Carbonyl Transformation in Medicinal Chemistry: Trends
JUL 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbonyl Chemistry Evolution
Carbonyl chemistry has undergone significant evolution in medicinal chemistry over the past century. The transformation of carbonyl compounds has been a cornerstone in the synthesis of complex molecules, particularly in drug discovery and development. This evolution can be traced through several key phases, each marked by groundbreaking discoveries and methodological advancements.
In the early 20th century, carbonyl chemistry was primarily focused on simple reactions such as aldol condensations and Grignard additions. These reactions laid the foundation for more complex transformations but were limited in their scope and selectivity. The mid-20th century saw a surge in the development of new carbonyl transformations, including the Wittig reaction and its variants, which revolutionized the synthesis of alkenes from carbonyl compounds.
The late 20th century brought about a paradigm shift with the advent of asymmetric catalysis. This period saw the development of chiral catalysts that could selectively produce enantiomerically pure compounds from prochiral carbonyl substrates. Notably, the work of Knowles, Noyori, and Sharpless on asymmetric hydrogenation and oxidation reactions of carbonyl compounds earned them the Nobel Prize in Chemistry in 2001.
As the field progressed into the 21st century, there was a growing emphasis on green chemistry and sustainable practices. This led to the development of more environmentally friendly carbonyl transformations, including organocatalytic methods and the use of renewable feedstocks. The emergence of biocatalysis also provided new avenues for selective carbonyl transformations under mild conditions.
Recent trends in carbonyl chemistry have focused on expanding the toolbox of available transformations. Cross-coupling reactions involving carbonyl compounds have gained prominence, allowing for the formation of complex carbon-carbon bonds. Additionally, photoredox catalysis has opened up new possibilities for carbonyl transformations under mild conditions, often with high levels of selectivity.
The evolution of carbonyl chemistry in medicinal chemistry has also been marked by advances in computational methods. These tools have enabled researchers to predict reaction outcomes, design more efficient catalysts, and understand complex reaction mechanisms. This has led to more rational approaches in drug design and synthesis, where carbonyl transformations play a crucial role.
Looking forward, the field of carbonyl chemistry continues to evolve with emerging technologies such as flow chemistry and artificial intelligence. These advancements promise to further expand the scope and efficiency of carbonyl transformations, potentially revolutionizing drug discovery processes and enabling the synthesis of previously inaccessible molecular architectures.
In the early 20th century, carbonyl chemistry was primarily focused on simple reactions such as aldol condensations and Grignard additions. These reactions laid the foundation for more complex transformations but were limited in their scope and selectivity. The mid-20th century saw a surge in the development of new carbonyl transformations, including the Wittig reaction and its variants, which revolutionized the synthesis of alkenes from carbonyl compounds.
The late 20th century brought about a paradigm shift with the advent of asymmetric catalysis. This period saw the development of chiral catalysts that could selectively produce enantiomerically pure compounds from prochiral carbonyl substrates. Notably, the work of Knowles, Noyori, and Sharpless on asymmetric hydrogenation and oxidation reactions of carbonyl compounds earned them the Nobel Prize in Chemistry in 2001.
As the field progressed into the 21st century, there was a growing emphasis on green chemistry and sustainable practices. This led to the development of more environmentally friendly carbonyl transformations, including organocatalytic methods and the use of renewable feedstocks. The emergence of biocatalysis also provided new avenues for selective carbonyl transformations under mild conditions.
Recent trends in carbonyl chemistry have focused on expanding the toolbox of available transformations. Cross-coupling reactions involving carbonyl compounds have gained prominence, allowing for the formation of complex carbon-carbon bonds. Additionally, photoredox catalysis has opened up new possibilities for carbonyl transformations under mild conditions, often with high levels of selectivity.
The evolution of carbonyl chemistry in medicinal chemistry has also been marked by advances in computational methods. These tools have enabled researchers to predict reaction outcomes, design more efficient catalysts, and understand complex reaction mechanisms. This has led to more rational approaches in drug design and synthesis, where carbonyl transformations play a crucial role.
