How Ammonium Hydroxide Modifies the Viscosity of Polymer Solutions
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ammonium Hydroxide and Polymer Viscosity Modification
The modification of polymer solution viscosity using ammonium hydroxide is a critical area of study in polymer science and engineering. This process involves the interaction between ammonium hydroxide (NH4OH) and various polymer structures, resulting in changes to the solution's rheological properties. The mechanism behind this modification is complex and depends on several factors, including the polymer type, concentration, and environmental conditions.
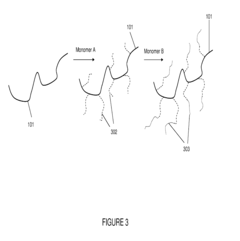
Ammonium hydroxide, a weak base, can alter the pH of the polymer solution, which in turn affects the polymer's conformation and intermolecular interactions. In many cases, the addition of NH4OH leads to an increase in viscosity due to the expansion of polymer chains and enhanced polymer-solvent interactions. This phenomenon is particularly pronounced in polyelectrolytes and pH-responsive polymers.
The viscosity modification process typically begins with the dissociation of ammonium hydroxide in the solution, releasing hydroxide ions (OH-). These ions can interact with functional groups on the polymer chains, such as carboxylic acid or amine groups, leading to changes in the polymer's charge distribution and hydrophilicity. As a result, the polymer chains may undergo conformational changes, expanding or contracting depending on the specific polymer-solvent system.
For acidic polymers, such as polyacrylic acid, the addition of ammonium hydroxide neutralizes the acid groups, causing the polymer chains to extend due to electrostatic repulsion between the newly formed carboxylate anions. This extension increases the hydrodynamic volume of the polymer, thereby increasing the solution's viscosity. Conversely, for basic polymers, the addition of NH4OH may lead to protonation of amine groups, potentially causing chain collapse and a decrease in viscosity.
The concentration of ammonium hydroxide plays a crucial role in determining the extent of viscosity modification. At low concentrations, the effect may be minimal, while at higher concentrations, significant changes in viscosity can be observed. However, there is often an optimal concentration range beyond which further addition of NH4OH may not result in additional viscosity increases or may even lead to a decrease in viscosity due to screening effects or changes in polymer-solvent interactions.
Temperature also influences the viscosity modification process. Higher temperatures generally lead to increased molecular motion and reduced viscosity. However, the presence of ammonium hydroxide can complicate this relationship, as it may affect the temperature-dependent behavior of the polymer solution through changes in hydrogen bonding and other intermolecular forces.
Understanding and controlling the viscosity modification of polymer solutions using ammonium hydroxide has important implications for various industrial applications. These include the formulation of paints and coatings, the production of adhesives, and the development of drug delivery systems. By carefully manipulating the NH4OH concentration and other parameters, researchers and engineers can tailor the rheological properties of polymer solutions to meet specific performance requirements in these and other applications.
Ammonium hydroxide, a weak base, can alter the pH of the polymer solution, which in turn affects the polymer's conformation and intermolecular interactions. In many cases, the addition of NH4OH leads to an increase in viscosity due to the expansion of polymer chains and enhanced polymer-solvent interactions. This phenomenon is particularly pronounced in polyelectrolytes and pH-responsive polymers.
The viscosity modification process typically begins with the dissociation of ammonium hydroxide in the solution, releasing hydroxide ions (OH-). These ions can interact with functional groups on the polymer chains, such as carboxylic acid or amine groups, leading to changes in the polymer's charge distribution and hydrophilicity. As a result, the polymer chains may undergo conformational changes, expanding or contracting depending on the specific polymer-solvent system.
For acidic polymers, such as polyacrylic acid, the addition of ammonium hydroxide neutralizes the acid groups, causing the polymer chains to extend due to electrostatic repulsion between the newly formed carboxylate anions. This extension increases the hydrodynamic volume of the polymer, thereby increasing the solution's viscosity. Conversely, for basic polymers, the addition of NH4OH may lead to protonation of amine groups, potentially causing chain collapse and a decrease in viscosity.
The concentration of ammonium hydroxide plays a crucial role in determining the extent of viscosity modification. At low concentrations, the effect may be minimal, while at higher concentrations, significant changes in viscosity can be observed. However, there is often an optimal concentration range beyond which further addition of NH4OH may not result in additional viscosity increases or may even lead to a decrease in viscosity due to screening effects or changes in polymer-solvent interactions.
Temperature also influences the viscosity modification process. Higher temperatures generally lead to increased molecular motion and reduced viscosity. However, the presence of ammonium hydroxide can complicate this relationship, as it may affect the temperature-dependent behavior of the polymer solution through changes in hydrogen bonding and other intermolecular forces.
Understanding and controlling the viscosity modification of polymer solutions using ammonium hydroxide has important implications for various industrial applications. These include the formulation of paints and coatings, the production of adhesives, and the development of drug delivery systems. By carefully manipulating the NH4OH concentration and other parameters, researchers and engineers can tailor the rheological properties of polymer solutions to meet specific performance requirements in these and other applications.
