The Stability of Muscimol Under Various Environmental Conditions
JUL 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Muscimol Stability Background and Objectives
Muscimol, a potent GABA receptor agonist, has garnered significant attention in neuroscience and pharmacology due to its potential therapeutic applications. The study of muscimol's stability under various environmental conditions is crucial for understanding its efficacy, storage requirements, and potential for long-term use in medical treatments.
The historical context of muscimol research dates back to its isolation from the Amanita muscaria mushroom in the 1960s. Since then, researchers have been exploring its pharmacological properties and potential uses in treating neurological disorders. However, the stability of muscimol in different environments has remained a critical area of investigation, as it directly impacts the compound's effectiveness and safety in clinical applications.
The primary objective of studying muscimol stability is to determine the optimal conditions for its storage, handling, and administration. This includes investigating how factors such as temperature, pH, light exposure, and humidity affect the compound's chemical structure and biological activity. Understanding these parameters is essential for developing stable formulations and ensuring the consistency of muscimol-based treatments.
Another key goal is to identify potential degradation products and pathways. This knowledge is crucial for assessing the safety profile of muscimol-based therapies, as degradation products may have different pharmacological properties or toxicity profiles. By characterizing these degradation processes, researchers can develop strategies to mitigate instability and extend the shelf life of muscimol preparations.
The technological evolution in analytical techniques has significantly contributed to advancing muscimol stability studies. High-performance liquid chromatography (HPLC), mass spectrometry, and nuclear magnetic resonance (NMR) spectroscopy have enabled more precise and sensitive analyses of muscimol and its degradation products. These advancements have allowed researchers to detect even minor changes in the compound's structure and purity under various environmental conditions.
Current trends in muscimol stability research focus on developing novel formulations and delivery systems that enhance the compound's stability. This includes exploring the use of nanoencapsulation, lyophilization, and other advanced drug delivery technologies to protect muscimol from environmental factors that may compromise its integrity.
The outcomes of these stability studies have far-reaching implications for the pharmaceutical industry, particularly in the development of muscimol-based drugs for neurological disorders. By establishing robust stability profiles, researchers can optimize drug formulations, storage conditions, and administration protocols, ultimately improving the efficacy and safety of muscimol treatments.
The historical context of muscimol research dates back to its isolation from the Amanita muscaria mushroom in the 1960s. Since then, researchers have been exploring its pharmacological properties and potential uses in treating neurological disorders. However, the stability of muscimol in different environments has remained a critical area of investigation, as it directly impacts the compound's effectiveness and safety in clinical applications.
The primary objective of studying muscimol stability is to determine the optimal conditions for its storage, handling, and administration. This includes investigating how factors such as temperature, pH, light exposure, and humidity affect the compound's chemical structure and biological activity. Understanding these parameters is essential for developing stable formulations and ensuring the consistency of muscimol-based treatments.
Another key goal is to identify potential degradation products and pathways. This knowledge is crucial for assessing the safety profile of muscimol-based therapies, as degradation products may have different pharmacological properties or toxicity profiles. By characterizing these degradation processes, researchers can develop strategies to mitigate instability and extend the shelf life of muscimol preparations.
The technological evolution in analytical techniques has significantly contributed to advancing muscimol stability studies. High-performance liquid chromatography (HPLC), mass spectrometry, and nuclear magnetic resonance (NMR) spectroscopy have enabled more precise and sensitive analyses of muscimol and its degradation products. These advancements have allowed researchers to detect even minor changes in the compound's structure and purity under various environmental conditions.
Current trends in muscimol stability research focus on developing novel formulations and delivery systems that enhance the compound's stability. This includes exploring the use of nanoencapsulation, lyophilization, and other advanced drug delivery technologies to protect muscimol from environmental factors that may compromise its integrity.
The outcomes of these stability studies have far-reaching implications for the pharmaceutical industry, particularly in the development of muscimol-based drugs for neurological disorders. By establishing robust stability profiles, researchers can optimize drug formulations, storage conditions, and administration protocols, ultimately improving the efficacy and safety of muscimol treatments.
Market Analysis for Muscimol-Based Products
The market for muscimol-based products is experiencing significant growth, driven by increasing interest in novel psychoactive compounds and potential therapeutic applications. Muscimol, a psychoactive compound found in Amanita muscaria mushrooms, has garnered attention for its unique pharmacological properties and potential uses in various industries.