Looking forward, the field of carbonyl chemistry continues to evolve with emerging technologies such as flow chemistry and artificial intelligence. These advancements promise to further expand the scope and efficiency of carbonyl transformations, potentially revolutionizing drug discovery processes and enabling the synthesis of previously inaccessible molecular architectures.
Pharmaceutical Market Needs
The pharmaceutical industry is experiencing a growing demand for innovative carbonyl transformation techniques in medicinal chemistry. This trend is driven by the need for more efficient and cost-effective drug development processes, as well as the pursuit of novel therapeutic compounds with enhanced efficacy and reduced side effects. The market for carbonyl transformation technologies is expanding rapidly, with a particular focus on green chemistry approaches and sustainable manufacturing methods.
One of the key drivers of this market demand is the increasing prevalence of chronic diseases and the need for new treatments. As the global population ages and lifestyle-related health issues become more common, pharmaceutical companies are under pressure to develop new drugs that can address these challenges. Carbonyl transformations play a crucial role in the synthesis of many important drug molecules, making advancements in this field highly valuable to the industry.
Another significant factor influencing market needs is the push for more environmentally friendly and sustainable drug manufacturing processes. Regulatory bodies and consumers alike are demanding greener approaches to pharmaceutical production. This has led to a surge in interest for carbonyl transformation methods that utilize less toxic reagents, reduce waste generation, and minimize energy consumption. Companies that can develop and implement such technologies are likely to gain a competitive edge in the market.
The pharmaceutical industry is also seeking ways to streamline drug discovery and development processes to reduce time-to-market and overall costs. Advanced carbonyl transformation techniques that enable more efficient synthesis of complex molecules are in high demand. These methods can significantly shorten the lead optimization phase and accelerate the progression of drug candidates through preclinical and clinical trials.
Furthermore, there is a growing interest in precision medicine and personalized therapies. This trend requires the ability to synthesize diverse libraries of compounds quickly and efficiently for screening purposes. Carbonyl transformations that offer high versatility and compatibility with a wide range of functional groups are particularly valuable in this context.
The market is also seeing increased demand for technologies that can facilitate the synthesis of previously challenging or inaccessible molecular structures. As drug targets become more complex, pharmaceutical companies are looking for innovative carbonyl transformation methods that can overcome synthetic hurdles and open up new chemical space for drug discovery.
Lastly, there is a significant market need for scalable carbonyl transformation processes that can seamlessly transition from laboratory-scale synthesis to industrial-scale production. Technologies that maintain high efficiency and selectivity across different scales are highly sought after, as they can significantly reduce the time and resources required for process development and scale-up in drug manufacturing.
One of the key drivers of this market demand is the increasing prevalence of chronic diseases and the need for new treatments. As the global population ages and lifestyle-related health issues become more common, pharmaceutical companies are under pressure to develop new drugs that can address these challenges. Carbonyl transformations play a crucial role in the synthesis of many important drug molecules, making advancements in this field highly valuable to the industry.
Another significant factor influencing market needs is the push for more environmentally friendly and sustainable drug manufacturing processes. Regulatory bodies and consumers alike are demanding greener approaches to pharmaceutical production. This has led to a surge in interest for carbonyl transformation methods that utilize less toxic reagents, reduce waste generation, and minimize energy consumption. Companies that can develop and implement such technologies are likely to gain a competitive edge in the market.
The pharmaceutical industry is also seeking ways to streamline drug discovery and development processes to reduce time-to-market and overall costs. Advanced carbonyl transformation techniques that enable more efficient synthesis of complex molecules are in high demand. These methods can significantly shorten the lead optimization phase and accelerate the progression of drug candidates through preclinical and clinical trials.
