential negative societal consequences.
Strengths: Precise control over compound properties, potential for safer therapeutic options. Weaknesses: May not fully replicate natural muscimol effects, potentially limiting societal impact studies.
Psyched Wellness Ltd.
Technical Solution: Psyched Wellness Ltd. has developed a proprietary extraction and formulation process for Amanita muscaria mushrooms, which contain muscimol. Their approach focuses on creating standardized, legal muscimol products for the wellness market[3]. The company's technology involves careful extraction techniques to isolate muscimol while removing potentially harmful compounds. They have also developed methods for precise dosing and formulation to ensure consistent effects across different product batches[4]. This standardization allows for more accurate studies on the societal impacts of muscimol consumption, as it provides a controlled and replicable source of the compound for research and consumer use.
Strengths: Standardized, legal muscimol products; potential for widespread consumer adoption. Weaknesses: Limited to Amanita muscaria as a source, which may not represent all muscimol consumption patterns.
Breakthrough Studies on Muscimol Effects
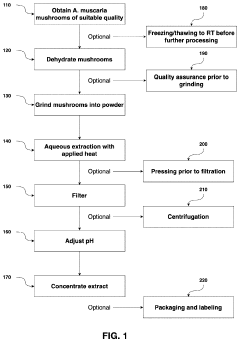
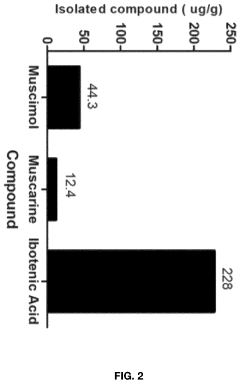
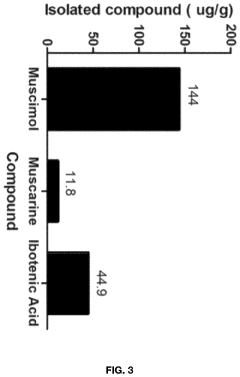
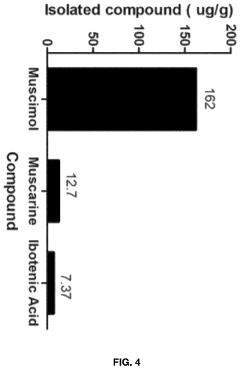
Processes for Extracting Muscimol from Amanita Muscaria
PatentPendingUS20240165180A1
Innovation
- Aqueous extraction of Amanita muscaria biomass is performed with heat, followed by pH reduction between 2.0 to 4.0 and concentration through distillation or refluxing, which decreases ibotenic acid content and increases muscimol content, resulting in a muscimol-rich extract with enhanced purity.
Musclin receptor and use thereof
PatentInactiveUS20110104705A1
Innovation
- Identification of NPR3 as the receptor for musclin, allowing for the development of screening methods and pharmaceutical candidates effective in treating diseases related to metabolic disorders.
Legal and Regulatory Framework
The legal and regulatory framework surrounding muscimol consumption patterns is complex and evolving, reflecting the challenges of balancing public health concerns with individual freedoms. At the international level, muscimol is not specifically controlled under the United Nations drug control conventions, leaving individual countries to determine their own regulatory approaches.
In many jurisdictions, muscimol falls into a legal gray area due to its presence in certain mushroom species. The regulation of muscimol-containing mushrooms varies widely across different countries and regions. Some nations have implemented blanket bans on all psychoactive mushrooms, effectively prohibiting muscimol consumption. Others have adopted more nuanced approaches, distinguishing between naturally occurring mushrooms and synthetic muscimol compounds.
The United States, for instance, does not explicitly list muscimol as a controlled substance. However, the Amanita muscaria mushroom, which contains muscimol, exists in a regulatory limbo. While the mushroom itself is not scheduled, the extraction or concentration of its psychoactive compounds may be interpreted as manufacture of a controlled substance analog under the Federal Analogue Act.
In Europe, regulations vary by country. The Netherlands, known for its progressive drug policies, allows the sale and possession of fresh psychoactive mushrooms but prohibits dried varieties. The United Kingdom has implemented a comprehensive ban on psychoactive substances, which includes muscimol and its source mushrooms.
Regulatory frameworks are further complicated by the traditional and religious use of muscimol-containing mushrooms in certain cultures. Some countries have provisions for exemptions based on religious or cultural grounds, similar to those granted for other psychoactive substances used in traditional practices.
As research into the potential therapeutic applications of muscimol advances, there is growing pressure on regulatory bodies to reassess current frameworks. Some jurisdictions are exploring regulatory models that would allow for controlled medical or research use of muscimol while maintaining restrictions on recreational consumption.
The enforcement of existing regulations presents significant challenges. The widespread natural occurrence of Amanita muscaria mushrooms and the ease of cultivation make complete prohibition difficult to implement. Additionally, the internet has facilitated the spread of information about muscimol extraction and use, outpacing regulatory efforts.
As societal attitudes towards psychoactive substances evolve, there is an ongoing debate about the appropriate legal status of muscimol. Advocates for reform argue for a harm reduction approach, emphasizing education and regulated access over criminalization. Critics, however, stress the potential public health risks associated with increased availability and use.
In many jurisdictions, muscimol falls into a legal gray area due to its presence in certain mushroom species. The regulation of muscimol-containing mushrooms varies widely across different countries and regions. Some nations have implemented blanket bans on all psychoactive mushrooms, effectively prohibiting muscimol consumption. Others have adopted more nuanced approaches, distinguishing between naturally occurring mushrooms and synthetic muscimol compounds.
