Understanding Muscimol's Risk Factors in Human Consumption
JUL 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Muscimol Background and Research Objectives
Muscimol, a potent psychoactive compound found primarily in Amanita mushroom species, has a rich history of traditional use and scientific interest. This naturally occurring GABA agonist has been utilized in various cultural practices for centuries, particularly in Siberian shamanic rituals. The compound's unique pharmacological properties have attracted significant attention from researchers in recent decades, leading to a growing body of scientific literature exploring its potential therapeutic applications and associated risks.
The evolution of muscimol research has been marked by several key milestones. Early ethnobotanical studies in the mid-20th century documented its traditional use, while subsequent pharmacological investigations in the 1960s and 1970s elucidated its mechanism of action as a GABA receptor agonist. More recent research has focused on understanding muscimol's effects on the central nervous system, its potential for treating neurological disorders, and the development of synthetic analogues for therapeutic use.
Despite its long history of human consumption, the safety profile of muscimol remains a subject of ongoing investigation. The compound's potent psychoactive effects, coupled with the potential for toxicity when consumed in high doses or in combination with certain mushroom species, underscore the need for a comprehensive understanding of its risk factors in human consumption.
The primary objectives of this technical research report are to critically examine the current state of knowledge regarding muscimol's risk factors, identify gaps in our understanding, and propose directions for future research. Specifically, we aim to:
1. Synthesize existing data on muscimol's pharmacokinetics, pharmacodynamics, and dose-response relationships in humans.
2. Evaluate the potential for acute and chronic toxicity, including interactions with other substances and pre-existing medical conditions.
3. Assess the variability in muscimol content across different Amanita species and environmental conditions, and its implications for risk assessment.
4. Explore the psychological and physiological effects of muscimol consumption, including both desired and adverse outcomes.
5. Investigate potential long-term health consequences associated with repeated or prolonged muscimol use.
6. Examine the legal and regulatory landscape surrounding muscimol and its source organisms.
By addressing these objectives, we seek to provide a comprehensive framework for understanding and mitigating the risks associated with muscimol consumption. This research will not only contribute to the scientific knowledge base but also inform public health policies, clinical practices, and harm reduction strategies related to the use of muscimol-containing substances.
The evolution of muscimol research has been marked by several key milestones. Early ethnobotanical studies in the mid-20th century documented its traditional use, while subsequent pharmacological investigations in the 1960s and 1970s elucidated its mechanism of action as a GABA receptor agonist. More recent research has focused on understanding muscimol's effects on the central nervous system, its potential for treating neurological disorders, and the development of synthetic analogues for therapeutic use.
Despite its long history of human consumption, the safety profile of muscimol remains a subject of ongoing investigation. The compound's potent psychoactive effects, coupled with the potential for toxicity when consumed in high doses or in combination with certain mushroom species, underscore the need for a comprehensive understanding of its risk factors in human consumption.
The primary objectives of this technical research report are to critically examine the current state of knowledge regarding muscimol's risk factors, identify gaps in our understanding, and propose directions for future research. Specifically, we aim to:
1. Synthesize existing data on muscimol's pharmacokinetics, pharmacodynamics, and dose-response relationships in humans.
2. Evaluate the potential for acute and chronic toxicity, including interactions with other substances and pre-existing medical conditions.
3. Assess the variability in muscimol content across different Amanita species and environmental conditions, and its implications for risk assessment.
4. Explore the psychological and physiological effects of muscimol consumption, including both desired and adverse outcomes.
5. Investigate potential long-term health consequences associated with repeated or prolonged muscimol use.
6. Examine the legal and regulatory landscape surrounding muscimol and its source organisms.
By addressing these objectives, we seek to provide a comprehensive framework for understanding and mitigating the risks associated with muscimol consumption. This research will not only contribute to the scientific knowledge base but also inform public health policies, clinical practices, and harm reduction strategies related to the use of muscimol-containing substances.
Market Analysis of Muscimol-Containing Products
The market for muscimol-containing products has been experiencing significant growth in recent years, driven by increasing consumer interest in alternative wellness solutions and psychoactive substances. Muscimol, a psychoactive compound found primarily in Amanita muscaria mushrooms, has garnered attention for its potential therapeutic applications and recreational use.
The global market for muscimol-containing products can be segmented into several categories, including dietary supplements, functional foods and beverages, and pharmaceutical formulations. The dietary supplement sector has seen the most rapid expansion, with muscimol-based products being marketed for stress relief, sleep enhancement, and cognitive support. This segment is expected to continue its growth trajectory as consumers seek natural alternatives to conventional medications.