Furthermore, there is a growing interest in precision medicine and personalized therapies. This trend requires the ability to synthesize diverse libraries of compounds quickly and efficiently for screening purposes. Carbonyl transformations that offer high versatility and compatibility with a wide range of functional groups are particularly valuable in this context.
The market is also seeing increased demand for technologies that can facilitate the synthesis of previously challenging or inaccessible molecular structures. As drug targets become more complex, pharmaceutical companies are looking for innovative carbonyl transformation methods that can overcome synthetic hurdles and open up new chemical space for drug discovery.
Lastly, there is a significant market need for scalable carbonyl transformation processes that can seamlessly transition from laboratory-scale synthesis to industrial-scale production. Technologies that maintain high efficiency and selectivity across different scales are highly sought after, as they can significantly reduce the time and resources required for process development and scale-up in drug manufacturing.
Current Challenges
Carbonyl transformation in medicinal chemistry faces several significant challenges that hinder its full potential in drug discovery and development. One of the primary obstacles is the selectivity of carbonyl reactions, particularly in complex molecular environments. Achieving site-specific transformations without affecting other functional groups remains a persistent issue, often requiring extensive protecting group strategies that can complicate synthetic routes and reduce overall efficiency.
The reactivity of carbonyl groups also presents a double-edged sword. While their electrophilic nature makes them versatile synthetic handles, it also renders them susceptible to undesired side reactions. This can lead to decreased yields, formation of byproducts, and difficulties in purification processes. Moreover, controlling the stereochemistry of carbonyl transformations, especially in asymmetric synthesis, continues to be a significant challenge, impacting the development of enantiopure drug candidates.
Another critical issue is the limited scope of some carbonyl transformations. Certain reactions may work well with model compounds but fail when applied to more complex, drug-like molecules. This lack of robustness and generality can severely limit the applicability of otherwise promising synthetic methodologies in real-world medicinal chemistry scenarios.
The use of harsh reaction conditions in many carbonyl transformations poses additional challenges. High temperatures, strong acids or bases, and sensitive organometallic reagents are often required, which can be incompatible with sensitive functional groups present in advanced intermediates or lead compounds. This incompatibility can necessitate longer, less efficient synthetic routes or limit the structural diversity of accessible compounds.
Sustainability and green chemistry considerations are becoming increasingly important in the pharmaceutical industry. Many traditional carbonyl transformations rely on toxic reagents, generate significant waste, or require energy-intensive processes. Developing more environmentally friendly alternatives that maintain or improve efficiency remains a significant challenge.
Lastly, the scalability of carbonyl transformations from laboratory to industrial scale presents ongoing difficulties. Reactions that perform well on a small scale may encounter issues when scaled up, such as heat transfer problems, mixing inefficiencies, or unexpected side reactions. This can lead to increased costs, reduced yields, and potential safety concerns in large-scale production of pharmaceutical intermediates and active pharmaceutical ingredients.
The reactivity of carbonyl groups also presents a double-edged sword. While their electrophilic nature makes them versatile synthetic handles, it also renders them susceptible to undesired side reactions. This can lead to decreased yields, formation of byproducts, and difficulties in purification processes. Moreover, controlling the stereochemistry of carbonyl transformations, especially in asymmetric synthesis, continues to be a significant challenge, impacting the development of enantiopure drug candidates.
Another critical issue is the limited scope of some carbonyl transformations. Certain reactions may work well with model compounds but fail when applied to more complex, drug-like molecules. This lack of robustness and generality can severely limit the applicability of otherwise promising synthetic methodologies in real-world medicinal chemistry scenarios.
The use of harsh reaction conditions in many carbonyl transformations poses additional challenges. High temperatures, strong acids or bases, and sensitive organometallic reagents are often required, which can be incompatible with sensitive functional groups present in advanced intermediates or lead compounds. This incompatibility can necessitate longer, less efficient synthetic routes or limit the structural diversity of accessible compounds.