Market Applications of Modified Polymer Solutions
The modification of polymer solutions using ammonium hydroxide has opened up a wide range of market applications across various industries. In the textile sector, this technology has revolutionized the production of high-performance fabrics. By altering the viscosity of polymer solutions, manufacturers can create textiles with enhanced moisture-wicking properties, improved durability, and increased comfort. This has led to the development of advanced sportswear, outdoor gear, and protective clothing that offer superior performance in challenging environments.
In the construction industry, ammonium hydroxide-modified polymer solutions have found applications in the production of advanced concrete admixtures. These modified solutions enhance the workability and flow characteristics of concrete, allowing for easier placement and improved finishing. The resulting concrete structures exhibit increased strength, reduced permeability, and enhanced resistance to chemical attack, making them ideal for use in demanding infrastructure projects such as bridges, tunnels, and high-rise buildings.
The automotive sector has also benefited from this technology, particularly in the development of high-performance coatings and adhesives. Modified polymer solutions enable the creation of durable, weather-resistant coatings that protect vehicle exteriors from environmental damage. Additionally, these solutions have improved the bonding strength of adhesives used in vehicle assembly, contributing to lighter and more fuel-efficient designs.
In the field of personal care and cosmetics, ammonium hydroxide-modified polymer solutions have enabled the formulation of innovative hair care and skincare products. These modified solutions provide enhanced stability, improved texture, and better delivery of active ingredients. As a result, consumers can enjoy more effective and longer-lasting beauty products, such as hair styling gels, moisturizers, and anti-aging creams.
The pharmaceutical industry has leveraged this technology to develop advanced drug delivery systems. By modifying the viscosity of polymer solutions, researchers have created controlled-release formulations that can precisely regulate the release of active pharmaceutical ingredients over extended periods. This has led to the development of more effective medications with reduced side effects and improved patient compliance.
In the electronics sector, ammonium hydroxide-modified polymer solutions have found applications in the production of advanced printed circuit boards (PCBs) and flexible electronics. The modified solutions enable the creation of thinner, more flexible, and more durable electronic components, paving the way for the development of wearable technology, foldable displays, and other innovative electronic devices.
In the construction industry, ammonium hydroxide-modified polymer solutions have found applications in the production of advanced concrete admixtures. These modified solutions enhance the workability and flow characteristics of concrete, allowing for easier placement and improved finishing. The resulting concrete structures exhibit increased strength, reduced permeability, and enhanced resistance to chemical attack, making them ideal for use in demanding infrastructure projects such as bridges, tunnels, and high-rise buildings.
The automotive sector has also benefited from this technology, particularly in the development of high-performance coatings and adhesives. Modified polymer solutions enable the creation of durable, weather-resistant coatings that protect vehicle exteriors from environmental damage. Additionally, these solutions have improved the bonding strength of adhesives used in vehicle assembly, contributing to lighter and more fuel-efficient designs.
In the field of personal care and cosmetics, ammonium hydroxide-modified polymer solutions have enabled the formulation of innovative hair care and skincare products. These modified solutions provide enhanced stability, improved texture, and better delivery of active ingredients. As a result, consumers can enjoy more effective and longer-lasting beauty products, such as hair styling gels, moisturizers, and anti-aging creams.
The pharmaceutical industry has leveraged this technology to develop advanced drug delivery systems. By modifying the viscosity of polymer solutions, researchers have created controlled-release formulations that can precisely regulate the release of active pharmaceutical ingredients over extended periods. This has led to the development of more effective medications with reduced side effects and improved patient compliance.
In the electronics sector, ammonium hydroxide-modified polymer solutions have found applications in the production of advanced printed circuit boards (PCBs) and flexible electronics. The modified solutions enable the creation of thinner, more flexible, and more durable electronic components, paving the way for the development of wearable technology, foldable displays, and other innovative electronic devices.
Current Challenges in Polymer Viscosity Control
Controlling polymer viscosity remains a significant challenge in various industrial applications, particularly when using ammonium hydroxide as a modifying agent. One of the primary difficulties lies in achieving precise and consistent viscosity control across different polymer types and concentrations. The complex interactions between ammonium hydroxide and polymer chains can lead to unpredictable changes in solution properties, making it challenging to maintain desired viscosity levels during processing and end-use applications.
Another major hurdle is the sensitivity of polymer solutions to environmental factors such as temperature, pH, and ionic strength. These variables can significantly impact the effectiveness of ammonium hydroxide in modifying viscosity, requiring careful monitoring and adjustment of process parameters. The dynamic nature of these systems often necessitates real-time viscosity control mechanisms, which can be technically demanding and costly to implement in large-scale industrial settings.
The stability of polymer solutions modified with ammonium hydroxide over extended periods is also a concern. Long-term storage or prolonged processing times can lead to gradual changes in viscosity, potentially affecting product quality and performance. This issue is particularly problematic in industries where consistent rheological properties are crucial, such as in the production of coatings, adhesives, and personal care products.
