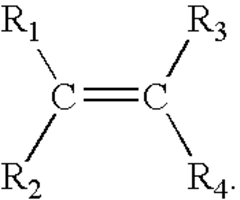
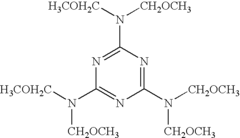
Ammonium Hydroxide's Role in Water-Based Coating Formulation
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
NH4OH in Coatings: Background and Objectives
Ammonium hydroxide, commonly known as aqueous ammonia, has played a significant role in the development of water-based coating formulations. This versatile compound has been utilized in the coatings industry for decades, primarily due to its unique properties and multifaceted functionality. The evolution of water-based coatings has been driven by increasing environmental regulations and a growing demand for more sustainable and eco-friendly products.
The primary objective of incorporating ammonium hydroxide in water-based coating formulations is to enhance the overall performance and stability of the coating system. As a weak base, NH4OH serves as an effective pH regulator, maintaining the alkalinity of the formulation within the desired range. This pH control is crucial for ensuring the stability of the dispersion and preventing premature coagulation of the coating components.
Furthermore, ammonium hydroxide acts as a neutralizing agent for acidic components in the formulation, such as carboxylic acid-functional resins. This neutralization process is essential for achieving proper film formation and adhesion properties in the final coating. The volatile nature of ammonia also contributes to its effectiveness, as it evaporates during the drying process, leaving behind a neutral film.
In recent years, the coatings industry has witnessed a shift towards low-VOC (Volatile Organic Compound) and zero-VOC formulations. Ammonium hydroxide has emerged as a valuable tool in this transition, as it can partially or fully replace traditional organic co-solvents in many water-based systems. This substitution helps reduce the overall VOC content of the coating, aligning with stringent environmental regulations and consumer preferences for greener products.
The use of ammonium hydroxide in water-based coatings extends beyond its primary functions. It also serves as a wetting agent, improving the flow and leveling characteristics of the coating. This property is particularly beneficial in applications where a smooth, uniform finish is desired. Additionally, NH4OH can enhance the freeze-thaw stability of water-based formulations, a critical factor for products that may be exposed to varying temperatures during storage and transportation.
As the coatings industry continues to evolve, researchers and formulators are exploring new ways to optimize the use of ammonium hydroxide in water-based systems. Current objectives include developing formulations with improved durability, faster drying times, and enhanced resistance to environmental factors. There is also a focus on understanding the long-term effects of NH4OH on coating performance and substrate interactions, particularly in challenging applications such as industrial and automotive coatings.
The primary objective of incorporating ammonium hydroxide in water-based coating formulations is to enhance the overall performance and stability of the coating system. As a weak base, NH4OH serves as an effective pH regulator, maintaining the alkalinity of the formulation within the desired range. This pH control is crucial for ensuring the stability of the dispersion and preventing premature coagulation of the coating components.
Furthermore, ammonium hydroxide acts as a neutralizing agent for acidic components in the formulation, such as carboxylic acid-functional resins. This neutralization process is essential for achieving proper film formation and adhesion properties in the final coating. The volatile nature of ammonia also contributes to its effectiveness, as it evaporates during the drying process, leaving behind a neutral film.
In recent years, the coatings industry has witnessed a shift towards low-VOC (Volatile Organic Compound) and zero-VOC formulations. Ammonium hydroxide has emerged as a valuable tool in this transition, as it can partially or fully replace traditional organic co-solvents in many water-based systems. This substitution helps reduce the overall VOC content of the coating, aligning with stringent environmental regulations and consumer preferences for greener products.
The use of ammonium hydroxide in water-based coatings extends beyond its primary functions. It also serves as a wetting agent, improving the flow and leveling characteristics of the coating. This property is particularly beneficial in applications where a smooth, uniform finish is desired. Additionally, NH4OH can enhance the freeze-thaw stability of water-based formulations, a critical factor for products that may be exposed to varying temperatures during storage and transportation.
