What Are Muscimol's Implications in Nutraceutical Research?
JUL 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Muscimol Background and Research Objectives
Muscimol, a potent GABA-A receptor agonist, has emerged as a compound of significant interest in the field of nutraceutical research. This naturally occurring psychoactive alkaloid, primarily found in Amanita mushroom species, has a rich history of traditional use in various cultures. The evolution of muscimol research has progressed from its initial discovery and characterization to its current status as a potential nutraceutical ingredient with diverse applications.
The primary objective of muscimol research in the nutraceutical context is to explore its potential therapeutic benefits while ensuring safety and efficacy. Researchers aim to harness muscimol's GABAergic properties to address various health concerns, including anxiety, sleep disorders, and cognitive function. The goal is to develop nutraceutical products that can provide these benefits without the psychoactive effects associated with higher doses of muscimol.
Recent technological advancements have significantly contributed to the progress in muscimol research. Improved extraction and purification techniques have enabled the production of high-quality muscimol isolates, facilitating more accurate and controlled studies. Additionally, developments in analytical methods have enhanced our ability to detect and quantify muscimol in various matrices, crucial for quality control and standardization in nutraceutical applications.
The nutraceutical industry's growing interest in natural bioactive compounds has further propelled muscimol research. As consumers increasingly seek natural alternatives to synthetic pharmaceuticals, muscimol's potential as a nutraceutical ingredient has gained traction. This trend has led to increased funding and resources dedicated to understanding muscimol's mechanisms of action, optimal dosing, and potential synergistic effects with other nutraceutical compounds.
However, the path to integrating muscimol into nutraceutical products faces several challenges. Regulatory hurdles, particularly concerning its psychoactive properties at higher doses, necessitate careful consideration and extensive safety studies. Furthermore, the development of stable formulations that maintain muscimol's bioactivity while ensuring consistent dosing presents technical challenges that researchers must overcome.
Looking ahead, the future of muscimol in nutraceutical research appears promising. Ongoing studies are exploring its potential in areas such as neuroprotection, stress reduction, and mood regulation. The development of novel delivery systems, such as microencapsulation or targeted release formulations, may unlock new possibilities for muscimol-based nutraceuticals. As research progresses, the goal is to establish a comprehensive understanding of muscimol's benefits and limitations, paving the way for innovative nutraceutical products that can positively impact human health and well-being.
The primary objective of muscimol research in the nutraceutical context is to explore its potential therapeutic benefits while ensuring safety and efficacy. Researchers aim to harness muscimol's GABAergic properties to address various health concerns, including anxiety, sleep disorders, and cognitive function. The goal is to develop nutraceutical products that can provide these benefits without the psychoactive effects associated with higher doses of muscimol.
Recent technological advancements have significantly contributed to the progress in muscimol research. Improved extraction and purification techniques have enabled the production of high-quality muscimol isolates, facilitating more accurate and controlled studies. Additionally, developments in analytical methods have enhanced our ability to detect and quantify muscimol in various matrices, crucial for quality control and standardization in nutraceutical applications.
The nutraceutical industry's growing interest in natural bioactive compounds has further propelled muscimol research. As consumers increasingly seek natural alternatives to synthetic pharmaceuticals, muscimol's potential as a nutraceutical ingredient has gained traction. This trend has led to increased funding and resources dedicated to understanding muscimol's mechanisms of action, optimal dosing, and potential synergistic effects with other nutraceutical compounds.
However, the path to integrating muscimol into nutraceutical products faces several challenges. Regulatory hurdles, particularly concerning its psychoactive properties at higher doses, necessitate careful consideration and extensive safety studies. Furthermore, the development of stable formulations that maintain muscimol's bioactivity while ensuring consistent dosing presents technical challenges that researchers must overcome.
Looking ahead, the future of muscimol in nutraceutical research appears promising. Ongoing studies are exploring its potential in areas such as neuroprotection, stress reduction, and mood regulation. The development of novel delivery systems, such as microencapsulation or targeted release formulations, may unlock new possibilities for muscimol-based nutraceuticals. As research progresses, the goal is to establish a comprehensive understanding of muscimol's benefits and limitations, paving the way for innovative nutraceutical products that can positively impact human health and well-being.