In the functional food and beverage market, muscimol-infused products are emerging as a niche but rapidly growing category. Manufacturers are incorporating muscimol extracts into various consumables, such as teas, energy drinks, and snack bars, targeting health-conscious consumers looking for novel experiences. This trend aligns with the broader shift towards functional foods and beverages that offer benefits beyond basic nutrition.
The pharmaceutical industry has also shown interest in muscimol's potential therapeutic applications. Research is ongoing to explore its efficacy in treating various neurological and psychiatric conditions, including anxiety disorders, depression, and epilepsy. While still in early stages, the development of muscimol-based pharmaceuticals could represent a significant market opportunity in the future.
Geographically, North America and Europe currently dominate the muscimol product market, with the United States and Canada leading in terms of product innovation and consumer adoption. However, Asia-Pacific is emerging as a rapidly growing market, particularly in countries like Japan and South Korea, where there is a strong cultural affinity for mushroom-based products and traditional medicine.
Consumer demographics for muscimol-containing products skew towards younger adults, particularly those in the 25-40 age range, who are more likely to experiment with alternative wellness solutions. This demographic tends to be well-educated, health-conscious, and open to trying new products that promise cognitive or emotional benefits.
Despite the growing market potential, the muscimol product industry faces significant regulatory challenges. The legal status of muscimol varies widely across jurisdictions, with some countries classifying it as a controlled substance. This regulatory uncertainty has led to a fragmented market landscape and poses a significant barrier to large-scale commercialization.
Looking ahead, the market for muscimol-containing products is poised for continued growth, driven by increasing consumer awareness, ongoing research into its therapeutic potential, and the broader trend towards natural and plant-based wellness solutions. However, the industry's future will largely depend on navigating complex regulatory environments and addressing safety concerns associated with muscimol consumption.
The global market for muscimol-containing products can be segmented into several categories, including dietary supplements, functional foods and beverages, and pharmaceutical formulations. The dietary supplement sector has seen the most rapid expansion, with muscimol-based products being marketed for stress relief, sleep enhancement, and cognitive support. This segment is expected to continue its growth trajectory as consumers seek natural alternatives to conventional medications.
In the functional food and beverage market, muscimol-infused products are emerging as a niche but rapidly growing category. Manufacturers are incorporating muscimol extracts into various consumables, such as teas, energy drinks, and snack bars, targeting health-conscious consumers looking for novel experiences. This trend aligns with the broader shift towards functional foods and beverages that offer benefits beyond basic nutrition.
The pharmaceutical industry has also shown interest in muscimol's potential therapeutic applications. Research is ongoing to explore its efficacy in treating various neurological and psychiatric conditions, including anxiety disorders, depression, and epilepsy. While still in early stages, the development of muscimol-based pharmaceuticals could represent a significant market opportunity in the future.
Geographically, North America and Europe currently dominate the muscimol product market, with the United States and Canada leading in terms of product innovation and consumer adoption. However, Asia-Pacific is emerging as a rapidly growing market, particularly in countries like Japan and South Korea, where there is a strong cultural affinity for mushroom-based products and traditional medicine.
Consumer demographics for muscimol-containing products skew towards younger adults, particularly those in the 25-40 age range, who are more likely to experiment with alternative wellness solutions. This demographic tends to be well-educated, health-conscious, and open to trying new products that promise cognitive or emotional benefits.
Despite the growing market potential, the muscimol product industry faces significant regulatory challenges. The legal status of muscimol varies widely across jurisdictions, with some countries classifying it as a controlled substance. This regulatory uncertainty has led to a fragmented market landscape and poses a significant barrier to large-scale commercialization.
Looking ahead, the market for muscimol-containing products is poised for continued growth, driven by increasing consumer awareness, ongoing research into its therapeutic potential, and the broader trend towards natural and plant-based wellness solutions. However, the industry's future will largely depend on navigating complex regulatory environments and addressing safety concerns associated with muscimol consumption.
Current Understanding and Challenges in Muscimol Safety
The current understanding of muscimol safety in human consumption is limited, primarily due to its classification as a psychoactive compound and its restricted legal status in many jurisdictions. Muscimol, the primary psychoactive constituent of Amanita muscaria mushrooms, has been used traditionally in some cultures but remains largely unstudied in controlled clinical settings.
One of the main challenges in assessing muscimol's safety profile is the lack of comprehensive human trials. Most available data come from case reports, animal studies, and limited human experiences, which provide an incomplete picture of its risk factors. The variability in mushroom potency and preparation methods further complicates the assessment of safe dosage levels.