As the coatings industry continues to evolve, researchers and formulators are exploring new ways to optimize the use of ammonium hydroxide in water-based systems. Current objectives include developing formulations with improved durability, faster drying times, and enhanced resistance to environmental factors. There is also a focus on understanding the long-term effects of NH4OH on coating performance and substrate interactions, particularly in challenging applications such as industrial and automotive coatings.
Market Analysis for Water-Based Coatings
The water-based coatings market has experienced significant growth in recent years, driven by increasing environmental regulations and consumer demand for eco-friendly products. This segment of the coating industry has shown robust expansion, with a compound annual growth rate (CAGR) exceeding global GDP growth. The market size for water-based coatings has reached substantial figures, with projections indicating continued strong performance in the coming years.
Key factors contributing to this market growth include stringent volatile organic compound (VOC) emission regulations, growing awareness of health and environmental issues, and the shift towards sustainable manufacturing practices. Industries such as construction, automotive, and furniture have been at the forefront of adopting water-based coatings, further propelling market expansion.
The construction sector, in particular, has been a major driver for water-based coating demand. Rapid urbanization and infrastructure development in emerging economies have led to increased use of these coatings in both residential and commercial applications. The automotive industry has also shown a growing preference for water-based coatings, especially in OEM and refinish segments, due to their lower environmental impact and improved performance characteristics.
Geographically, North America and Europe have been leading markets for water-based coatings, primarily due to strict environmental regulations and high consumer awareness. However, the Asia-Pacific region is emerging as the fastest-growing market, driven by rapid industrialization, urbanization, and increasing disposable incomes in countries like China and India.
The role of ammonium hydroxide in water-based coating formulations has been crucial in this market landscape. As a key ingredient, it serves multiple functions including pH adjustment, viscosity control, and enhancing the stability and performance of the coatings. The demand for ammonium hydroxide in this context has grown in tandem with the overall water-based coatings market.
Looking ahead, the water-based coatings market is expected to continue its growth trajectory. Factors such as ongoing research and development in coating technologies, increasing focus on bio-based and renewable raw materials, and the expansion of end-use industries in developing economies are likely to sustain this trend. The market is also witnessing a shift towards high-performance water-based coatings that can compete with solvent-based alternatives in terms of durability and application properties.
Key factors contributing to this market growth include stringent volatile organic compound (VOC) emission regulations, growing awareness of health and environmental issues, and the shift towards sustainable manufacturing practices. Industries such as construction, automotive, and furniture have been at the forefront of adopting water-based coatings, further propelling market expansion.
The construction sector, in particular, has been a major driver for water-based coating demand. Rapid urbanization and infrastructure development in emerging economies have led to increased use of these coatings in both residential and commercial applications. The automotive industry has also shown a growing preference for water-based coatings, especially in OEM and refinish segments, due to their lower environmental impact and improved performance characteristics.
Geographically, North America and Europe have been leading markets for water-based coatings, primarily due to strict environmental regulations and high consumer awareness. However, the Asia-Pacific region is emerging as the fastest-growing market, driven by rapid industrialization, urbanization, and increasing disposable incomes in countries like China and India.
The role of ammonium hydroxide in water-based coating formulations has been crucial in this market landscape. As a key ingredient, it serves multiple functions including pH adjustment, viscosity control, and enhancing the stability and performance of the coatings. The demand for ammonium hydroxide in this context has grown in tandem with the overall water-based coatings market.
Looking ahead, the water-based coatings market is expected to continue its growth trajectory. Factors such as ongoing research and development in coating technologies, increasing focus on bio-based and renewable raw materials, and the expansion of end-use industries in developing economies are likely to sustain this trend. The market is also witnessing a shift towards high-performance water-based coatings that can compete with solvent-based alternatives in terms of durability and application properties.