Nutraceutical Market Demand Analysis
The nutraceutical market has been experiencing significant growth in recent years, driven by increasing consumer awareness of health and wellness, as well as a growing preference for natural and preventive healthcare solutions. Within this expanding market, there is a rising interest in novel compounds with potential cognitive and neurological benefits, creating a unique opportunity for muscimol-based products.
Muscimol, a psychoactive compound found in certain mushroom species, has garnered attention in nutraceutical research due to its potential neuroprotective and cognitive-enhancing properties. The demand for muscimol-based nutraceuticals is primarily fueled by the aging population seeking solutions for cognitive decline and neurological disorders, as well as younger consumers looking for natural nootropics to enhance mental performance.
Market research indicates that the global brain health supplements market, which includes cognitive enhancers and neuroprotective agents, is projected to grow at a compound annual growth rate (CAGR) of over 8% in the coming years. This growth is partly attributed to the increasing prevalence of neurodegenerative diseases and the rising demand for natural alternatives to pharmaceutical interventions.
Consumer surveys reveal a growing interest in functional foods and supplements that offer cognitive benefits. A significant portion of consumers, particularly in developed markets, express willingness to pay premium prices for nutraceuticals with proven neurological benefits. This trend aligns well with the potential applications of muscimol in cognitive enhancement and neuroprotection.
The sports nutrition and performance enhancement sectors also present opportunities for muscimol-based products. Athletes and fitness enthusiasts are increasingly seeking natural supplements that can improve focus, reaction time, and mental endurance. This segment of the nutraceutical market has shown robust growth, with consumers demonstrating a high willingness to try innovative products.
However, the market demand for muscimol-based nutraceuticals faces several challenges. Regulatory hurdles and safety concerns surrounding psychoactive compounds pose significant barriers to market entry. Additionally, consumer education regarding the benefits and proper use of muscimol-derived products will be crucial for market acceptance and growth.
Despite these challenges, the potential therapeutic applications of muscimol in areas such as anxiety reduction, sleep improvement, and neuroprotection continue to drive research and development efforts. As clinical evidence accumulates and regulatory frameworks evolve, the market demand for muscimol-based nutraceuticals is expected to expand, particularly in regions with progressive regulatory environments and health-conscious consumer bases.
Muscimol, a psychoactive compound found in certain mushroom species, has garnered attention in nutraceutical research due to its potential neuroprotective and cognitive-enhancing properties. The demand for muscimol-based nutraceuticals is primarily fueled by the aging population seeking solutions for cognitive decline and neurological disorders, as well as younger consumers looking for natural nootropics to enhance mental performance.
Market research indicates that the global brain health supplements market, which includes cognitive enhancers and neuroprotective agents, is projected to grow at a compound annual growth rate (CAGR) of over 8% in the coming years. This growth is partly attributed to the increasing prevalence of neurodegenerative diseases and the rising demand for natural alternatives to pharmaceutical interventions.
Consumer surveys reveal a growing interest in functional foods and supplements that offer cognitive benefits. A significant portion of consumers, particularly in developed markets, express willingness to pay premium prices for nutraceuticals with proven neurological benefits. This trend aligns well with the potential applications of muscimol in cognitive enhancement and neuroprotection.
The sports nutrition and performance enhancement sectors also present opportunities for muscimol-based products. Athletes and fitness enthusiasts are increasingly seeking natural supplements that can improve focus, reaction time, and mental endurance. This segment of the nutraceutical market has shown robust growth, with consumers demonstrating a high willingness to try innovative products.
However, the market demand for muscimol-based nutraceuticals faces several challenges. Regulatory hurdles and safety concerns surrounding psychoactive compounds pose significant barriers to market entry. Additionally, consumer education regarding the benefits and proper use of muscimol-derived products will be crucial for market acceptance and growth.