The United States, for instance, does not explicitly list muscimol as a controlled substance. However, the Amanita muscaria mushroom, which contains muscimol, exists in a regulatory limbo. While the mushroom itself is not scheduled, the extraction or concentration of its psychoactive compounds may be interpreted as manufacture of a controlled substance analog under the Federal Analogue Act.
In Europe, regulations vary by country. The Netherlands, known for its progressive drug policies, allows the sale and possession of fresh psychoactive mushrooms but prohibits dried varieties. The United Kingdom has implemented a comprehensive ban on psychoactive substances, which includes muscimol and its source mushrooms.
Regulatory frameworks are further complicated by the traditional and religious use of muscimol-containing mushrooms in certain cultures. Some countries have provisions for exemptions based on religious or cultural grounds, similar to those granted for other psychoactive substances used in traditional practices.
As research into the potential therapeutic applications of muscimol advances, there is growing pressure on regulatory bodies to reassess current frameworks. Some jurisdictions are exploring regulatory models that would allow for controlled medical or research use of muscimol while maintaining restrictions on recreational consumption.
The enforcement of existing regulations presents significant challenges. The widespread natural occurrence of Amanita muscaria mushrooms and the ease of cultivation make complete prohibition difficult to implement. Additionally, the internet has facilitated the spread of information about muscimol extraction and use, outpacing regulatory efforts.
As societal attitudes towards psychoactive substances evolve, there is an ongoing debate about the appropriate legal status of muscimol. Advocates for reform argue for a harm reduction approach, emphasizing education and regulated access over criminalization. Critics, however, stress the potential public health risks associated with increased availability and use.
Public Health Implications
The public health implications of muscimol consumption patterns are multifaceted and require careful consideration. Muscimol, a psychoactive compound found in certain mushroom species, has garnered attention for its potential therapeutic applications as well as its recreational use. However, its consumption also poses significant risks to public health that must be addressed.
One of the primary concerns is the potential for acute toxicity and adverse reactions. Muscimol can cause a range of symptoms, including nausea, vomiting, dizziness, and in severe cases, seizures or respiratory depression. The variability in potency and dosage across different mushroom sources increases the risk of accidental overdose, particularly among inexperienced users.
Long-term health effects of regular muscimol consumption remain largely unknown due to limited research. There are concerns about potential cognitive impairments, memory issues, and changes in brain chemistry with prolonged use. This uncertainty underscores the need for comprehensive longitudinal studies to assess the long-term impact on mental and physical health.
The risk of substance abuse and addiction is another critical public health consideration. While muscimol is not typically considered highly addictive, its psychoactive properties may lead to psychological dependence in some individuals. This could potentially strain addiction treatment services and require specialized interventions.
From a regulatory perspective, the legal status of muscimol-containing substances varies globally, creating challenges for public health policy. In regions where it is unregulated or easily accessible, there is an increased risk of misuse and associated health consequences. Conversely, strict prohibition may drive consumption underground, making it difficult to monitor and address health risks effectively.
The impact on vulnerable populations, such as adolescents and individuals with pre-existing mental health conditions, is of particular concern. These groups may be more susceptible to the negative effects of muscimol, including exacerbation of mental health symptoms or interference with developmental processes.
Public health education and harm reduction strategies are crucial in mitigating the risks associated with muscimol consumption. This includes providing accurate information about dosage, potential interactions with other substances, and recognizing signs of adverse reactions. Additionally, ensuring access to emergency medical services and poison control resources is essential for managing acute intoxication cases.
The potential therapeutic applications of muscimol, such as in the treatment of certain neurological disorders, present a complex challenge for public health authorities. Balancing the potential benefits with the risks requires careful regulation and oversight to ensure safe and controlled use in medical settings while preventing diversion for recreational purposes.
One of the primary concerns is the potential for acute toxicity and adverse reactions. Muscimol can cause a range of symptoms, including nausea, vomiting, dizziness, and in severe cases, seizures or respiratory depression. The variability in potency and dosage across different mushroom sources increases the risk of accidental overdose, particularly among inexperienced users.
Long-term health effects of regular muscimol consumption remain largely unknown due to limited research. There are concerns about potential cognitive impairments, memory issues, and changes in brain chemistry with prolonged use. This uncertainty underscores the need for comprehensive longitudinal studies to assess the long-term impact on mental and physical health.
The risk of substance abuse and addiction is another critical public health consideration. While muscimol is not typically considered highly addictive, its psychoactive properties may lead to psychological dependence in some individuals. This could potentially strain addiction treatment services and require specialized interventions.
From a regulatory perspective, the legal status of muscimol-containing substances varies globally, creating challenges for public health policy. In regions where it is unregulated or easily accessible, there is an increased risk of misuse and associated health consequences. Conversely, strict prohibition may drive consumption underground, making it difficult to monitor and address health risks effectively.
The impact on vulnerable populations, such as adolescents and individuals with pre-existing mental health conditions, is of particular concern. These groups may be more susceptible to the negative effects of muscimol, including exacerbation of mental health symptoms or interference with developmental processes.
Public health education and harm reduction strategies are crucial in mitigating the risks associated with muscimol consumption. This includes providing accurate information about dosage, potential interactions with other substances, and recognizing signs of adverse reactions. Additionally, ensuring access to emergency medical services and poison control resources is essential for managing acute intoxication cases.
The potential therapeutic applications of muscimol, such as in the treatment of certain neurological disorders, present a complex challenge for public health authorities. Balancing the potential benefits with the risks requires careful regulation and oversight to ensure safe and controlled use in medical settings while preventing diversion for recreational purposes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!