Current Challenges in NH4OH Formulation
The formulation of water-based coatings using ammonium hydroxide (NH4OH) presents several significant challenges that researchers and manufacturers must address. One of the primary issues is maintaining the stability of the coating formulation over time. NH4OH, being volatile, tends to evaporate from the mixture, leading to pH fluctuations and potential instability of the dispersion. This volatility can result in inconsistent product quality and reduced shelf life, necessitating careful consideration of storage conditions and packaging materials.
Another challenge lies in achieving the optimal balance between pH adjustment and other coating properties. While NH4OH effectively raises the pH of the formulation, excessive amounts can negatively impact adhesion, durability, and film formation. Formulators must carefully titrate the NH4OH content to ensure proper neutralization of acidic components without compromising the overall performance of the coating.
The environmental and health concerns associated with NH4OH usage also pose significant challenges. As regulations become more stringent, manufacturers are under pressure to reduce volatile organic compound (VOC) emissions. NH4OH, despite being considered a low-VOC additive, still contributes to overall emissions and odor issues. This necessitates the development of alternative formulation strategies or the implementation of advanced emission control technologies in production facilities.
Compatibility with other coating components is another area of concern. NH4OH can interact with certain pigments, binders, and additives, potentially causing color shifts, viscosity changes, or even chemical reactions that alter the coating's properties. Formulators must conduct extensive compatibility testing and optimize the entire formulation to mitigate these interactions.
The impact of NH4OH on the drying and curing process of water-based coatings also presents challenges. Its presence can affect the rate of water evaporation and film formation, potentially leading to issues such as blistering, cracking, or inadequate crosslinking in the final coating. Balancing the NH4OH content with other factors that influence drying and curing, such as temperature and humidity, requires careful consideration and process control.
Lastly, the variability in raw material quality and the need for precise dosing of NH4OH in industrial-scale production pose significant challenges. Ensuring consistent product quality across different batches and production runs requires sophisticated process control systems and robust quality assurance protocols. Manufacturers must invest in advanced mixing and dosing equipment, as well as implement rigorous testing procedures to maintain the desired formulation properties.
Another challenge lies in achieving the optimal balance between pH adjustment and other coating properties. While NH4OH effectively raises the pH of the formulation, excessive amounts can negatively impact adhesion, durability, and film formation. Formulators must carefully titrate the NH4OH content to ensure proper neutralization of acidic components without compromising the overall performance of the coating.
The environmental and health concerns associated with NH4OH usage also pose significant challenges. As regulations become more stringent, manufacturers are under pressure to reduce volatile organic compound (VOC) emissions. NH4OH, despite being considered a low-VOC additive, still contributes to overall emissions and odor issues. This necessitates the development of alternative formulation strategies or the implementation of advanced emission control technologies in production facilities.
Compatibility with other coating components is another area of concern. NH4OH can interact with certain pigments, binders, and additives, potentially causing color shifts, viscosity changes, or even chemical reactions that alter the coating's properties. Formulators must conduct extensive compatibility testing and optimize the entire formulation to mitigate these interactions.
The impact of NH4OH on the drying and curing process of water-based coatings also presents challenges. Its presence can affect the rate of water evaporation and film formation, potentially leading to issues such as blistering, cracking, or inadequate crosslinking in the final coating. Balancing the NH4OH content with other factors that influence drying and curing, such as temperature and humidity, requires careful consideration and process control.
Lastly, the variability in raw material quality and the need for precise dosing of NH4OH in industrial-scale production pose significant challenges. Ensuring consistent product quality across different batches and production runs requires sophisticated process control systems and robust quality assurance protocols. Manufacturers must invest in advanced mixing and dosing equipment, as well as implement rigorous testing procedures to maintain the desired formulation properties.