Despite these challenges, the potential therapeutic applications of muscimol in areas such as anxiety reduction, sleep improvement, and neuroprotection continue to drive research and development efforts. As clinical evidence accumulates and regulatory frameworks evolve, the market demand for muscimol-based nutraceuticals is expected to expand, particularly in regions with progressive regulatory environments and health-conscious consumer bases.
Current State of Muscimol Research
Muscimol, a psychoactive compound found in certain mushroom species, has gained significant attention in nutraceutical research due to its potential therapeutic properties. The current state of muscimol research is characterized by a growing body of scientific evidence supporting its diverse applications in health and wellness.
Recent studies have focused on muscimol's neuroprotective effects, particularly in the context of neurodegenerative disorders. Researchers have observed that muscimol can modulate GABAergic signaling, potentially offering benefits in conditions such as Alzheimer's disease and Parkinson's disease. These findings have sparked interest in developing muscimol-based nutraceuticals for cognitive enhancement and neuroprotection.
In the field of mental health, muscimol has shown promise as a potential anxiolytic and antidepressant agent. Preclinical studies have demonstrated its ability to reduce anxiety-like behaviors and improve mood in animal models. This has led to increased exploration of muscimol's potential as a natural alternative to conventional psychiatric medications, with ongoing research aimed at optimizing its delivery and efficacy.
Another area of active investigation is muscimol's role in pain management. Several studies have reported its analgesic properties, suggesting potential applications in the development of nutraceutical products for chronic pain conditions. Researchers are particularly interested in muscimol's ability to modulate pain perception without the addictive potential associated with opioid-based treatments.
The anti-inflammatory properties of muscimol have also garnered attention in nutraceutical research. Preliminary studies indicate that muscimol may help reduce inflammation in various tissues, opening up possibilities for its use in formulations targeting inflammatory disorders and promoting overall wellness.
As research progresses, scientists are exploring novel delivery methods to enhance muscimol's bioavailability and targeted action. Nanotechnology-based approaches and advanced formulation techniques are being investigated to optimize the compound's stability and efficacy in nutraceutical applications.
However, it is important to note that while the current state of muscimol research shows promise, many studies are still in preclinical stages. Rigorous clinical trials are needed to fully elucidate its safety profile and therapeutic potential in humans. Regulatory considerations also play a crucial role in shaping the future of muscimol-based nutraceuticals, as authorities carefully evaluate its status and potential reclassification.
In conclusion, the current state of muscimol research in nutraceuticals is characterized by growing scientific interest and expanding potential applications. As researchers continue to unravel its mechanisms of action and optimize its delivery, muscimol holds promise as a versatile compound in the development of innovative nutraceutical products for various health conditions.
Recent studies have focused on muscimol's neuroprotective effects, particularly in the context of neurodegenerative disorders. Researchers have observed that muscimol can modulate GABAergic signaling, potentially offering benefits in conditions such as Alzheimer's disease and Parkinson's disease. These findings have sparked interest in developing muscimol-based nutraceuticals for cognitive enhancement and neuroprotection.
In the field of mental health, muscimol has shown promise as a potential anxiolytic and antidepressant agent. Preclinical studies have demonstrated its ability to reduce anxiety-like behaviors and improve mood in animal models. This has led to increased exploration of muscimol's potential as a natural alternative to conventional psychiatric medications, with ongoing research aimed at optimizing its delivery and efficacy.
Another area of active investigation is muscimol's role in pain management. Several studies have reported its analgesic properties, suggesting potential applications in the development of nutraceutical products for chronic pain conditions. Researchers are particularly interested in muscimol's ability to modulate pain perception without the addictive potential associated with opioid-based treatments.
The anti-inflammatory properties of muscimol have also garnered attention in nutraceutical research. Preliminary studies indicate that muscimol may help reduce inflammation in various tissues, opening up possibilities for its use in formulations targeting inflammatory disorders and promoting overall wellness.
As research progresses, scientists are exploring novel delivery methods to enhance muscimol's bioavailability and targeted action. Nanotechnology-based approaches and advanced formulation techniques are being investigated to optimize the compound's stability and efficacy in nutraceutical applications.