Sustainability and green chemistry considerations are becoming increasingly important in the pharmaceutical industry. Many traditional carbonyl transformations rely on toxic reagents, generate significant waste, or require energy-intensive processes. Developing more environmentally friendly alternatives that maintain or improve efficiency remains a significant challenge.
Lastly, the scalability of carbonyl transformations from laboratory to industrial scale presents ongoing difficulties. Reactions that perform well on a small scale may encounter issues when scaled up, such as heat transfer problems, mixing inefficiencies, or unexpected side reactions. This can lead to increased costs, reduced yields, and potential safety concerns in large-scale production of pharmaceutical intermediates and active pharmaceutical ingredients.
Established Methodologies
01 Carbonyl reduction methods
Various methods for reducing carbonyl compounds to their corresponding alcohols or hydrocarbons. These transformations often involve catalytic hydrogenation or the use of reducing agents such as metal hydrides. The processes can be selective and are important in organic synthesis and industrial applications.- Carbonyl reduction methods: Various methods for reducing carbonyl compounds to their corresponding alcohols or hydrocarbons. These transformations often involve catalytic hydrogenation or the use of reducing agents such as metal hydrides. The processes can be selective and are important in organic synthesis and industrial applications.
- Oxidation of carbonyl compounds: Techniques for oxidizing carbonyl compounds to form carboxylic acids, esters, or other higher oxidation state products. These transformations may involve the use of oxidizing agents, catalysts, or enzymatic processes. Such reactions are crucial in the synthesis of various organic compounds and pharmaceuticals.
- Carbonyl addition reactions: Methods for adding nucleophiles to carbonyl compounds, resulting in the formation of new carbon-carbon or carbon-heteroatom bonds. These reactions include aldol condensations, Grignard reactions, and other nucleophilic additions. Such transformations are fundamental in organic synthesis and the production of complex molecules.
- Carbonyl rearrangement reactions: Processes involving the rearrangement of carbonyl compounds to form new structural isomers or different functional groups. These transformations may include pinacol rearrangements, Beckmann rearrangements, or other skeletal reorganizations. Such reactions are valuable in the synthesis of complex organic molecules and natural products.
- Carbonyl protection and deprotection: Techniques for protecting carbonyl groups during multi-step syntheses and subsequently removing the protecting groups. These methods are essential for controlling reactivity and selectivity in complex organic transformations. Various protecting groups and conditions for their installation and removal are employed depending on the specific requirements of the synthesis.
02 Oxidation of carbonyl compounds
Techniques for oxidizing carbonyl compounds to form carboxylic acids, esters, or other higher oxidation state products. These transformations may involve the use of oxidizing agents, catalysts, or electrochemical methods. The processes are crucial in the synthesis of various organic compounds and pharmaceuticals.Expand Specific Solutions03 Carbonyl addition reactions
Methods for adding nucleophiles to carbonyl compounds, resulting in the formation of new carbon-carbon or carbon-heteroatom bonds. These reactions include aldol condensations, Grignard reactions, and other nucleophilic additions. The processes are fundamental in organic synthesis for creating complex molecules.Expand Specific Solutions04 Carbonyl rearrangement reactions
Processes involving the rearrangement of carbonyl compounds to form new structural isomers or different functional groups. These transformations may include pinacol rearrangements, Beckmann rearrangements, or other skeletal reorganizations. The reactions are valuable for synthesizing complex organic structures.Expand Specific Solutions05 Carbonyl protection and deprotection
Techniques for protecting carbonyl groups during multi-step syntheses and subsequently removing the protecting groups. These methods are essential for controlling reactivity and selectivity in complex organic transformations. Various protecting groups and conditions are employed depending on the specific requirements of the synthesis.Expand Specific Solutions
Key Pharma Companies
The field of carbonyl transformation in medicinal chemistry is in a mature stage of development, with a well-established market and significant ongoing research. The global pharmaceutical industry, valued at over $1 trillion, drives continuous innovation in this area. Major players include both academic institutions and pharmaceutical companies, reflecting a blend of fundamental research and commercial applications. Universities like Zurich, Bonn, and Rutgers contribute significantly to basic research, while companies such as Merck & Co., Kaneka Corp., and ViiV Healthcare focus on translating these insights into drug development. The involvement of research foundations like Wisconsin Alumni Research Foundation and government institutions like the Spanish National Research Council underscores the field's importance. The technology's maturity is evident in the diverse range of applications across multiple therapeutic areas, with ongoing efforts to enhance efficiency and develop novel transformations for drug discovery and synthesis.
