What are the Safety Measures for Muriatic Acid Storage?
JUL 18, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Muriatic Acid Overview
Muriatic acid, also known as hydrochloric acid, is a highly corrosive and versatile chemical compound widely used in various industries. It is a strong mineral acid with the chemical formula HCl, consisting of hydrogen and chlorine atoms. In its pure form, muriatic acid is a colorless, transparent liquid with a pungent odor.
The concentration of muriatic acid typically ranges from 31.5% to 38% by weight in industrial and commercial applications. This concentration is often referred to as "technical grade" or "industrial strength" muriatic acid. Lower concentrations are available for household use, usually around 10-12%.
Muriatic acid plays a crucial role in numerous industrial processes and applications. In the chemical industry, it serves as a key reagent for the production of various chemicals, including PVC, fertilizers, and dyes. The metal processing sector utilizes muriatic acid for metal cleaning, pickling, and surface treatment of steel and other metals. In the food industry, it is used in the production of gelatin and as a food additive (E507) for pH regulation.
The construction and maintenance industries employ muriatic acid for cleaning masonry, concrete, and tiles, as well as for adjusting the pH of swimming pools. In the oil and gas sector, it is used in well acidizing to enhance oil and gas production. Additionally, muriatic acid finds applications in water treatment, leather processing, and as a laboratory reagent in scientific research.
Despite its widespread use, muriatic acid poses significant safety risks due to its corrosive nature. It can cause severe burns to skin and eyes upon contact and produce harmful fumes when exposed to air. Proper handling, storage, and disposal procedures are essential to mitigate these risks and ensure safe usage.
The global market for muriatic acid is substantial, driven by its diverse applications across multiple industries. The Asia-Pacific region dominates the market, followed by North America and Europe. Factors such as industrialization, urbanization, and the growth of end-use industries contribute to the increasing demand for muriatic acid worldwide.
Environmental concerns and regulations regarding the production and use of muriatic acid have led to the development of more sustainable and eco-friendly alternatives in some applications. However, its unique properties and cost-effectiveness continue to make it an indispensable chemical in many industrial processes.
The concentration of muriatic acid typically ranges from 31.5% to 38% by weight in industrial and commercial applications. This concentration is often referred to as "technical grade" or "industrial strength" muriatic acid. Lower concentrations are available for household use, usually around 10-12%.
Muriatic acid plays a crucial role in numerous industrial processes and applications. In the chemical industry, it serves as a key reagent for the production of various chemicals, including PVC, fertilizers, and dyes. The metal processing sector utilizes muriatic acid for metal cleaning, pickling, and surface treatment of steel and other metals. In the food industry, it is used in the production of gelatin and as a food additive (E507) for pH regulation.
The construction and maintenance industries employ muriatic acid for cleaning masonry, concrete, and tiles, as well as for adjusting the pH of swimming pools. In the oil and gas sector, it is used in well acidizing to enhance oil and gas production. Additionally, muriatic acid finds applications in water treatment, leather processing, and as a laboratory reagent in scientific research.
Despite its widespread use, muriatic acid poses significant safety risks due to its corrosive nature. It can cause severe burns to skin and eyes upon contact and produce harmful fumes when exposed to air. Proper handling, storage, and disposal procedures are essential to mitigate these risks and ensure safe usage.
The global market for muriatic acid is substantial, driven by its diverse applications across multiple industries. The Asia-Pacific region dominates the market, followed by North America and Europe. Factors such as industrialization, urbanization, and the growth of end-use industries contribute to the increasing demand for muriatic acid worldwide.
Environmental concerns and regulations regarding the production and use of muriatic acid have led to the development of more sustainable and eco-friendly alternatives in some applications. However, its unique properties and cost-effectiveness continue to make it an indispensable chemical in many industrial processes.
Safety Regulations
Safety regulations for muriatic acid storage are critical to ensure the protection of workers, facilities, and the environment. These regulations are typically established by governmental agencies and industry organizations to provide comprehensive guidelines for the safe handling, storage, and use of this hazardous substance.