In the pharmaceutical sector, muscimol is being explored for its potential in treating neurological disorders, anxiety, and sleep disturbances. The compound's ability to act as a potent GABA receptor agonist has sparked interest among researchers and drug developers. This has led to a growing demand for stable, high-quality muscimol for clinical trials and drug development processes.
The nutraceutical and functional food industries are also showing interest in muscimol-based products. As consumers increasingly seek natural alternatives for cognitive enhancement and stress relief, muscimol-infused supplements and beverages are emerging as a niche market segment. However, the stability of muscimol under various environmental conditions remains a critical factor in product development and commercialization.
The recreational drug market, while controversial, represents another potential avenue for muscimol-based products. As legal frameworks around psychoactive substances evolve in some regions, there is growing demand for novel, naturally-derived compounds that offer unique experiences. This market segment, however, faces significant regulatory challenges and ethical considerations.
Agricultural applications of muscimol are being explored, particularly in pest control. The compound's insecticidal properties have led to research into its potential as an eco-friendly alternative to synthetic pesticides. This market segment is still in its early stages but shows promise for future growth.
The stability of muscimol under various environmental conditions directly impacts its market potential across these sectors. Products that can maintain muscimol's efficacy and safety profile under different storage and usage conditions are likely to gain a competitive edge. This has led to increased investment in research and development focused on enhancing muscimol's stability through various formulation techniques and delivery systems.
Market growth is also influenced by regulatory landscapes, which vary significantly across regions. As research progresses and regulatory bodies evaluate the safety and efficacy of muscimol-based products, market dynamics are expected to evolve. Companies that can navigate these regulatory challenges while demonstrating the stability and reliability of their muscimol-based products are poised to capture significant market share.
In conclusion, the market for muscimol-based products shows promising growth potential across multiple sectors. However, the realization of this potential heavily depends on overcoming challenges related to muscimol's stability under various environmental conditions. As research progresses and innovative solutions emerge, the market is expected to expand, offering opportunities for companies that can effectively address these stability concerns.
In the pharmaceutical sector, muscimol is being explored for its potential in treating neurological disorders, anxiety, and sleep disturbances. The compound's ability to act as a potent GABA receptor agonist has sparked interest among researchers and drug developers. This has led to a growing demand for stable, high-quality muscimol for clinical trials and drug development processes.
The nutraceutical and functional food industries are also showing interest in muscimol-based products. As consumers increasingly seek natural alternatives for cognitive enhancement and stress relief, muscimol-infused supplements and beverages are emerging as a niche market segment. However, the stability of muscimol under various environmental conditions remains a critical factor in product development and commercialization.
The recreational drug market, while controversial, represents another potential avenue for muscimol-based products. As legal frameworks around psychoactive substances evolve in some regions, there is growing demand for novel, naturally-derived compounds that offer unique experiences. This market segment, however, faces significant regulatory challenges and ethical considerations.
Agricultural applications of muscimol are being explored, particularly in pest control. The compound's insecticidal properties have led to research into its potential as an eco-friendly alternative to synthetic pesticides. This market segment is still in its early stages but shows promise for future growth.
The stability of muscimol under various environmental conditions directly impacts its market potential across these sectors. Products that can maintain muscimol's efficacy and safety profile under different storage and usage conditions are likely to gain a competitive edge. This has led to increased investment in research and development focused on enhancing muscimol's stability through various formulation techniques and delivery systems.
Market growth is also influenced by regulatory landscapes, which vary significantly across regions. As research progresses and regulatory bodies evaluate the safety and efficacy of muscimol-based products, market dynamics are expected to evolve. Companies that can navigate these regulatory challenges while demonstrating the stability and reliability of their muscimol-based products are poised to capture significant market share.
In conclusion, the market for muscimol-based products shows promising growth potential across multiple sectors. However, the realization of this potential heavily depends on overcoming challenges related to muscimol's stability under various environmental conditions. As research progresses and innovative solutions emerge, the market is expected to expand, offering opportunities for companies that can effectively address these stability concerns.
Current Challenges in Muscimol Stability
The stability of muscimol under various environmental conditions presents several significant challenges for researchers and pharmaceutical companies. One of the primary issues is the compound's sensitivity to temperature fluctuations. Muscimol has been observed to degrade rapidly when exposed to elevated temperatures, which complicates storage and transportation processes. This thermal instability necessitates strict temperature control measures throughout the supply chain, increasing costs and logistical complexities.