However, it is important to note that while the current state of muscimol research shows promise, many studies are still in preclinical stages. Rigorous clinical trials are needed to fully elucidate its safety profile and therapeutic potential in humans. Regulatory considerations also play a crucial role in shaping the future of muscimol-based nutraceuticals, as authorities carefully evaluate its status and potential reclassification.
In conclusion, the current state of muscimol research in nutraceuticals is characterized by growing scientific interest and expanding potential applications. As researchers continue to unravel its mechanisms of action and optimize its delivery, muscimol holds promise as a versatile compound in the development of innovative nutraceutical products for various health conditions.
Existing Muscimol-based Nutraceutical Solutions
01 Pharmaceutical compositions containing muscimol
Muscimol is used in pharmaceutical compositions for various therapeutic applications. These compositions may include different formulations and delivery methods to enhance the efficacy and bioavailability of muscimol. The compositions can be designed for specific medical conditions or to target particular biological pathways.- Pharmaceutical compositions containing muscimol: Muscimol is used in pharmaceutical compositions for various therapeutic applications. These formulations may include specific dosage forms, delivery methods, or combinations with other active ingredients to enhance efficacy or reduce side effects.
- Muscimol for neurological disorders: Muscimol is investigated for its potential in treating neurological disorders. Research focuses on its GABA-mimetic properties and ability to modulate neural activity, which may be beneficial in conditions such as epilepsy, anxiety, or sleep disorders.
- Synthesis and extraction methods for muscimol: Various methods are developed for the synthesis or extraction of muscimol from natural sources. These processes aim to improve yield, purity, or cost-effectiveness of muscimol production for research or pharmaceutical use.
- Muscimol analogs and derivatives: Research into muscimol analogs and derivatives aims to create compounds with improved pharmacological properties. These modified versions may offer enhanced selectivity, bioavailability, or reduced side effects compared to natural muscimol.
- Drug delivery systems for muscimol: Innovative drug delivery systems are developed to optimize the administration of muscimol. These may include controlled release formulations, targeted delivery methods, or novel routes of administration to enhance therapeutic efficacy and patient compliance.
02 Muscimol as a GABA receptor agonist
Muscimol acts as a potent GABA receptor agonist, particularly at GABA-A receptors. This property makes it useful in treating various neurological and psychiatric disorders. Research focuses on understanding its mechanism of action and potential therapeutic applications in conditions related to GABA system dysfunction.Expand Specific Solutions03 Synthesis and production methods for muscimol
Various methods for synthesizing and producing muscimol are explored to improve yield, purity, and cost-effectiveness. These may include chemical synthesis routes, biotechnological approaches, or extraction methods from natural sources. Innovations in production techniques aim to make muscimol more accessible for research and potential commercial applications.Expand Specific Solutions04 Muscimol in combination therapies
Muscimol is investigated for use in combination with other therapeutic agents to enhance treatment efficacy or reduce side effects. These combinations may target multiple pathways or provide synergistic effects in treating complex disorders, particularly in the field of neurology and psychiatry.Expand Specific Solutions05 Novel delivery systems for muscimol
Research is conducted on developing innovative delivery systems for muscimol to improve its pharmacokinetics, target specificity, and reduce potential side effects. These may include nanoparticle formulations, controlled release mechanisms, or novel routes of administration to enhance the compound's therapeutic potential.Expand Specific Solutions
Key Players in Muscimol Nutraceutical Research
The muscimol nutraceutical research field is in its early stages, with a growing market driven by increasing interest in natural psychoactive compounds. The technology is still developing, with varying levels of maturity across different applications. Key players like CaaMTech LLC and Psyched Wellness Ltd. are pioneering research into muscimol's potential benefits, while established institutions such as Louisiana State University and Beijing University of Chemical Technology contribute valuable scientific insights. Companies like Zhucheng Haotian Pharm Co., Ltd. and Shandong Fuyang Bio-Tech. Co., Ltd. may leverage their expertise in pharmaceutical manufacturing to advance muscimol-based products. As the field evolves, collaborations between academic institutions, biotech startups, and established pharmaceutical companies are likely to accelerate innovation and market growth.