The Occupational Safety and Health Administration (OSHA) in the United States has set forth specific standards for the storage of muriatic acid, also known as hydrochloric acid. These standards require employers to provide proper containment systems, ventilation, and personal protective equipment (PPE) for workers handling the acid. OSHA mandates that storage areas must be equipped with adequate spill containment measures, such as dikes or berms, to prevent accidental releases from spreading.
The Environmental Protection Agency (EPA) also plays a crucial role in regulating muriatic acid storage through its Resource Conservation and Recovery Act (RCRA). This act establishes requirements for the proper management of hazardous waste, including the storage of corrosive substances like muriatic acid. Facilities storing large quantities of muriatic acid may be subject to additional reporting and permitting requirements under the EPA's Risk Management Program.
International regulations, such as the European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation, impose strict controls on the storage and handling of hazardous substances, including muriatic acid. These regulations often require detailed safety data sheets, risk assessments, and specific storage conditions to be maintained.
Industry-specific standards, like those set by the American Chemistry Council, provide additional guidance on best practices for muriatic acid storage. These standards often go beyond regulatory requirements, addressing issues such as material compatibility, storage tank design, and emergency response procedures.
Local fire codes and building regulations also impact muriatic acid storage. Many jurisdictions require special fire-resistant construction for areas where corrosive chemicals are stored, as well as the installation of fire suppression systems and emergency eyewash stations and showers.
Compliance with these regulations often necessitates regular inspections, documentation, and employee training programs. Facilities must maintain accurate inventory records, conduct routine equipment checks, and ensure that all personnel are properly trained in the safe handling and emergency procedures related to muriatic acid.
As regulations evolve to address new safety concerns and technological advancements, companies must stay informed about changes in regulatory requirements. This may involve participating in industry associations, attending regulatory update seminars, and consulting with safety experts to ensure ongoing compliance and adoption of best practices in muriatic acid storage.
The Occupational Safety and Health Administration (OSHA) in the United States has set forth specific standards for the storage of muriatic acid, also known as hydrochloric acid. These standards require employers to provide proper containment systems, ventilation, and personal protective equipment (PPE) for workers handling the acid. OSHA mandates that storage areas must be equipped with adequate spill containment measures, such as dikes or berms, to prevent accidental releases from spreading.
The Environmental Protection Agency (EPA) also plays a crucial role in regulating muriatic acid storage through its Resource Conservation and Recovery Act (RCRA). This act establishes requirements for the proper management of hazardous waste, including the storage of corrosive substances like muriatic acid. Facilities storing large quantities of muriatic acid may be subject to additional reporting and permitting requirements under the EPA's Risk Management Program.
International regulations, such as the European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation, impose strict controls on the storage and handling of hazardous substances, including muriatic acid. These regulations often require detailed safety data sheets, risk assessments, and specific storage conditions to be maintained.
Industry-specific standards, like those set by the American Chemistry Council, provide additional guidance on best practices for muriatic acid storage. These standards often go beyond regulatory requirements, addressing issues such as material compatibility, storage tank design, and emergency response procedures.
Local fire codes and building regulations also impact muriatic acid storage. Many jurisdictions require special fire-resistant construction for areas where corrosive chemicals are stored, as well as the installation of fire suppression systems and emergency eyewash stations and showers.
Compliance with these regulations often necessitates regular inspections, documentation, and employee training programs. Facilities must maintain accurate inventory records, conduct routine equipment checks, and ensure that all personnel are properly trained in the safe handling and emergency procedures related to muriatic acid.
As regulations evolve to address new safety concerns and technological advancements, companies must stay informed about changes in regulatory requirements. This may involve participating in industry associations, attending regulatory update seminars, and consulting with safety experts to ensure ongoing compliance and adoption of best practices in muriatic acid storage.
Current Storage Challenges
The storage of muriatic acid, also known as hydrochloric acid, presents several significant challenges that require careful consideration and management. One of the primary concerns is the highly corrosive nature of the acid, which can rapidly deteriorate standard storage containers and equipment. This corrosivity necessitates the use of specialized materials that can withstand prolonged exposure to the acid without degradation.