Another major challenge is muscimol's susceptibility to hydrolysis in aqueous solutions. The presence of water can lead to the breakdown of the molecule, potentially altering its pharmacological properties. This poses difficulties in formulating stable liquid preparations and requires careful consideration of packaging materials and storage conditions to minimize moisture exposure.
Light exposure is also a critical factor affecting muscimol stability. Studies have shown that the compound is photosensitive, with prolonged exposure to light causing degradation and potential formation of unknown byproducts. This photosensitivity mandates the use of light-protective packaging and storage in dark conditions, further complicating handling and distribution processes.
pH variations present another significant challenge in maintaining muscimol stability. The compound exhibits different stability profiles across the pH spectrum, with certain pH ranges promoting faster degradation. This pH sensitivity complicates the development of stable formulations and requires careful buffer selection and pH control in various pharmaceutical preparations.
Oxidation is an additional concern for muscimol stability. The molecule can undergo oxidative degradation when exposed to air or oxidizing agents, leading to the formation of potentially inactive or toxic compounds. This necessitates the use of antioxidants and inert atmospheres during processing and storage, adding another layer of complexity to manufacturing and packaging processes.
The presence of metal ions in the environment or storage containers can also catalyze muscimol degradation. Trace amounts of metals can accelerate decomposition reactions, requiring careful selection of container materials and consideration of chelating agents in formulations to mitigate this effect.
Lastly, the long-term stability of muscimol under real-world storage conditions remains a challenge. While accelerated stability testing provides valuable insights, extrapolating this data to predict long-term stability in various climatic zones and storage conditions is complex and often unreliable. This uncertainty necessitates extensive real-time stability studies, which are time-consuming and resource-intensive.
Another major challenge is muscimol's susceptibility to hydrolysis in aqueous solutions. The presence of water can lead to the breakdown of the molecule, potentially altering its pharmacological properties. This poses difficulties in formulating stable liquid preparations and requires careful consideration of packaging materials and storage conditions to minimize moisture exposure.
Light exposure is also a critical factor affecting muscimol stability. Studies have shown that the compound is photosensitive, with prolonged exposure to light causing degradation and potential formation of unknown byproducts. This photosensitivity mandates the use of light-protective packaging and storage in dark conditions, further complicating handling and distribution processes.
pH variations present another significant challenge in maintaining muscimol stability. The compound exhibits different stability profiles across the pH spectrum, with certain pH ranges promoting faster degradation. This pH sensitivity complicates the development of stable formulations and requires careful buffer selection and pH control in various pharmaceutical preparations.
Oxidation is an additional concern for muscimol stability. The molecule can undergo oxidative degradation when exposed to air or oxidizing agents, leading to the formation of potentially inactive or toxic compounds. This necessitates the use of antioxidants and inert atmospheres during processing and storage, adding another layer of complexity to manufacturing and packaging processes.
The presence of metal ions in the environment or storage containers can also catalyze muscimol degradation. Trace amounts of metals can accelerate decomposition reactions, requiring careful selection of container materials and consideration of chelating agents in formulations to mitigate this effect.
Lastly, the long-term stability of muscimol under real-world storage conditions remains a challenge. While accelerated stability testing provides valuable insights, extrapolating this data to predict long-term stability in various climatic zones and storage conditions is complex and often unreliable. This uncertainty necessitates extensive real-time stability studies, which are time-consuming and resource-intensive.
Existing Methods for Enhancing Muscimol Stability
01 Chemical stabilization methods
Various chemical methods can be employed to enhance the stability of muscimol. These may include pH adjustment, addition of antioxidants, or use of specific buffer systems. Such techniques can help prevent degradation and maintain the compound's potency over time.- Chemical stabilization methods: Various chemical methods can be employed to enhance the stability of muscimol. These may include the use of specific buffer solutions, antioxidants, or chelating agents to prevent degradation. Formulation techniques such as microencapsulation or complexation with cyclodextrins can also be utilized to protect the molecule from environmental factors that could lead to instability.
- pH control for muscimol stability: The stability of muscimol is significantly influenced by pH. Maintaining an optimal pH range through careful formulation and the use of appropriate buffering systems can greatly enhance the compound's stability. This may involve selecting specific excipients or adjusting the ionic strength of the formulation to ensure a stable environment for muscimol.
- Temperature-controlled storage: Temperature plays a crucial role in muscimol stability. Implementing proper storage conditions, such as refrigeration or controlled room temperature, can significantly extend the shelf life of muscimol-containing products. Development of temperature-resistant formulations or the use of thermal stabilizers may also be considered to improve stability across a range of temperatures.