University of Zurich
Technical Solution: The University of Zurich has made significant contributions to carbonyl transformation research in medicinal chemistry. Their approach focuses on developing sustainable and green chemistry methods for carbonyl modifications. They have pioneered the use of photocatalysis for carbonyl transformations, enabling light-driven reactions that reduce the need for harsh reagents[4]. The university's research team has also developed novel organocatalytic methods for asymmetric carbonyl transformations, which are particularly valuable in the synthesis of chiral drug molecules[5]. Additionally, they have explored the use of electrochemical methods for carbonyl functionalization, offering an environmentally friendly alternative to traditional synthetic routes[6].
Strengths: Strong focus on sustainable chemistry and innovative catalytic methods. Weaknesses: Potential challenges in scaling up academic research for industrial applications.
Merck & Co., Inc.
Technical Solution: Merck & Co., Inc. has been at the forefront of carbonyl transformation research in medicinal chemistry. They have developed innovative approaches to carbonyl modification, including selective reduction and oxidation techniques. Their research focuses on the use of transition metal catalysts for carbonyl transformations, particularly in the synthesis of complex pharmaceutical compounds[1]. Merck has also pioneered the use of biocatalysis for carbonyl transformations, employing engineered enzymes to achieve high selectivity and efficiency in drug synthesis[2]. Additionally, they have made significant advancements in flow chemistry applications for carbonyl transformations, enabling continuous production of key pharmaceutical intermediates[3].
Strengths: Strong R&D capabilities, extensive patent portfolio, and integration of cutting-edge technologies. Weaknesses: High research costs and potential regulatory challenges in implementing novel synthetic methods.
Innovative Catalytic Systems
Therapeutic delivery of carbon monoxide
PatentWO2007085806A2
Innovation
- Development of pharmaceutical compositions containing transition metal carbonyl compounds with specific ligands and substituents that ensure CO release without precipitation, maintaining solubility and stability in aqueous physiological fluids, thereby preventing undesirable side effects.
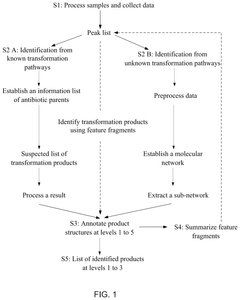
Method for identifying transformation products of antibiotics from known and potential unknown transformation pathways
PatentPendingUS20250095792A1
Innovation
- A method that combines known and potential unknown transformation pathways using ultra-high performance liquid chromatography-high resolution mass spectrometry, molecular networks, and feature fragments to comprehensively identify transformation products of antibiotics.
Green Chemistry Aspects
Green chemistry principles have become increasingly important in medicinal chemistry, particularly in carbonyl transformations. The pharmaceutical industry is actively seeking more sustainable and environmentally friendly approaches to drug synthesis. In the context of carbonyl transformations, several green chemistry aspects have gained prominence in recent years.
One key focus has been on the development of catalytic methods that reduce waste and improve atom economy. Traditional stoichiometric reagents are being replaced by catalytic systems that can achieve the same transformations with higher efficiency and lower environmental impact. For example, the use of organocatalysts and biocatalysts in carbonyl transformations has gained significant attention due to their ability to operate under mild conditions and their potential for recyclability.