Another major challenge is the potential for harmful fumes and vapors. Muriatic acid can release toxic gases, particularly when exposed to heat or other chemicals. These emissions pose serious health risks to workers and can contribute to air pollution if not properly contained. Adequate ventilation systems and air quality monitoring are essential to mitigate these risks, but implementing such measures can be complex and costly.
The reactivity of muriatic acid with various substances presents additional storage difficulties. It can react violently with certain metals, generating hydrogen gas, which is highly flammable and explosive. This reactivity necessitates careful selection of storage materials and strict segregation from incompatible chemicals, complicating storage logistics and increasing the risk of accidental mixing.
Temperature control is another critical factor in muriatic acid storage. Extreme temperatures can affect the acid's stability and increase the likelihood of container failure or pressure buildup. Maintaining a consistent, moderate temperature range requires sophisticated climate control systems, particularly in regions with variable weather conditions.
The potential for spills and leaks is a persistent concern in muriatic acid storage. Even small breaches can lead to significant environmental damage and pose severe health hazards. Implementing effective containment systems, such as secondary containment structures and spill pallets, is crucial but can be challenging in terms of design and space requirements.
Regulatory compliance adds another layer of complexity to muriatic acid storage. Stringent regulations govern the handling and storage of hazardous materials, including specific requirements for labeling, documentation, and emergency response planning. Keeping up with evolving regulations and ensuring consistent compliance across all storage facilities can be a significant operational challenge for organizations.
Lastly, the training and safety of personnel involved in handling and managing muriatic acid storage facilities is an ongoing challenge. Ensuring that all staff are adequately trained in safety protocols, emergency procedures, and proper handling techniques is critical but requires continuous effort and resources to maintain high standards of safety and competence.
Another major challenge is the potential for harmful fumes and vapors. Muriatic acid can release toxic gases, particularly when exposed to heat or other chemicals. These emissions pose serious health risks to workers and can contribute to air pollution if not properly contained. Adequate ventilation systems and air quality monitoring are essential to mitigate these risks, but implementing such measures can be complex and costly.
The reactivity of muriatic acid with various substances presents additional storage difficulties. It can react violently with certain metals, generating hydrogen gas, which is highly flammable and explosive. This reactivity necessitates careful selection of storage materials and strict segregation from incompatible chemicals, complicating storage logistics and increasing the risk of accidental mixing.
Temperature control is another critical factor in muriatic acid storage. Extreme temperatures can affect the acid's stability and increase the likelihood of container failure or pressure buildup. Maintaining a consistent, moderate temperature range requires sophisticated climate control systems, particularly in regions with variable weather conditions.
The potential for spills and leaks is a persistent concern in muriatic acid storage. Even small breaches can lead to significant environmental damage and pose severe health hazards. Implementing effective containment systems, such as secondary containment structures and spill pallets, is crucial but can be challenging in terms of design and space requirements.
Regulatory compliance adds another layer of complexity to muriatic acid storage. Stringent regulations govern the handling and storage of hazardous materials, including specific requirements for labeling, documentation, and emergency response planning. Keeping up with evolving regulations and ensuring consistent compliance across all storage facilities can be a significant operational challenge for organizations.
Lastly, the training and safety of personnel involved in handling and managing muriatic acid storage facilities is an ongoing challenge. Ensuring that all staff are adequately trained in safety protocols, emergency procedures, and proper handling techniques is critical but requires continuous effort and resources to maintain high standards of safety and competence.
Best Storage Practices
01 Personal protective equipment for handling muriatic acid
When working with muriatic acid, proper personal protective equipment (PPE) is essential for safety. This includes wearing chemical-resistant gloves, goggles or face shields, and protective clothing to prevent skin contact and eye exposure. Respiratory protection may also be necessary in poorly ventilated areas or when dealing with high concentrations of acid fumes.- Personal protective equipment for handling muriatic acid: When working with muriatic acid, proper personal protective equipment (PPE) is essential for safety. This includes wearing chemical-resistant gloves, goggles or face shields, and protective clothing to prevent skin contact and eye exposure. Respiratory protection may also be necessary in poorly ventilated areas or when dealing with high concentrations of acid fumes.