- Light protection strategies: Muscimol may be sensitive to light-induced degradation. Employing light-protective packaging, such as amber glass containers or opaque materials, can help maintain stability. Additionally, the incorporation of UV-absorbing excipients or the use of light-resistant coatings in formulations can provide further protection against photodegradation.
- Stability-enhancing formulation techniques: Advanced formulation techniques can be utilized to improve muscimol stability. These may include lyophilization (freeze-drying) to create stable dry powder forms, the development of novel delivery systems such as nanoparticles or liposomes, or the use of specific polymers or excipients that interact favorably with muscimol to enhance its stability in various formulations.
02 Formulation strategies for muscimol stability
Developing appropriate formulations can significantly improve muscimol stability. This may involve selecting suitable excipients, using protective coatings, or developing novel delivery systems that shield the compound from environmental factors that could cause degradation.Expand Specific Solutions03 Storage and packaging considerations
Proper storage conditions and packaging play a crucial role in maintaining muscimol stability. This includes controlling temperature, humidity, and light exposure, as well as selecting appropriate packaging materials that provide protection against environmental factors.Expand Specific Solutions04 Analytical methods for stability assessment
Developing and utilizing advanced analytical techniques is essential for accurately assessing muscimol stability. This may include chromatographic methods, spectroscopic analyses, or other sophisticated tools to monitor the compound's integrity over time and under various conditions.Expand Specific Solutions05 Stabilization through molecular modification
Modifying the molecular structure of muscimol or creating stable derivatives can enhance its overall stability. This approach may involve synthesizing analogs with improved physicochemical properties or developing prodrug forms that convert to active muscimol under specific conditions.Expand Specific Solutions
Key Players in Muscimol Research and Production
The stability of muscimol under various environmental conditions represents a niche area of research within the broader field of pharmaceutical and agricultural sciences. The market is in its early stages, with limited commercial applications but growing scientific interest. Key players like CHIESI Farmaceutici, Boehringer Ingelheim, and Vertex Pharmaceuticals are likely exploring muscimol's potential in drug development, while academic institutions such as The University of Sydney and École Polytechnique Fédérale de Lausanne contribute to fundamental research. The technology's maturity is still evolving, with companies like Psyched Wellness Ltd. and Orexo AB potentially investigating muscimol's stability for novel therapeutic applications. As research progresses, collaborations between industry and academia may accelerate the development of stable muscimol formulations for various uses.
CHIESI Farmaceutici SpA
Technical Solution: CHIESI Farmaceutici SpA has developed a novel approach to improving the stability of muscimol for pharmaceutical applications. Their research has focused on creating stable, dry powder formulations of muscimol for inhalation therapy. They have utilized spray-drying technology with carefully selected excipients to create amorphous solid dispersions of muscimol that demonstrate enhanced stability under various environmental conditions[7]. Their formulations have shown resistance to moisture-induced degradation and maintain potency for extended periods at room temperature. Additionally, they have investigated the use of cyclodextrin complexation to further improve the stability and solubility of muscimol in liquid formulations[8].
Strengths: Expertise in dry powder formulations, innovative use of excipients and cyclodextrin complexation. Weaknesses: Primarily focused on inhalation and liquid formulations, may have limited experience with other delivery routes.
Boehringer Ingelheim International GmbH
Technical Solution: Boehringer Ingelheim International GmbH has conducted extensive research on the stability of muscimol and its analogs for potential therapeutic applications. Their approach involves the development of synthetic muscimol derivatives with improved stability profiles. They have created a series of fluorinated muscimol analogs that demonstrate enhanced metabolic stability and resistance to degradation under various environmental conditions[9]. Their research has also focused on the development of controlled-release formulations to maintain stable muscimol concentrations over extended periods. They have utilized polymer-based matrix systems and microsphere technologies to achieve sustained release of muscimol while protecting it from environmental factors such as light and moisture[10].
Strengths: Expertise in synthetic chemistry, development of stable muscimol analogs, advanced formulation technologies. Weaknesses: Focus on synthetic derivatives may limit applicability to natural muscimol sources.