CaaMTech LLC
Technical Solution: CaaMTech LLC is at the forefront of muscimol research, focusing on its potential applications in mental health and neurological disorders. They have developed novel synthetic methods to produce pure muscimol and its derivatives, allowing for more precise dosing and reduced side effects compared to natural extracts[4]. CaaMTech's research extends to investigating muscimol's interactions with other neurotransmitter systems, particularly its effects on GABA receptors, which play a crucial role in anxiety and sleep disorders[5]. Their approach combines computational modeling with in vitro and in vivo studies to optimize muscimol's therapeutic potential while minimizing adverse effects.
Strengths: Advanced synthetic capabilities, comprehensive approach to muscimol research. Weaknesses: High development costs, potential regulatory hurdles for novel compounds.
Psyched Wellness Ltd.
Technical Solution: Psyched Wellness Ltd. is pioneering the use of muscimol, a psychoactive compound found in Amanita muscaria mushrooms, for nutraceutical applications. Their research focuses on developing a proprietary extraction and purification process to create a standardized form of muscimol suitable for consumer products. The company is exploring muscimol's potential benefits in areas such as stress reduction, sleep improvement, and cognitive enhancement[1][2]. They have conducted preclinical studies to assess the safety and efficacy of their muscimol extract, demonstrating its potential as a natural alternative to synthetic pharmaceuticals in the wellness market[3].
Strengths: First-mover advantage in muscimol-based nutraceuticals, proprietary extraction technology. Weaknesses: Limited clinical data on long-term effects, regulatory challenges due to psychoactive nature.
Core Muscimol Mechanisms and Effects
Amanita muscaria compounds
PatentPendingUS20240050502A1
Innovation
- Development of purified Amanita muscaria compound compositions and formulations comprising specific ratios of ibotenic acid, muscimol, and other compounds, which are structurally distinct and free from other Amanita muscaria compounds, combined with excipients and serotonergic drugs, psilocybin derivatives, or cannabinoids to create pharmaceutical formulations for therapeutic use.
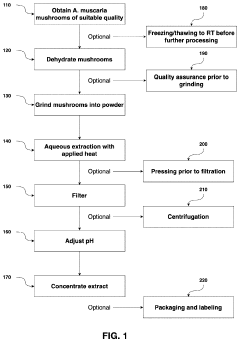
Processes for Extracting Muscimol from Amanita Muscaria
PatentPendingUS20240165180A1
Innovation
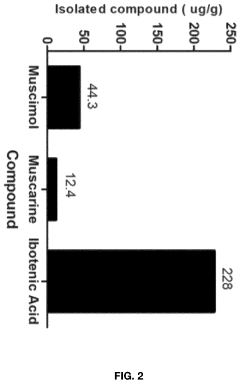
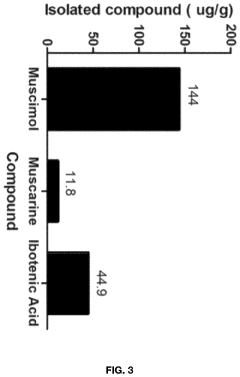
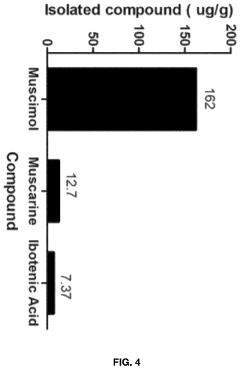
- Aqueous extraction of Amanita muscaria biomass is performed with heat, followed by pH reduction between 2.0 to 4.0 and concentration through distillation or refluxing, which decreases ibotenic acid content and increases muscimol content, resulting in a muscimol-rich extract with enhanced purity.
Regulatory Landscape for Muscimol-based Products
The regulatory landscape for muscimol-based products is complex and evolving, reflecting the unique challenges posed by this psychoactive compound derived from certain mushroom species. As muscimol gains attention in nutraceutical research, regulatory bodies worldwide are grappling with how to classify and control its use.