- Storage and handling precautions for muriatic acid: Proper storage and handling of muriatic acid are crucial for safety. The acid should be stored in well-ventilated areas, away from incompatible materials, and in corrosion-resistant containers. Handling should be done in well-ventilated spaces, using appropriate transfer methods to minimize spills and splashes. Emergency eyewash stations and safety showers should be readily available in areas where muriatic acid is used or stored.
- Neutralization and spill response procedures: In case of muriatic acid spills, proper neutralization and response procedures are essential. This includes using appropriate neutralizing agents such as sodium bicarbonate or lime, and following specific clean-up protocols. Adequate ventilation should be ensured during the neutralization process, and all contaminated materials should be disposed of properly according to local regulations.
- Safety equipment and engineering controls: Implementing proper safety equipment and engineering controls is crucial when working with muriatic acid. This includes the use of fume hoods, local exhaust ventilation systems, and acid-resistant work surfaces. Safety equipment such as emergency showers, eyewash stations, and spill containment kits should be readily available and regularly maintained to ensure their effectiveness in case of accidents.
- Training and emergency response planning: Comprehensive training programs and emergency response planning are essential for ensuring the safe handling of muriatic acid. This includes educating workers on the hazards associated with the acid, proper handling techniques, and emergency procedures. Developing and regularly reviewing emergency response plans, conducting drills, and maintaining up-to-date safety data sheets (SDS) are crucial components of a robust safety program for muriatic acid handling.
02 Proper storage and handling of muriatic acid
Safe storage and handling of muriatic acid involve using corrosion-resistant containers, maintaining proper ventilation, and implementing spill containment measures. It's crucial to store the acid away from incompatible materials and to use appropriate transfer methods to minimize the risk of spills or accidents during handling.Expand Specific Solutions03 Emergency response and first aid for muriatic acid exposure
In case of muriatic acid exposure, immediate action is critical. This includes flushing affected areas with copious amounts of water, removing contaminated clothing, and seeking medical attention. Having emergency eyewash stations and safety showers readily available in areas where muriatic acid is used is essential for quick response to accidental exposures.Expand Specific Solutions04 Proper disposal and neutralization of muriatic acid
Safe disposal of muriatic acid involves neutralization with appropriate bases and following local environmental regulations. Proper neutralization techniques and disposal methods are crucial to prevent environmental contamination and ensure worker safety during the disposal process.Expand Specific Solutions05 Engineering controls for muriatic acid safety
Implementing engineering controls such as proper ventilation systems, enclosed processes, and automated handling equipment can significantly reduce the risk of exposure to muriatic acid. These controls help minimize worker contact with the acid and its fumes, improving overall safety in industrial settings where muriatic acid is used.Expand Specific Solutions
Key Industry Players
The safety measures for muriatic acid storage are part of a mature and well-established industry practice, with a competitive landscape shaped by major chemical manufacturers and industrial suppliers. The market for acid storage solutions is substantial, driven by widespread use in various industries. Companies like BASF Corp., China Petroleum & Chemical Corp., and Ecolab USA, Inc. are key players, offering advanced storage systems and safety protocols. The technology for safe acid storage is well-developed, with ongoing innovations focusing on improving containment, monitoring, and handling processes. These advancements aim to enhance worker safety, environmental protection, and operational efficiency in industries relying on muriatic acid.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has implemented comprehensive safety measures for muriatic acid storage. They utilize double-walled tanks with leak detection systems, ensuring a secondary containment capacity of at least 110% of the largest tank volume[1]. The company employs advanced corrosion-resistant materials like fiber-reinforced plastic (FRP) or rubber-lined steel for tank construction. Automated monitoring systems continuously track temperature, pressure, and pH levels, with alarms set for any deviations. Sinopec has also developed a proprietary vapor recovery system that captures and neutralizes acid fumes, reducing environmental impact and worker exposure[2]. Regular integrity testing, including ultrasonic thickness measurements and hydrostatic testing, is conducted to maintain tank integrity. The company's storage facilities are equipped with emergency shower and eyewash stations, and employees undergo rigorous safety training specific to muriatic acid handling[3].