Existing NH4OH Formulation Techniques
01 Ammonium hydroxide in coating formulations
Ammonium hydroxide is used in various coating formulations as a pH regulator, neutralizing agent, or catalyst. It can improve the stability and performance of coatings, particularly in water-based systems. The addition of ammonium hydroxide can enhance the dispersion of pigments and binders, leading to improved coating properties.- Ammonium hydroxide in coating compositions: Ammonium hydroxide is used in various coating formulations as a pH regulator, neutralizing agent, or catalyst. It can improve the stability and performance of the coating, enhance adhesion, and control the curing process. The volatile nature of ammonium hydroxide allows it to evaporate during the drying process, leaving minimal residue in the final coating.
- Ammonium hydroxide in polymer coatings: In polymer-based coatings, ammonium hydroxide is utilized to neutralize acidic components, adjust pH, and improve dispersion stability. It can enhance the solubility of certain polymers in water-based systems, leading to better film formation and improved coating properties. The use of ammonium hydroxide in polymer coatings can result in enhanced durability and chemical resistance.
- Ammonium hydroxide in corrosion-resistant coatings: Ammonium hydroxide plays a role in formulating corrosion-resistant coatings. It can be used to adjust the pH of the coating solution, which is crucial for the formation of protective layers on metal surfaces. The alkaline nature of ammonium hydroxide can help in passivating metal surfaces and enhancing the overall corrosion resistance of the coating system.
- Ammonium hydroxide in sol-gel coatings: In sol-gel coating formulations, ammonium hydroxide serves as a catalyst for hydrolysis and condensation reactions. It can control the rate of gel formation and influence the final structure and properties of the coating. The use of ammonium hydroxide in sol-gel coatings can lead to improved hardness, scratch resistance, and optical properties.
- Ammonium hydroxide in environmentally friendly coatings: Ammonium hydroxide is used in the development of environmentally friendly coating formulations. It can replace more harmful alkaline compounds and volatile organic compounds (VOCs) in certain coating systems. The low toxicity and biodegradability of ammonium hydroxide make it a suitable choice for eco-friendly coating applications, particularly in water-based systems.
02 Ammonium hydroxide in polymer synthesis
Ammonium hydroxide plays a role in polymer synthesis and modification processes. It can be used as a catalyst or reactant in polymerization reactions, particularly in the production of water-soluble or water-dispersible polymers. These polymers may then be incorporated into coating formulations to improve adhesion, durability, or other properties.Expand Specific Solutions03 Ammonium hydroxide in nanoparticle coatings
Ammonium hydroxide is utilized in the synthesis and stabilization of nanoparticles for coating applications. It can control the pH during nanoparticle formation, influence particle size and morphology, and help in the dispersion of nanoparticles in coating formulations. These nanoparticle-enhanced coatings may exhibit improved mechanical, optical, or functional properties.Expand Specific Solutions04 Ammonium hydroxide in corrosion-resistant coatings
Ammonium hydroxide is incorporated into corrosion-resistant coating formulations. It can act as a complexing agent for metal ions, helping to form protective layers on metal surfaces. Additionally, it may be used to adjust the pH of the coating system, which can influence the corrosion protection mechanism and overall coating performance.Expand Specific Solutions05 Ammonium hydroxide in environmentally friendly coatings
Ammonium hydroxide is employed in the development of environmentally friendly coating formulations. It can serve as a volatile base in low-VOC (volatile organic compound) coatings, helping to reduce environmental impact. In some cases, it may replace traditional organic amines or other alkaline compounds in coating systems, contributing to more sustainable product formulations.Expand Specific Solutions
Key Players in Water-Based Coating Industry
The market for ammonium hydroxide in water-based coating formulations is in a mature stage, with a stable global market size. The technology is well-established, with key players like BASF Coatings GmbH, Kansai Paint Co., Ltd., and Asian Paints Ltd. leading innovation in this field. These companies have extensive experience in developing and refining water-based coating technologies, focusing on improving performance and environmental sustainability. The competitive landscape is characterized by ongoing research and development efforts to enhance coating properties, reduce VOC emissions, and meet increasingly stringent regulatory requirements. Smaller specialized firms like Safeguard Europe Ltd. are also contributing to niche applications, driving further innovation in the sector.