In the United States, the Food and Drug Administration (FDA) has not yet established specific regulations for muscimol-based products. However, the compound falls under the purview of the Dietary Supplement Health and Education Act (DSHEA) of 1994 if marketed as a dietary supplement. This classification requires manufacturers to ensure product safety and accurate labeling, but does not mandate pre-market approval.
The Drug Enforcement Administration (DEA) currently does not list muscimol as a controlled substance, unlike its precursor ibotenic acid. This regulatory gap has led to a gray area in which muscimol-containing products may be legally sold, albeit with careful marketing to avoid drug claims.
In the European Union, the European Food Safety Authority (EFSA) has not issued specific guidelines for muscimol. However, novel food regulations may apply, potentially requiring safety assessments before market authorization. Some EU member states have implemented stricter controls on mushroom-derived compounds, which could impact muscimol-based products.
Japan's regulatory approach is more permissive, with the Ministry of Health, Labour and Welfare allowing certain mushroom extracts in food products. This could potentially include muscimol, provided safety and quality standards are met.
Australia and New Zealand have a joint regulatory body, the Food Standards Australia New Zealand (FSANZ), which has not explicitly addressed muscimol. However, their strict regulations on novel foods and therapeutic goods would likely apply to muscimol-based nutraceuticals.
As research progresses, regulatory frameworks are expected to evolve. Key considerations for future regulations include establishing safe dosage levels, standardizing extraction and production methods, and developing accurate testing protocols. The potential therapeutic benefits of muscimol may also influence regulatory decisions, potentially leading to a more nuanced approach that balances safety concerns with medical potential.
Manufacturers and researchers must navigate this complex regulatory environment carefully. Compliance with current good manufacturing practices (cGMP), thorough safety assessments, and transparent communication with regulatory bodies will be crucial for the development and marketing of muscimol-based nutraceutical products.
In the United States, the Food and Drug Administration (FDA) has not yet established specific regulations for muscimol-based products. However, the compound falls under the purview of the Dietary Supplement Health and Education Act (DSHEA) of 1994 if marketed as a dietary supplement. This classification requires manufacturers to ensure product safety and accurate labeling, but does not mandate pre-market approval.
The Drug Enforcement Administration (DEA) currently does not list muscimol as a controlled substance, unlike its precursor ibotenic acid. This regulatory gap has led to a gray area in which muscimol-containing products may be legally sold, albeit with careful marketing to avoid drug claims.
In the European Union, the European Food Safety Authority (EFSA) has not issued specific guidelines for muscimol. However, novel food regulations may apply, potentially requiring safety assessments before market authorization. Some EU member states have implemented stricter controls on mushroom-derived compounds, which could impact muscimol-based products.
Japan's regulatory approach is more permissive, with the Ministry of Health, Labour and Welfare allowing certain mushroom extracts in food products. This could potentially include muscimol, provided safety and quality standards are met.
Australia and New Zealand have a joint regulatory body, the Food Standards Australia New Zealand (FSANZ), which has not explicitly addressed muscimol. However, their strict regulations on novel foods and therapeutic goods would likely apply to muscimol-based nutraceuticals.
As research progresses, regulatory frameworks are expected to evolve. Key considerations for future regulations include establishing safe dosage levels, standardizing extraction and production methods, and developing accurate testing protocols. The potential therapeutic benefits of muscimol may also influence regulatory decisions, potentially leading to a more nuanced approach that balances safety concerns with medical potential.
Manufacturers and researchers must navigate this complex regulatory environment carefully. Compliance with current good manufacturing practices (cGMP), thorough safety assessments, and transparent communication with regulatory bodies will be crucial for the development and marketing of muscimol-based nutraceutical products.
Safety and Toxicology Considerations
Safety and toxicology considerations are paramount in the exploration of muscimol's potential applications in nutraceutical research. As a naturally occurring psychoactive compound found in certain mushroom species, muscimol's use in dietary supplements raises significant concerns that must be thoroughly addressed before any commercial development can proceed.