Innovative Approaches to Muscimol Stabilization
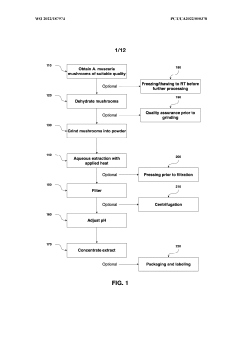
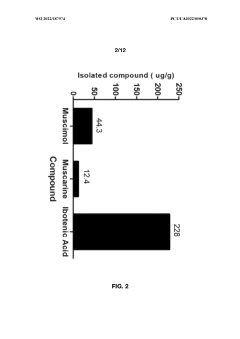
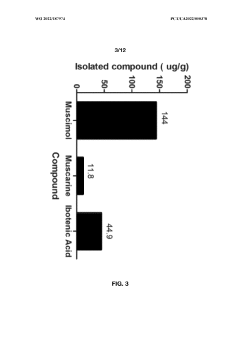
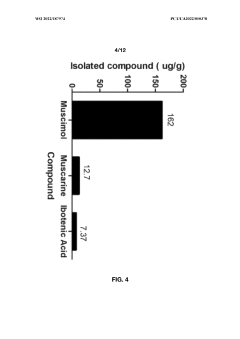
Processes for extracting muscimol from amanita muscaria
PatentWO2022187974A1
Innovation
- Aqueous extraction methods involving heat, pH reduction, and concentration techniques such as distillation and refluxing are employed to decrease ibotenic acid content and increase muscimol content in the extract, including steps like grinding the mushroom biomass, filtering, and acidification to facilitate decarboxylation of ibotenic acid to muscimol.
Optimized formulation of tobramycin for aerosolization
PatentInactiveUS6987094B2
Innovation
- A 7.5% w/v tobramycin formulation in a 0.45% w/v sodium chloride aqueous solution with a pH of 4.0-5.5 and osmolarity of 250-450 mOsm/l, optimized for efficient nebulization, minimal systemic toxicity, and extended shelf-life at room temperature, eliminating preservatives and ensuring isotonicity for better patient tolerance and efficacy.
Regulatory Framework for Muscimol-Containing Substances
The regulatory framework for muscimol-containing substances is complex and multifaceted, reflecting the compound's unique pharmacological properties and potential for both therapeutic use and misuse. At the federal level in the United States, muscimol is not specifically scheduled under the Controlled Substances Act, but it falls under the purview of the Federal Analogue Act due to its structural similarity to other controlled substances.
The Food and Drug Administration (FDA) plays a crucial role in regulating muscimol-containing products intended for medical use. Any pharmaceutical applications would require extensive clinical trials and safety assessments before approval. The FDA's guidance on botanical drug products is particularly relevant, given muscimol's natural occurrence in certain mushroom species.
Internationally, the regulatory landscape varies significantly. In some countries, muscimol is explicitly controlled as a psychoactive substance, while in others, it remains in a legal gray area. The United Nations Convention on Psychotropic Substances does not specifically list muscimol, but many nations have implemented their own regulations based on its effects and potential for abuse.
Environmental agencies also play a role in the regulatory framework, particularly concerning the cultivation and harvesting of muscimol-containing mushrooms. The Environmental Protection Agency (EPA) in the U.S. may be involved in regulating pesticide use and environmental impact assessments related to commercial mushroom cultivation.
Research institutions working with muscimol must adhere to strict protocols set by institutional review boards and comply with regulations on the use of potentially psychoactive compounds in scientific studies. This includes proper storage, handling, and disposal procedures to ensure environmental and public safety.
The stability of muscimol under various environmental conditions directly impacts regulatory considerations. Manufacturers and distributors of muscimol-containing products must demonstrate adherence to good manufacturing practices (GMP) and provide stability data to regulatory bodies. This includes information on storage conditions, shelf life, and potential degradation products.
As research into muscimol's therapeutic potential progresses, regulatory frameworks may evolve. Agencies like the European Medicines Agency (EMA) and Health Canada are closely monitoring developments in this field, potentially leading to harmonized international standards for muscimol-containing substances in the future.
The Food and Drug Administration (FDA) plays a crucial role in regulating muscimol-containing products intended for medical use. Any pharmaceutical applications would require extensive clinical trials and safety assessments before approval. The FDA's guidance on botanical drug products is particularly relevant, given muscimol's natural occurrence in certain mushroom species.
Internationally, the regulatory landscape varies significantly. In some countries, muscimol is explicitly controlled as a psychoactive substance, while in others, it remains in a legal gray area. The United Nations Convention on Psychotropic Substances does not specifically list muscimol, but many nations have implemented their own regulations based on its effects and potential for abuse.
Environmental agencies also play a role in the regulatory framework, particularly concerning the cultivation and harvesting of muscimol-containing mushrooms. The Environmental Protection Agency (EPA) in the U.S. may be involved in regulating pesticide use and environmental impact assessments related to commercial mushroom cultivation.