BASF Coatings GmbH
Technical Solution: BASF Coatings GmbH has developed innovative water-based coating formulations utilizing ammonium hydroxide as a key component. Their approach involves using ammonium hydroxide as a pH regulator and neutralizing agent for acid-functional binders in waterborne coatings[1]. This allows for the creation of stable emulsions and dispersions, crucial for coating performance. BASF's technology incorporates ammonium hydroxide to enhance film formation, improve adhesion, and increase the overall durability of the coating[2]. Their formulations typically contain 0.1-2% ammonium hydroxide by weight, which helps maintain an optimal pH range of 7.5-9.0 for most water-based coatings[3]. Additionally, BASF has developed proprietary additives that work synergistically with ammonium hydroxide to further improve coating properties such as gloss, leveling, and resistance to environmental factors[4].
Strengths: Excellent pH control, improved emulsion stability, enhanced film formation, and increased coating durability. Weaknesses: Potential ammonia odor during application, slight VOC contribution, and possible incompatibility with certain substrates or additives.
Dow Global Technologies LLC
Technical Solution: Dow Global Technologies LLC has developed cutting-edge water-based coating formulations that utilize ammonium hydroxide as a key ingredient. Their approach focuses on leveraging ammonium hydroxide's properties as a volatile base to neutralize acid-functional resins and create stable waterborne dispersions[1]. Dow's technology incorporates ammonium hydroxide to control pH, enhance pigment dispersion, and improve the overall stability of the coating system[2]. Their formulations typically contain 0.2-1.5% ammonium hydroxide by weight, carefully optimized to maintain a pH range of 7.8-9.2 for various coating applications[3]. Dow has also developed proprietary polymer technologies that synergize with ammonium hydroxide to enhance film formation, improve adhesion to diverse substrates, and increase the coating's resistance to environmental stressors[4]. Additionally, their formulations often include specially designed coalescent aids that work in tandem with ammonium hydroxide to ensure optimal film formation across a wide range of application conditions[5].
Strengths: Excellent pH control, improved dispersion stability, enhanced film properties, and broad substrate compatibility. Weaknesses: Potential ammonia odor during application, slight VOC contribution, and possible limitations in high-humidity environments.
Innovations in NH4OH Coating Applications
Water-based coating composition
PatentWO2009145223A1
Innovation
- A water-based coating composition containing a monomer mixture with a tertiary amino group-containing polymerizable unsaturated monomer and other polymerizable unsaturated monomers, dispersed in an aqueous medium with an ammonium salt-type anionic emulsifier to form a water-dispersible acrylic resin, which is then polymerized to create a coating film with improved sagging resistance and surface finish.
Water-based coating composition having carbamate-melamine cross-linking, method of preparing the same, and a cured film thereof
PatentInactiveUS6699943B2
Innovation
- A curable, water-based coating composition comprising a water-based copolymer with a carbamate functional group and a reactive cross-linking agent, such as hexamethoxymethyl melamine, that is free from co-solvents and surfactants, allowing for controlled free-radical polymerization to produce a stable and miscible copolymer suitable for waterborne basecoat, clearcoat, and primer systems.
Environmental Impact of NH4OH in Coatings
The environmental impact of ammonium hydroxide (NH4OH) in water-based coating formulations is a critical consideration for manufacturers and regulators alike. NH4OH, commonly used as a pH adjuster and neutralizing agent, plays a significant role in the performance and stability of coatings. However, its environmental implications cannot be overlooked.
One of the primary environmental concerns associated with NH4OH in coatings is its potential to contribute to atmospheric ammonia emissions. When coatings containing NH4OH are applied, the compound can volatilize, releasing ammonia into the air. This process not only reduces the effectiveness of the coating but also contributes to air pollution. Atmospheric ammonia is a precursor to fine particulate matter (PM2.5) formation, which has well-documented negative impacts on human health and ecosystems.