Strengths: Comprehensive containment systems, advanced materials usage, and automated monitoring enhance safety. The vapor recovery system demonstrates environmental responsibility. Weaknesses: High initial investment costs for advanced systems and potential complexity in maintenance and operation.
BASF Corp.
Technical Solution: BASF Corp. has developed a multi-layered approach to muriatic acid storage safety. Their storage tanks are constructed with specially formulated acid-resistant polymers and feature a patented double-wall design with interstitial space monitoring[1]. The company utilizes a computerized inventory management system that tracks acid levels in real-time, optimizing storage capacity and minimizing the risk of overfilling. BASF has implemented a closed-loop transfer system for loading and unloading, significantly reducing the potential for spills and worker exposure[2]. Their storage areas are equipped with sophisticated ventilation systems that maintain negative air pressure, preventing the escape of acid vapors. Additionally, BASF has developed a proprietary neutralization system that can quickly respond to spills, automatically dispensing neutralizing agents to contain and mitigate acid releases[3]. The company also employs remote-operated valves and emergency shutdown systems that can be activated from a safe distance in case of incidents.
Strengths: Advanced materials and design in storage tanks, automated inventory management, and innovative spill response systems enhance safety. Weaknesses: High technological dependence may lead to potential vulnerabilities in case of system failures or cyber threats.
Innovative Containment Solutions
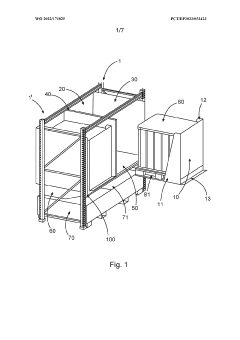
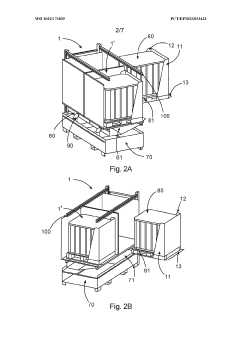
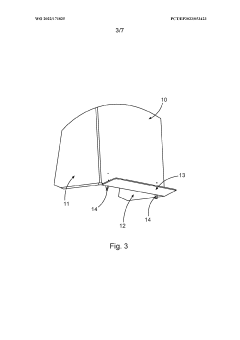
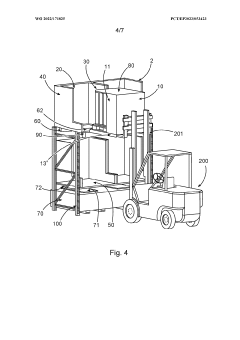
Device for securely storing liquid hazardous material, corresponding storage system and method
PatentWO2022171825A1
Innovation
- A device with a housing comprising a front protective wall, rear protective wall, lateral protective walls, and a fluid-permeable platform that can be transported with the storage container, providing protection during transport and storage, and includes a collection area connected to the housing interior to contain escaped liquids, along with additional features like flame arrestors and heat protection measures.
Storage medium for storing hydrogen chloride and method for separating and storing hydrogen chloride hcl from hcl containing gas
PatentPendingUS20240216889A1
Innovation
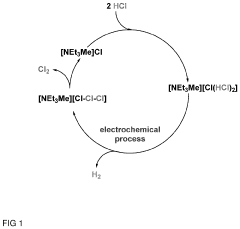
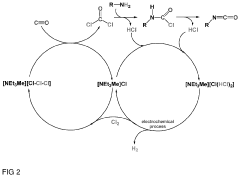
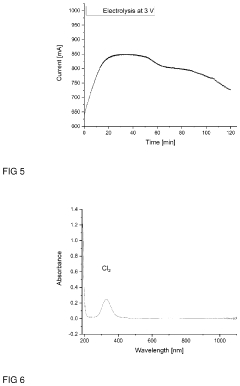
- A storage medium comprising an ionic liquid compound with an asymmetrically substituted ammonium cation and poly(HCl)chloride anion, which allows for reversible absorption and storage of HCl, enabling efficient separation and subsequent release of hydrogen and chlorine through electrolysis, with potential for low-cost and energy-efficient HCl storage and purification.