Acute toxicity of muscimol is generally considered low compared to other psychoactive substances, but its effects can be unpredictable. Short-term risks include disorientation, confusion, and impaired motor coordination, which can lead to accidents or injuries. More severe reactions, though rare, may include respiratory depression, seizures, and loss of consciousness.
The potential for psychological distress is a significant concern, particularly in individuals with pre-existing mental health conditions. Muscimol's GABAergic activity can induce intense alterations in perception and cognition, which may trigger anxiety, panic attacks, or exacerbate underlying psychiatric disorders.
Long-term effects of muscimol consumption remain largely unknown. While some studies suggest potential neuroprotective properties, the impact of chronic use on cognitive function, memory, and overall brain health requires further investigation. The risk of developing tolerance or dependence, though believed to be low, has not been thoroughly evaluated in human populations.
Interactions with other substances, particularly those affecting the GABAergic system, pose additional safety concerns. Combining muscimol with alcohol, benzodiazepines, or other CNS depressants may potentiate sedative effects and increase the risk of adverse reactions.
The challenge of accurate identification and dosing of Amanita muscaria mushrooms in their natural form presents a significant safety issue. Misidentification can lead to the consumption of highly toxic look-alike species, while improper preparation may fail to convert ibotenic acid to muscimol, potentially increasing toxicity.
Addressing these challenges requires a multifaceted approach. Controlled clinical trials are needed to establish a comprehensive safety profile and determine appropriate dosing guidelines. Improved analytical methods for quantifying muscimol content in mushrooms and standardized extraction procedures could enhance consistency and safety in potential therapeutic applications.
One of the main challenges in assessing muscimol's safety profile is the lack of comprehensive human trials. Most available data come from case reports, animal studies, and limited human experiences, which provide an incomplete picture of its risk factors. The variability in mushroom potency and preparation methods further complicates the assessment of safe dosage levels.
Acute toxicity of muscimol is generally considered low compared to other psychoactive substances, but its effects can be unpredictable. Short-term risks include disorientation, confusion, and impaired motor coordination, which can lead to accidents or injuries. More severe reactions, though rare, may include respiratory depression, seizures, and loss of consciousness.
The potential for psychological distress is a significant concern, particularly in individuals with pre-existing mental health conditions. Muscimol's GABAergic activity can induce intense alterations in perception and cognition, which may trigger anxiety, panic attacks, or exacerbate underlying psychiatric disorders.
Long-term effects of muscimol consumption remain largely unknown. While some studies suggest potential neuroprotective properties, the impact of chronic use on cognitive function, memory, and overall brain health requires further investigation. The risk of developing tolerance or dependence, though believed to be low, has not been thoroughly evaluated in human populations.
Interactions with other substances, particularly those affecting the GABAergic system, pose additional safety concerns. Combining muscimol with alcohol, benzodiazepines, or other CNS depressants may potentiate sedative effects and increase the risk of adverse reactions.
The challenge of accurate identification and dosing of Amanita muscaria mushrooms in their natural form presents a significant safety issue. Misidentification can lead to the consumption of highly toxic look-alike species, while improper preparation may fail to convert ibotenic acid to muscimol, potentially increasing toxicity.
Addressing these challenges requires a multifaceted approach. Controlled clinical trials are needed to establish a comprehensive safety profile and determine appropriate dosing guidelines. Improved analytical methods for quantifying muscimol content in mushrooms and standardized extraction procedures could enhance consistency and safety in potential therapeutic applications.
Existing Safety Protocols for Muscimol Consumption
01 Pharmacological effects and potential risks
Muscimol, a psychoactive compound found in certain mushrooms, acts as a potent GABA receptor agonist. While it has potential therapeutic applications, it also carries risks such as hallucinations, confusion, and impaired motor function. Understanding these effects is crucial for assessing its safety profile and potential medical use.- Pharmacological effects and potential risks: Muscimol, a psychoactive compound found in certain mushrooms, acts as a potent GABA receptor agonist. While it has potential therapeutic applications, it also carries risks such as hallucinations, confusion, and altered perception. Understanding its pharmacological profile is crucial for assessing potential benefits and hazards in medical use.
- Drug interactions and contraindications: Muscimol may interact with other medications, particularly those affecting the central nervous system. Potential contraindications include use with alcohol, sedatives, or in individuals with certain psychiatric conditions. Careful consideration of a patient's medical history and current medications is essential when considering muscimol-based treatments.