Water contamination is another significant environmental risk posed by NH4OH in coatings. During the application process or through weathering of coated surfaces, NH4OH can leach into water bodies. Elevated levels of ammonia in aquatic environments can lead to eutrophication, causing algal blooms and subsequent oxygen depletion, which is detrimental to aquatic life. Furthermore, ammonia is directly toxic to fish and other aquatic organisms at high concentrations.
The production and transportation of NH4OH for coating formulations also contribute to its environmental footprint. The manufacturing process of NH4OH typically involves the Haber-Bosch process, which is energy-intensive and relies heavily on fossil fuels, contributing to greenhouse gas emissions. Additionally, the transportation of NH4OH poses risks of accidental spills, which can have localized but severe environmental impacts.
Regulatory bodies worldwide are increasingly scrutinizing the use of volatile organic compounds (VOCs) in coatings, and while NH4OH is not classified as a VOC in many jurisdictions, its ammonia emissions are coming under similar scrutiny. This regulatory pressure is driving research into alternative pH adjusters and neutralizing agents that have lower environmental impacts.
To mitigate these environmental concerns, coating manufacturers are exploring several strategies. These include developing low-ammonia or ammonia-free formulations, improving application techniques to minimize volatilization, and investigating bio-based alternatives to NH4OH. Additionally, advancements in encapsulation technologies are being pursued to reduce the release of ammonia during and after coating application.
In conclusion, while NH4OH plays a crucial role in water-based coating formulations, its environmental impact necessitates careful consideration and ongoing research to develop more sustainable alternatives. The balance between coating performance and environmental protection remains a key challenge for the industry, driving innovation in formulation and application technologies.
One of the primary environmental concerns associated with NH4OH in coatings is its potential to contribute to atmospheric ammonia emissions. When coatings containing NH4OH are applied, the compound can volatilize, releasing ammonia into the air. This process not only reduces the effectiveness of the coating but also contributes to air pollution. Atmospheric ammonia is a precursor to fine particulate matter (PM2.5) formation, which has well-documented negative impacts on human health and ecosystems.
Water contamination is another significant environmental risk posed by NH4OH in coatings. During the application process or through weathering of coated surfaces, NH4OH can leach into water bodies. Elevated levels of ammonia in aquatic environments can lead to eutrophication, causing algal blooms and subsequent oxygen depletion, which is detrimental to aquatic life. Furthermore, ammonia is directly toxic to fish and other aquatic organisms at high concentrations.
The production and transportation of NH4OH for coating formulations also contribute to its environmental footprint. The manufacturing process of NH4OH typically involves the Haber-Bosch process, which is energy-intensive and relies heavily on fossil fuels, contributing to greenhouse gas emissions. Additionally, the transportation of NH4OH poses risks of accidental spills, which can have localized but severe environmental impacts.
Regulatory bodies worldwide are increasingly scrutinizing the use of volatile organic compounds (VOCs) in coatings, and while NH4OH is not classified as a VOC in many jurisdictions, its ammonia emissions are coming under similar scrutiny. This regulatory pressure is driving research into alternative pH adjusters and neutralizing agents that have lower environmental impacts.
To mitigate these environmental concerns, coating manufacturers are exploring several strategies. These include developing low-ammonia or ammonia-free formulations, improving application techniques to minimize volatilization, and investigating bio-based alternatives to NH4OH. Additionally, advancements in encapsulation technologies are being pursued to reduce the release of ammonia during and after coating application.
In conclusion, while NH4OH plays a crucial role in water-based coating formulations, its environmental impact necessitates careful consideration and ongoing research to develop more sustainable alternatives. The balance between coating performance and environmental protection remains a key challenge for the industry, driving innovation in formulation and application technologies.