Environmental Impact
The storage and handling of muriatic acid, also known as hydrochloric acid, pose significant environmental risks that require careful consideration and management. When improperly stored or accidentally released, muriatic acid can have severe impacts on soil, water bodies, and air quality.
Soil contamination is a primary concern in the event of a muriatic acid spill. The highly corrosive nature of the acid can alter soil pH levels dramatically, leading to the destruction of soil microorganisms and rendering the affected area inhospitable for plant growth. This can result in long-term ecological damage and potential loss of agricultural productivity in contaminated areas.
Water pollution is another critical environmental issue associated with muriatic acid storage. Accidental discharge into water systems can cause rapid acidification, severely impacting aquatic ecosystems. Fish and other aquatic organisms are particularly vulnerable to changes in pH levels, and even small quantities of muriatic acid can lead to mass die-offs in affected water bodies. Furthermore, the acid can react with minerals in the water, potentially releasing harmful heavy metals and further exacerbating environmental damage.
Air quality can also be compromised by improper storage of muriatic acid. Fumes from the acid can contribute to the formation of acid rain when released into the atmosphere. This can have far-reaching effects on ecosystems, damaging forests, crops, and aquatic environments even at considerable distances from the source of emission.
To mitigate these environmental risks, stringent storage and handling protocols must be implemented. These include using corrosion-resistant storage containers, installing secondary containment systems to prevent spills from spreading, and ensuring proper ventilation in storage areas to minimize the accumulation of fumes. Regular inspections and maintenance of storage facilities are crucial to identify and address potential leaks or structural weaknesses before they lead to environmental incidents.
In the event of a spill or release, rapid response protocols should be in place to contain and neutralize the acid, minimizing its spread into the environment. This may involve the use of specialized absorbent materials and neutralizing agents designed specifically for acid spills. Additionally, comprehensive environmental monitoring programs should be established around storage facilities to detect any early signs of contamination and allow for prompt remediation efforts.
Proper disposal of muriatic acid and its containers is also essential to prevent environmental contamination. This often involves neutralization processes and specialized waste management procedures to ensure that the acid does not end up in landfills or water treatment systems ill-equipped to handle such corrosive materials.
Soil contamination is a primary concern in the event of a muriatic acid spill. The highly corrosive nature of the acid can alter soil pH levels dramatically, leading to the destruction of soil microorganisms and rendering the affected area inhospitable for plant growth. This can result in long-term ecological damage and potential loss of agricultural productivity in contaminated areas.
Water pollution is another critical environmental issue associated with muriatic acid storage. Accidental discharge into water systems can cause rapid acidification, severely impacting aquatic ecosystems. Fish and other aquatic organisms are particularly vulnerable to changes in pH levels, and even small quantities of muriatic acid can lead to mass die-offs in affected water bodies. Furthermore, the acid can react with minerals in the water, potentially releasing harmful heavy metals and further exacerbating environmental damage.
Air quality can also be compromised by improper storage of muriatic acid. Fumes from the acid can contribute to the formation of acid rain when released into the atmosphere. This can have far-reaching effects on ecosystems, damaging forests, crops, and aquatic environments even at considerable distances from the source of emission.
To mitigate these environmental risks, stringent storage and handling protocols must be implemented. These include using corrosion-resistant storage containers, installing secondary containment systems to prevent spills from spreading, and ensuring proper ventilation in storage areas to minimize the accumulation of fumes. Regular inspections and maintenance of storage facilities are crucial to identify and address potential leaks or structural weaknesses before they lead to environmental incidents.
In the event of a spill or release, rapid response protocols should be in place to contain and neutralize the acid, minimizing its spread into the environment. This may involve the use of specialized absorbent materials and neutralizing agents designed specifically for acid spills. Additionally, comprehensive environmental monitoring programs should be established around storage facilities to detect any early signs of contamination and allow for prompt remediation efforts.
Proper disposal of muriatic acid and its containers is also essential to prevent environmental contamination. This often involves neutralization processes and specialized waste management procedures to ensure that the acid does not end up in landfills or water treatment systems ill-equipped to handle such corrosive materials.