Solvent selection has also become a critical consideration in green carbonyl chemistry. Water-based reactions and the use of bio-derived solvents are being explored as alternatives to traditional organic solvents. These approaches not only reduce the environmental footprint but also often lead to improved reaction outcomes, such as enhanced selectivity and easier product isolation.
The application of flow chemistry techniques to carbonyl transformations has emerged as another important green chemistry aspect. Continuous flow processes offer advantages in terms of improved heat and mass transfer, leading to more efficient reactions and reduced energy consumption. Additionally, flow chemistry enables precise control over reaction parameters, facilitating process optimization and scale-up.
Photochemical and electrochemical methods for carbonyl transformations have also gained traction in the green chemistry context. These approaches often allow for milder reaction conditions and can be powered by renewable energy sources, aligning well with sustainability goals. Photocatalytic carbonyl transformations, in particular, have shown promise in enabling previously challenging reactions under environmentally benign conditions.
The principles of green chemistry are also being applied to the development of new protecting group strategies in carbonyl chemistry. Efforts are being made to design protecting groups that can be easily removed under mild conditions, reducing the need for harsh reagents and minimizing waste generation. This approach not only improves the overall sustainability of synthetic routes but also enhances the compatibility with sensitive functional groups.
As the field of medicinal chemistry continues to evolve, the integration of green chemistry principles in carbonyl transformations is likely to become even more prevalent. Future trends may include the development of more efficient and selective catalysts, the exploration of novel reaction media, and the implementation of artificial intelligence and machine learning to optimize sustainable synthetic routes. These advancements will contribute to the ongoing efforts to make drug discovery and development processes more environmentally friendly and economically viable.
One key focus has been on the development of catalytic methods that reduce waste and improve atom economy. Traditional stoichiometric reagents are being replaced by catalytic systems that can achieve the same transformations with higher efficiency and lower environmental impact. For example, the use of organocatalysts and biocatalysts in carbonyl transformations has gained significant attention due to their ability to operate under mild conditions and their potential for recyclability.
Solvent selection has also become a critical consideration in green carbonyl chemistry. Water-based reactions and the use of bio-derived solvents are being explored as alternatives to traditional organic solvents. These approaches not only reduce the environmental footprint but also often lead to improved reaction outcomes, such as enhanced selectivity and easier product isolation.
The application of flow chemistry techniques to carbonyl transformations has emerged as another important green chemistry aspect. Continuous flow processes offer advantages in terms of improved heat and mass transfer, leading to more efficient reactions and reduced energy consumption. Additionally, flow chemistry enables precise control over reaction parameters, facilitating process optimization and scale-up.
Photochemical and electrochemical methods for carbonyl transformations have also gained traction in the green chemistry context. These approaches often allow for milder reaction conditions and can be powered by renewable energy sources, aligning well with sustainability goals. Photocatalytic carbonyl transformations, in particular, have shown promise in enabling previously challenging reactions under environmentally benign conditions.
The principles of green chemistry are also being applied to the development of new protecting group strategies in carbonyl chemistry. Efforts are being made to design protecting groups that can be easily removed under mild conditions, reducing the need for harsh reagents and minimizing waste generation. This approach not only improves the overall sustainability of synthetic routes but also enhances the compatibility with sensitive functional groups.
As the field of medicinal chemistry continues to evolve, the integration of green chemistry principles in carbonyl transformations is likely to become even more prevalent. Future trends may include the development of more efficient and selective catalysts, the exploration of novel reaction media, and the implementation of artificial intelligence and machine learning to optimize sustainable synthetic routes. These advancements will contribute to the ongoing efforts to make drug discovery and development processes more environmentally friendly and economically viable.
Computational Approaches
Computational approaches have revolutionized the field of carbonyl transformation in medicinal chemistry, offering powerful tools for predicting and optimizing chemical reactions. These methods leverage advanced algorithms and machine learning techniques to simulate molecular interactions, predict reaction outcomes, and guide the design of novel compounds.