Regulatory Framework for NH4OH Usage
The regulatory framework for ammonium hydroxide (NH4OH) usage in water-based coating formulations is complex and multifaceted, involving various governmental agencies and international bodies. At the forefront of this regulatory landscape is the Environmental Protection Agency (EPA) in the United States, which sets guidelines for the use of NH4OH under the Toxic Substances Control Act (TSCA). The EPA mandates strict reporting and record-keeping requirements for manufacturers and importers of NH4OH, particularly when used in significant quantities in coating formulations.
In the European Union, the regulatory framework is governed by the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation. Under REACH, NH4OH is subject to registration requirements, with manufacturers and importers required to submit detailed safety data and exposure scenarios. The European Chemicals Agency (ECHA) plays a crucial role in assessing the risks associated with NH4OH and determining appropriate risk management measures.
Occupational safety regulations also form a significant part of the NH4OH regulatory framework. The Occupational Safety and Health Administration (OSHA) in the U.S. sets permissible exposure limits (PELs) for NH4OH in the workplace, requiring employers to implement appropriate engineering controls and personal protective equipment to minimize worker exposure. Similarly, the European Agency for Safety and Health at Work (EU-OSHA) provides guidelines for safe handling and use of NH4OH in industrial settings.
Environmental regulations are another critical aspect of the NH4OH regulatory framework. The Clean Water Act in the U.S. and the Water Framework Directive in the EU set limits on the discharge of ammonia-containing compounds into water bodies. Manufacturers of water-based coatings must ensure their formulations and production processes comply with these regulations to prevent environmental contamination.
Transportation of NH4OH is regulated by the Department of Transportation (DOT) in the U.S. and the European Agreement concerning the International Carriage of Dangerous Goods by Road (ADR) in Europe. These regulations specify requirements for packaging, labeling, and documentation when transporting NH4OH, which is classified as a hazardous material.
Globally, the Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to communicating chemical hazards. Under GHS, NH4OH is classified and labeled according to its hazardous properties, ensuring consistent safety information across different countries and regions.
As the regulatory landscape continues to evolve, manufacturers and users of NH4OH in water-based coating formulations must stay informed of changes and updates to ensure ongoing compliance. This may involve regular training, audits, and engagement with regulatory bodies to adapt to new requirements and best practices in the use of NH4OH.
In the European Union, the regulatory framework is governed by the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation. Under REACH, NH4OH is subject to registration requirements, with manufacturers and importers required to submit detailed safety data and exposure scenarios. The European Chemicals Agency (ECHA) plays a crucial role in assessing the risks associated with NH4OH and determining appropriate risk management measures.
Occupational safety regulations also form a significant part of the NH4OH regulatory framework. The Occupational Safety and Health Administration (OSHA) in the U.S. sets permissible exposure limits (PELs) for NH4OH in the workplace, requiring employers to implement appropriate engineering controls and personal protective equipment to minimize worker exposure. Similarly, the European Agency for Safety and Health at Work (EU-OSHA) provides guidelines for safe handling and use of NH4OH in industrial settings.
Environmental regulations are another critical aspect of the NH4OH regulatory framework. The Clean Water Act in the U.S. and the Water Framework Directive in the EU set limits on the discharge of ammonia-containing compounds into water bodies. Manufacturers of water-based coatings must ensure their formulations and production processes comply with these regulations to prevent environmental contamination.
Transportation of NH4OH is regulated by the Department of Transportation (DOT) in the U.S. and the European Agreement concerning the International Carriage of Dangerous Goods by Road (ADR) in Europe. These regulations specify requirements for packaging, labeling, and documentation when transporting NH4OH, which is classified as a hazardous material.
Globally, the Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to communicating chemical hazards. Under GHS, NH4OH is classified and labeled according to its hazardous properties, ensuring consistent safety information across different countries and regions.
As the regulatory landscape continues to evolve, manufacturers and users of NH4OH in water-based coating formulations must stay informed of changes and updates to ensure ongoing compliance. This may involve regular training, audits, and engagement with regulatory bodies to adapt to new requirements and best practices in the use of NH4OH.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!