Emergency Response Protocols
Emergency response protocols are critical for ensuring the safety of personnel and minimizing environmental impact in the event of a muriatic acid spill or leak. These protocols should be comprehensive, well-documented, and regularly practiced to ensure swift and effective action during an emergency.
A key component of emergency response is the establishment of a clear chain of command and communication channels. Designated personnel should be trained to lead the response efforts, coordinate with emergency services, and provide accurate information to relevant authorities. Regular drills and simulations help reinforce these roles and identify potential weaknesses in the response plan.
Immediate containment measures are essential to prevent the spread of muriatic acid. Emergency response teams should be equipped with appropriate personal protective equipment (PPE), including chemical-resistant suits, gloves, and respiratory protection. Spill control kits containing neutralizing agents, absorbent materials, and containment barriers should be readily accessible in storage areas.
Evacuation procedures must be clearly defined and communicated to all personnel. Evacuation routes and assembly points should be strategically located away from potential acid exposure areas. Automated alarm systems and clear signage can facilitate rapid evacuation in case of an emergency.
Decontamination protocols are crucial for treating individuals who may have been exposed to muriatic acid. Emergency showers and eyewash stations should be installed in close proximity to storage areas, and personnel must be trained in their proper use. A designated decontamination area should be established for thorough cleaning and medical assessment of affected individuals.
Coordination with local emergency services is vital for an effective response. Regular communication and joint training exercises with fire departments, hazardous materials teams, and medical services ensure a coordinated approach to acid-related emergencies. Providing detailed facility layouts and chemical inventories to these services can significantly improve response times and effectiveness.
Environmental protection measures should be integrated into emergency response protocols. This includes procedures for containing and neutralizing acid spills to prevent contamination of soil and water sources. Proper disposal methods for contaminated materials and neutralized acid solutions should be outlined and followed to comply with environmental regulations.
Post-incident analysis and reporting procedures are essential for continuous improvement of emergency response protocols. Thorough investigations of any incidents or near-misses should be conducted to identify root causes and implement corrective actions. Lessons learned from these analyses should be incorporated into revised protocols and shared across the organization to prevent similar occurrences in the future.
A key component of emergency response is the establishment of a clear chain of command and communication channels. Designated personnel should be trained to lead the response efforts, coordinate with emergency services, and provide accurate information to relevant authorities. Regular drills and simulations help reinforce these roles and identify potential weaknesses in the response plan.
Immediate containment measures are essential to prevent the spread of muriatic acid. Emergency response teams should be equipped with appropriate personal protective equipment (PPE), including chemical-resistant suits, gloves, and respiratory protection. Spill control kits containing neutralizing agents, absorbent materials, and containment barriers should be readily accessible in storage areas.
Evacuation procedures must be clearly defined and communicated to all personnel. Evacuation routes and assembly points should be strategically located away from potential acid exposure areas. Automated alarm systems and clear signage can facilitate rapid evacuation in case of an emergency.
Decontamination protocols are crucial for treating individuals who may have been exposed to muriatic acid. Emergency showers and eyewash stations should be installed in close proximity to storage areas, and personnel must be trained in their proper use. A designated decontamination area should be established for thorough cleaning and medical assessment of affected individuals.
Coordination with local emergency services is vital for an effective response. Regular communication and joint training exercises with fire departments, hazardous materials teams, and medical services ensure a coordinated approach to acid-related emergencies. Providing detailed facility layouts and chemical inventories to these services can significantly improve response times and effectiveness.
Environmental protection measures should be integrated into emergency response protocols. This includes procedures for containing and neutralizing acid spills to prevent contamination of soil and water sources. Proper disposal methods for contaminated materials and neutralized acid solutions should be outlined and followed to comply with environmental regulations.
Post-incident analysis and reporting procedures are essential for continuous improvement of emergency response protocols. Thorough investigations of any incidents or near-misses should be conducted to identify root causes and implement corrective actions. Lessons learned from these analyses should be incorporated into revised protocols and shared across the organization to prevent similar occurrences in the future.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!