- Dosage and administration risks: Determining the appropriate dosage of muscimol is critical, as overdose can lead to severe adverse effects. Factors such as body weight, tolerance, and individual sensitivity must be considered. The method of administration (e.g., oral, intravenous) also impacts the risk profile and effectiveness of the compound.
- Long-term effects and addiction potential: Research into the long-term effects of muscimol use is ongoing. There are concerns about potential cognitive impairment, changes in brain chemistry, and the development of tolerance or dependence with prolonged use. Understanding these risks is crucial for developing safe treatment protocols and patient monitoring strategies.
- Legal and regulatory considerations: The legal status of muscimol varies by jurisdiction, impacting its research and potential medical applications. Regulatory frameworks for its use in clinical settings are still evolving. Compliance with local laws and international regulations is crucial for researchers and healthcare providers working with muscimol-based treatments.
02 Drug interactions and contraindications
Muscimol may interact with other medications, particularly those affecting the central nervous system. It is contraindicated in certain medical conditions and may exacerbate pre-existing mental health disorders. Careful consideration of a patient's medical history and current medications is essential when evaluating the use of muscimol-containing compounds.Expand Specific Solutions03 Dosage and administration considerations
The potency of muscimol necessitates precise dosing to minimize risks while maximizing potential benefits. Factors such as body weight, age, and individual sensitivity must be considered. Proper administration methods and monitoring protocols are crucial to ensure patient safety and efficacy of treatment.Expand Specific Solutions04 Environmental and ecological factors
The presence of muscimol in certain mushroom species raises concerns about accidental ingestion and environmental contamination. Understanding the ecological distribution of muscimol-containing fungi and their potential impact on wildlife and ecosystems is important for risk assessment and conservation efforts.Expand Specific Solutions05 Regulatory and legal considerations
The legal status of muscimol varies across jurisdictions, impacting research, medical use, and potential therapeutic applications. Regulatory frameworks must balance the compound's potential benefits with its risks, considering issues of controlled substance classification, research permissions, and medical use authorizations.Expand Specific Solutions
Key Players in Muscimol Research and Production
The market for understanding muscimol's risk factors in human consumption is in an early developmental stage, characterized by limited commercial applications and ongoing research. The market size remains relatively small, primarily driven by academic and pharmaceutical research interests. Technologically, the field is still evolving, with companies like Psyched Wellness Ltd. and CaaMTech LLC leading innovative efforts in psychedelic compound research and development. Established players such as Suntory Holdings Ltd. and Takeda Pharmaceutical Co., Ltd. may leverage their expertise in related fields to contribute to this emerging area. Academic institutions like Cornell University and Zhejiang University are also playing crucial roles in advancing the scientific understanding of muscimol's effects and potential applications.
Psyched Wellness Ltd.
Technical Solution: Psyched Wellness Ltd. has developed a proprietary extraction and purification process for Amanita Muscaria mushrooms, which contain muscimol. Their approach focuses on creating a safe, standardized extract called AME-1 for potential use in consumer products. The company has conducted extensive toxicology studies to understand the risk factors and establish safe dosage levels for human consumption[1][2]. They have also invested in research to explore the potential health benefits of muscimol, including its anxiolytic and sleep-promoting properties, while carefully assessing its safety profile[3].
Strengths: Specialized focus on Amanita Muscaria and muscimol, proprietary extraction process, extensive safety studies. Weaknesses: Limited product range, regulatory challenges in different markets.
CaaMTech LLC
Technical Solution: CaaMTech LLC has developed a comprehensive approach to studying muscimol and other compounds found in psychoactive mushrooms. Their research involves synthesizing and analyzing various derivatives of muscimol to understand its pharmacological properties and potential therapeutic applications. The company utilizes advanced analytical techniques, including X-ray crystallography, to characterize the molecular structure of muscimol and its analogs[4]. This approach allows for a deeper understanding of the compound's interaction with human physiology and potential risk factors[5].
Strengths: Advanced analytical capabilities, focus on molecular-level understanding. Weaknesses: Early-stage research, potential regulatory hurdles for novel compounds.
Critical Studies on Muscimol's Pharmacological Effects
Amanita muscaria compounds
PatentPendingUS20240050502A1
Innovation
- Development of purified Amanita muscaria compound compositions and formulations comprising specific ratios of ibotenic acid, muscimol, and other compounds, which are structurally distinct and free from other Amanita muscaria compounds, combined with excipients and serotonergic drugs, psilocybin derivatives, or cannabinoids to create pharmaceutical formulations for therapeutic use.