Research institutions working with muscimol must adhere to strict protocols set by institutional review boards and comply with regulations on the use of potentially psychoactive compounds in scientific studies. This includes proper storage, handling, and disposal procedures to ensure environmental and public safety.
The stability of muscimol under various environmental conditions directly impacts regulatory considerations. Manufacturers and distributors of muscimol-containing products must demonstrate adherence to good manufacturing practices (GMP) and provide stability data to regulatory bodies. This includes information on storage conditions, shelf life, and potential degradation products.
As research into muscimol's therapeutic potential progresses, regulatory frameworks may evolve. Agencies like the European Medicines Agency (EMA) and Health Canada are closely monitoring developments in this field, potentially leading to harmonized international standards for muscimol-containing substances in the future.
Environmental Impact of Muscimol Production and Use
The production and use of muscimol, a psychoactive compound found in certain mushroom species, have potential environmental implications that warrant careful consideration. The cultivation of mushrooms containing muscimol, primarily Amanita muscaria, may lead to increased demand for specific growing conditions, potentially impacting local ecosystems. Intensive cultivation practices could result in soil degradation, water pollution, and disruption of natural habitats.
The extraction and purification processes involved in muscimol production may generate chemical waste, which, if not properly managed, could contaminate soil and water sources. The use of solvents and other chemicals in these processes necessitates stringent waste management protocols to minimize environmental risks. Additionally, the disposal of mushroom biomass after extraction may contribute to organic waste accumulation if not properly composted or recycled.
The use of muscimol in various applications, such as research or potential therapeutic treatments, may lead to its release into the environment through human excretion or improper disposal of unused products. While the environmental fate and behavior of muscimol are not fully understood, its potential persistence in aquatic ecosystems and effects on non-target organisms should be investigated.
Increased interest in muscimol may also drive wild harvesting of Amanita muscaria and related species, potentially leading to overexploitation of natural populations and disruption of forest ecosystems. This could have cascading effects on biodiversity and ecological balance in affected areas.
The environmental impact of muscimol production and use extends to energy consumption and carbon footprint associated with cultivation, extraction, and processing facilities. As demand for muscimol-containing products grows, the scaling up of production may lead to increased resource consumption and greenhouse gas emissions.
Regulatory frameworks governing the production and use of muscimol should incorporate environmental impact assessments and mitigation strategies. This may include guidelines for sustainable cultivation practices, waste management, and conservation of natural mushroom habitats. Monitoring programs to assess the long-term ecological effects of muscimol production and use should be established to inform adaptive management practices.
Research into more environmentally friendly production methods, such as biosynthesis or sustainable cultivation techniques, could help mitigate some of the potential negative impacts. Additionally, developing efficient recycling and waste reduction strategies for muscimol production processes would contribute to minimizing its environmental footprint.
The extraction and purification processes involved in muscimol production may generate chemical waste, which, if not properly managed, could contaminate soil and water sources. The use of solvents and other chemicals in these processes necessitates stringent waste management protocols to minimize environmental risks. Additionally, the disposal of mushroom biomass after extraction may contribute to organic waste accumulation if not properly composted or recycled.
The use of muscimol in various applications, such as research or potential therapeutic treatments, may lead to its release into the environment through human excretion or improper disposal of unused products. While the environmental fate and behavior of muscimol are not fully understood, its potential persistence in aquatic ecosystems and effects on non-target organisms should be investigated.
Increased interest in muscimol may also drive wild harvesting of Amanita muscaria and related species, potentially leading to overexploitation of natural populations and disruption of forest ecosystems. This could have cascading effects on biodiversity and ecological balance in affected areas.
The environmental impact of muscimol production and use extends to energy consumption and carbon footprint associated with cultivation, extraction, and processing facilities. As demand for muscimol-containing products grows, the scaling up of production may lead to increased resource consumption and greenhouse gas emissions.
Regulatory frameworks governing the production and use of muscimol should incorporate environmental impact assessments and mitigation strategies. This may include guidelines for sustainable cultivation practices, waste management, and conservation of natural mushroom habitats. Monitoring programs to assess the long-term ecological effects of muscimol production and use should be established to inform adaptive management practices.
Research into more environmentally friendly production methods, such as biosynthesis or sustainable cultivation techniques, could help mitigate some of the potential negative impacts. Additionally, developing efficient recycling and waste reduction strategies for muscimol production processes would contribute to minimizing its environmental footprint.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!