The primary focus of safety assessments revolves around muscimol's potent GABA-A receptor agonist properties. This mechanism of action, while potentially beneficial for certain therapeutic applications, also presents risks of adverse effects, particularly on the central nervous system. Researchers must carefully evaluate the dose-dependent effects of muscimol, establishing clear thresholds for both efficacy and toxicity.
Acute toxicity studies in animal models have provided initial insights into muscimol's safety profile. However, more comprehensive long-term studies are essential to understand the potential for chronic toxicity, accumulation in tissues, and any delayed onset effects. These investigations should encompass a wide range of physiological parameters, including liver function, kidney health, and neurological impacts.
The potential for drug interactions is another critical area of concern. Given muscimol's effects on GABA neurotransmission, its interaction with other CNS depressants, including alcohol and benzodiazepines, must be thoroughly examined. Additionally, its impact on the metabolism of other medications through potential enzyme induction or inhibition needs careful evaluation.
Reproductive and developmental toxicity studies are crucial, especially considering the potential for nutraceutical products to be consumed by diverse populations, including pregnant women. These studies should assess muscimol's effects on fertility, embryonic development, and postnatal growth across multiple generations.
The development of sensitive and specific analytical methods for detecting and quantifying muscimol in various matrices is essential for quality control and safety monitoring. These methods will be crucial for ensuring consistent dosing in nutraceutical products and for detecting potential contamination or adulteration.
Regulatory considerations play a significant role in the safety assessment of muscimol-containing nutraceuticals. Compliance with FDA regulations, particularly those governing dietary supplements, will be necessary. This includes adhering to Good Manufacturing Practices (GMP) and potentially conducting clinical trials to demonstrate safety in human subjects.
In conclusion, while muscimol shows promise in nutraceutical research, its path to market is contingent upon rigorous safety and toxicology evaluations. These studies must be comprehensive, addressing both short-term and long-term effects, potential interactions, and specific population considerations. Only through such thorough investigation can the true potential of muscimol in nutraceuticals be responsibly explored and realized.
The primary focus of safety assessments revolves around muscimol's potent GABA-A receptor agonist properties. This mechanism of action, while potentially beneficial for certain therapeutic applications, also presents risks of adverse effects, particularly on the central nervous system. Researchers must carefully evaluate the dose-dependent effects of muscimol, establishing clear thresholds for both efficacy and toxicity.
Acute toxicity studies in animal models have provided initial insights into muscimol's safety profile. However, more comprehensive long-term studies are essential to understand the potential for chronic toxicity, accumulation in tissues, and any delayed onset effects. These investigations should encompass a wide range of physiological parameters, including liver function, kidney health, and neurological impacts.
The potential for drug interactions is another critical area of concern. Given muscimol's effects on GABA neurotransmission, its interaction with other CNS depressants, including alcohol and benzodiazepines, must be thoroughly examined. Additionally, its impact on the metabolism of other medications through potential enzyme induction or inhibition needs careful evaluation.
Reproductive and developmental toxicity studies are crucial, especially considering the potential for nutraceutical products to be consumed by diverse populations, including pregnant women. These studies should assess muscimol's effects on fertility, embryonic development, and postnatal growth across multiple generations.
The development of sensitive and specific analytical methods for detecting and quantifying muscimol in various matrices is essential for quality control and safety monitoring. These methods will be crucial for ensuring consistent dosing in nutraceutical products and for detecting potential contamination or adulteration.
Regulatory considerations play a significant role in the safety assessment of muscimol-containing nutraceuticals. Compliance with FDA regulations, particularly those governing dietary supplements, will be necessary. This includes adhering to Good Manufacturing Practices (GMP) and potentially conducting clinical trials to demonstrate safety in human subjects.
In conclusion, while muscimol shows promise in nutraceutical research, its path to market is contingent upon rigorous safety and toxicology evaluations. These studies must be comprehensive, addressing both short-term and long-term effects, potential interactions, and specific population considerations. Only through such thorough investigation can the true potential of muscimol in nutraceuticals be responsibly explored and realized.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!