Processes for Extracting Muscimol from Amanita Muscaria
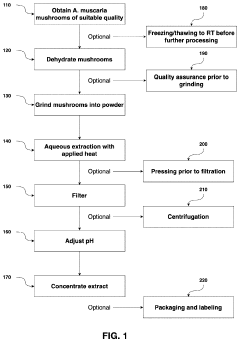
PatentPendingUS20240165180A1
Innovation
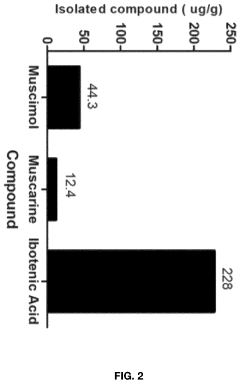
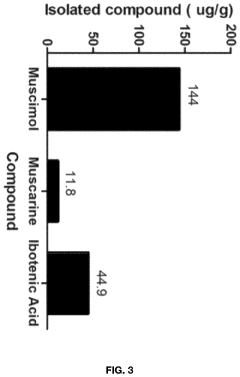
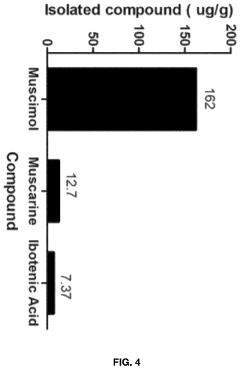
- Aqueous extraction of Amanita muscaria biomass is performed with heat, followed by pH reduction between 2.0 to 4.0 and concentration through distillation or refluxing, which decreases ibotenic acid content and increases muscimol content, resulting in a muscimol-rich extract with enhanced purity.
Regulatory Framework for Muscimol-Related Substances
The regulatory framework for muscimol-related substances is complex and multifaceted, reflecting the unique challenges posed by this psychoactive compound. At the international level, muscimol is not specifically scheduled under the United Nations Convention on Psychotropic Substances. However, many countries have implemented their own regulations to control its use and distribution.
In the United States, muscimol is not explicitly listed as a controlled substance under the Controlled Substances Act. Nevertheless, it falls under the Federal Analogue Act when intended for human consumption, as it is considered structurally similar to other controlled substances. This classification allows for prosecution of individuals involved in its manufacture, distribution, or possession.
The European Union has taken a varied approach to muscimol regulation. Some member states have explicitly banned the substance, while others have implemented restrictions on its sale and distribution. The European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) closely monitors the prevalence and use of muscimol-containing products, providing guidance to member states on potential health risks and regulatory measures.
In Japan, muscimol is regulated under the Pharmaceutical Affairs Law, which restricts its use to approved medical and research purposes. Similarly, Australia has classified muscimol as a Schedule 9 substance under the Poisons Standard, effectively prohibiting its use outside of authorized research contexts.
Regulatory bodies worldwide are increasingly focusing on the broader category of novel psychoactive substances (NPS), which often includes muscimol and related compounds. This approach aims to address the challenges posed by rapidly emerging synthetic analogues and natural products with psychoactive properties.
The Food and Drug Administration (FDA) in the United States has issued warnings about the risks associated with muscimol-containing products, particularly those derived from Amanita muscaria mushrooms. These warnings emphasize the potential for severe adverse effects and the lack of approved medical uses for muscimol-based preparations.
As research into the therapeutic potential of muscimol continues, regulatory frameworks may evolve to accommodate controlled medical applications while maintaining restrictions on recreational use. This balance will require ongoing collaboration between scientific institutions, regulatory bodies, and policymakers to ensure public safety while facilitating legitimate research and potential medical advancements.
In the United States, muscimol is not explicitly listed as a controlled substance under the Controlled Substances Act. Nevertheless, it falls under the Federal Analogue Act when intended for human consumption, as it is considered structurally similar to other controlled substances. This classification allows for prosecution of individuals involved in its manufacture, distribution, or possession.
The European Union has taken a varied approach to muscimol regulation. Some member states have explicitly banned the substance, while others have implemented restrictions on its sale and distribution. The European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) closely monitors the prevalence and use of muscimol-containing products, providing guidance to member states on potential health risks and regulatory measures.
In Japan, muscimol is regulated under the Pharmaceutical Affairs Law, which restricts its use to approved medical and research purposes. Similarly, Australia has classified muscimol as a Schedule 9 substance under the Poisons Standard, effectively prohibiting its use outside of authorized research contexts.