One of the key computational approaches in this domain is molecular docking, which allows researchers to predict the binding affinity between small molecules and target proteins. This technique has proven invaluable in identifying potential lead compounds for drug discovery, particularly in the context of carbonyl-containing molecules. By simulating the interaction between a ligand and a receptor, researchers can rapidly screen large libraries of compounds and prioritize those with the highest likelihood of success.
Quantum mechanical calculations have also played a crucial role in understanding the mechanisms of carbonyl transformations. Density Functional Theory (DFT) methods, in particular, have been widely employed to elucidate reaction pathways, transition states, and energetics of carbonyl-based reactions. These calculations provide valuable insights into the electronic structure and reactivity of carbonyl compounds, enabling researchers to rationalize experimental observations and guide the development of new synthetic strategies.
Machine learning algorithms have emerged as powerful tools for predicting the outcomes of carbonyl transformations. By training on large datasets of known reactions, these models can learn complex patterns and relationships, allowing them to make accurate predictions for novel reactions. This approach has been particularly successful in retrosynthesis planning, where AI-powered systems can suggest viable synthetic routes for target molecules containing carbonyl groups.
Molecular dynamics simulations have provided valuable insights into the behavior of carbonyl-containing compounds in biological systems. These simulations allow researchers to study the conformational changes, solvation effects, and intermolecular interactions of carbonyl-based drugs, helping to optimize their pharmacokinetic properties and improve their efficacy.
High-throughput virtual screening has become an essential tool in the discovery of new carbonyl-based drugs. By combining computational approaches with large chemical libraries, researchers can rapidly identify promising lead compounds for further experimental validation. This approach has significantly accelerated the drug discovery process, reducing the time and resources required to bring new medications to market.
In conclusion, computational approaches have become indispensable in the field of carbonyl transformation in medicinal chemistry. These methods continue to evolve, driven by advances in hardware capabilities and algorithm development, promising even greater insights and innovations in the future of drug discovery and design.
One of the key computational approaches in this domain is molecular docking, which allows researchers to predict the binding affinity between small molecules and target proteins. This technique has proven invaluable in identifying potential lead compounds for drug discovery, particularly in the context of carbonyl-containing molecules. By simulating the interaction between a ligand and a receptor, researchers can rapidly screen large libraries of compounds and prioritize those with the highest likelihood of success.
Quantum mechanical calculations have also played a crucial role in understanding the mechanisms of carbonyl transformations. Density Functional Theory (DFT) methods, in particular, have been widely employed to elucidate reaction pathways, transition states, and energetics of carbonyl-based reactions. These calculations provide valuable insights into the electronic structure and reactivity of carbonyl compounds, enabling researchers to rationalize experimental observations and guide the development of new synthetic strategies.
Machine learning algorithms have emerged as powerful tools for predicting the outcomes of carbonyl transformations. By training on large datasets of known reactions, these models can learn complex patterns and relationships, allowing them to make accurate predictions for novel reactions. This approach has been particularly successful in retrosynthesis planning, where AI-powered systems can suggest viable synthetic routes for target molecules containing carbonyl groups.
Molecular dynamics simulations have provided valuable insights into the behavior of carbonyl-containing compounds in biological systems. These simulations allow researchers to study the conformational changes, solvation effects, and intermolecular interactions of carbonyl-based drugs, helping to optimize their pharmacokinetic properties and improve their efficacy.
High-throughput virtual screening has become an essential tool in the discovery of new carbonyl-based drugs. By combining computational approaches with large chemical libraries, researchers can rapidly identify promising lead compounds for further experimental validation. This approach has significantly accelerated the drug discovery process, reducing the time and resources required to bring new medications to market.
In conclusion, computational approaches have become indispensable in the field of carbonyl transformation in medicinal chemistry. These methods continue to evolve, driven by advances in hardware capabilities and algorithm development, promising even greater insights and innovations in the future of drug discovery and design.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!