Regulatory bodies worldwide are increasingly focusing on the broader category of novel psychoactive substances (NPS), which often includes muscimol and related compounds. This approach aims to address the challenges posed by rapidly emerging synthetic analogues and natural products with psychoactive properties.
The Food and Drug Administration (FDA) in the United States has issued warnings about the risks associated with muscimol-containing products, particularly those derived from Amanita muscaria mushrooms. These warnings emphasize the potential for severe adverse effects and the lack of approved medical uses for muscimol-based preparations.
As research into the therapeutic potential of muscimol continues, regulatory frameworks may evolve to accommodate controlled medical applications while maintaining restrictions on recreational use. This balance will require ongoing collaboration between scientific institutions, regulatory bodies, and policymakers to ensure public safety while facilitating legitimate research and potential medical advancements.
Ethical Considerations in Muscimol Research and Use
The ethical considerations surrounding muscimol research and use are multifaceted and require careful examination. One primary concern is the potential for abuse and addiction, given muscimol's psychoactive properties. Researchers must prioritize participant safety in clinical trials, implementing rigorous screening processes and monitoring protocols to mitigate risks associated with altered states of consciousness.
Another critical ethical issue is informed consent. Due to muscimol's mind-altering effects, ensuring that participants fully comprehend the potential risks and consequences of their involvement in studies is paramount. This necessitates clear, comprehensive communication and may require additional safeguards to protect vulnerable populations.
The long-term effects of muscimol consumption remain largely unknown, raising ethical questions about the justification for human trials. Researchers must carefully weigh the potential benefits against the risks of exposing subjects to a substance with uncertain long-term impacts. This ethical dilemma extends to the broader societal implications of muscimol research, including the potential for misuse or unintended consequences if the substance becomes more widely available.
Cultural sensitivity is another crucial ethical consideration. Muscimol has historical significance in certain indigenous practices, and research efforts must respect these cultural contexts. This includes acknowledging traditional knowledge, involving indigenous communities in research decisions, and ensuring that scientific pursuits do not exploit or diminish cultural heritage.
The ethical use of animals in preclinical muscimol studies also warrants attention. Researchers must adhere to strict guidelines for animal welfare, minimizing suffering and using alternatives to animal testing whenever possible. Balancing the need for scientific advancement with ethical treatment of research subjects is a constant challenge in this field.
Data privacy and confidentiality present additional ethical concerns, particularly given the sensitive nature of information collected during muscimol studies. Researchers must implement robust data protection measures and clearly communicate how participants' information will be used and protected.
Lastly, there are ethical considerations surrounding the potential commercialization of muscimol-based products. Researchers and companies must navigate the fine line between scientific advancement and profit motives, ensuring that the pursuit of financial gain does not compromise safety or ethical standards. This includes transparent reporting of research findings, including negative results, to prevent bias in the scientific literature.
Another critical ethical issue is informed consent. Due to muscimol's mind-altering effects, ensuring that participants fully comprehend the potential risks and consequences of their involvement in studies is paramount. This necessitates clear, comprehensive communication and may require additional safeguards to protect vulnerable populations.
The long-term effects of muscimol consumption remain largely unknown, raising ethical questions about the justification for human trials. Researchers must carefully weigh the potential benefits against the risks of exposing subjects to a substance with uncertain long-term impacts. This ethical dilemma extends to the broader societal implications of muscimol research, including the potential for misuse or unintended consequences if the substance becomes more widely available.
Cultural sensitivity is another crucial ethical consideration. Muscimol has historical significance in certain indigenous practices, and research efforts must respect these cultural contexts. This includes acknowledging traditional knowledge, involving indigenous communities in research decisions, and ensuring that scientific pursuits do not exploit or diminish cultural heritage.
The ethical use of animals in preclinical muscimol studies also warrants attention. Researchers must adhere to strict guidelines for animal welfare, minimizing suffering and using alternatives to animal testing whenever possible. Balancing the need for scientific advancement with ethical treatment of research subjects is a constant challenge in this field.
Data privacy and confidentiality present additional ethical concerns, particularly given the sensitive nature of information collected during muscimol studies. Researchers must implement robust data protection measures and clearly communicate how participants' information will be used and protected.
Lastly, there are ethical considerations surrounding the potential commercialization of muscimol-based products. Researchers and companies must navigate the fine line between scientific advancement and profit motives, ensuring that the pursuit of financial gain does not compromise safety or ethical standards. This includes transparent reporting of research findings, including negative results, to prevent bias in the scientific literature.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!