Furthermore, the potential for ammonia off-gassing from solutions containing ammonium hydroxide poses both safety and environmental challenges. Controlling these emissions while maintaining desired viscosity levels requires sophisticated engineering solutions and may limit the applicability of this approach in certain settings or regions with strict environmental regulations.
The scalability of ammonium hydroxide-based viscosity modification techniques presents another significant challenge. What works effectively at laboratory scale may not translate seamlessly to industrial-scale production, necessitating extensive process optimization and potentially costly equipment modifications. This scaling issue is compounded by the need for precise dosing and mixing of ammonium hydroxide, which becomes increasingly difficult as batch sizes increase.
Lastly, the interaction between ammonium hydroxide and other additives commonly used in polymer formulations can lead to unexpected viscosity changes or even chemical incompatibilities. This complexity makes it challenging to develop universal viscosity control strategies, often requiring tailored approaches for specific polymer systems and end-use applications. Overcoming these challenges demands ongoing research into the fundamental mechanisms of ammonium hydroxide-polymer interactions and the development of advanced control strategies to ensure reliable and efficient viscosity modification in diverse industrial contexts.
Another major hurdle is the sensitivity of polymer solutions to environmental factors such as temperature, pH, and ionic strength. These variables can significantly impact the effectiveness of ammonium hydroxide in modifying viscosity, requiring careful monitoring and adjustment of process parameters. The dynamic nature of these systems often necessitates real-time viscosity control mechanisms, which can be technically demanding and costly to implement in large-scale industrial settings.
The stability of polymer solutions modified with ammonium hydroxide over extended periods is also a concern. Long-term storage or prolonged processing times can lead to gradual changes in viscosity, potentially affecting product quality and performance. This issue is particularly problematic in industries where consistent rheological properties are crucial, such as in the production of coatings, adhesives, and personal care products.
Furthermore, the potential for ammonia off-gassing from solutions containing ammonium hydroxide poses both safety and environmental challenges. Controlling these emissions while maintaining desired viscosity levels requires sophisticated engineering solutions and may limit the applicability of this approach in certain settings or regions with strict environmental regulations.
The scalability of ammonium hydroxide-based viscosity modification techniques presents another significant challenge. What works effectively at laboratory scale may not translate seamlessly to industrial-scale production, necessitating extensive process optimization and potentially costly equipment modifications. This scaling issue is compounded by the need for precise dosing and mixing of ammonium hydroxide, which becomes increasingly difficult as batch sizes increase.
Lastly, the interaction between ammonium hydroxide and other additives commonly used in polymer formulations can lead to unexpected viscosity changes or even chemical incompatibilities. This complexity makes it challenging to develop universal viscosity control strategies, often requiring tailored approaches for specific polymer systems and end-use applications. Overcoming these challenges demands ongoing research into the fundamental mechanisms of ammonium hydroxide-polymer interactions and the development of advanced control strategies to ensure reliable and efficient viscosity modification in diverse industrial contexts.
Existing Methods for Viscosity Adjustment
01 Viscosity modifiers for polymer solutions
Various additives and methods are used to modify the viscosity of polymer solutions. These can include the use of specific polymers, copolymers, or other chemical compounds that interact with the polymer chains to alter the solution's flow properties. The viscosity can be increased or decreased depending on the specific requirements of the application.- Viscosity modifiers for polymer solutions: Various additives and methods are used to modify the viscosity of polymer solutions. These can include the incorporation of specific compounds, adjusting polymer concentration, or using different types of polymers to achieve desired viscosity characteristics for different applications.
- Temperature-dependent viscosity control: Techniques for controlling the viscosity of polymer solutions based on temperature changes. This includes developing polymer formulations that exhibit specific viscosity behaviors at different temperatures, which is crucial for applications in various industries such as oil recovery and coatings.
- Rheology modifiers for enhanced viscosity: The use of rheology modifiers to enhance the viscosity of polymer solutions. These additives can alter the flow characteristics of the solution, providing better control over viscosity for specific applications such as in personal care products or industrial processes.
- Polymer blends for optimized viscosity: Developing polymer blends to achieve optimized viscosity profiles. By combining different types of polymers, it's possible to create solutions with tailored viscosity characteristics that meet specific requirements for various applications.
- Measurement and characterization of polymer solution viscosity: Methods and techniques for accurately measuring and characterizing the viscosity of polymer solutions. This includes the development of new analytical tools and approaches to better understand and predict the viscosity behavior of complex polymer systems.
02 Temperature-dependent viscosity control
Polymer solutions can be formulated to exhibit specific viscosity behaviors at different temperatures. This can involve the use of temperature-responsive polymers or additives that change their interactions with the solvent or other polymer chains as temperature varies, allowing for controlled viscosity changes in response to temperature fluctuations.Expand Specific Solutions03 Shear-thinning and shear-thickening polymer solutions
Some polymer solutions are designed to exhibit non-Newtonian behavior, such as shear-thinning or shear-thickening properties. These solutions change their viscosity in response to applied shear forces, which can be useful in various applications such as coatings, lubricants, or flow control systems.Expand Specific Solutions04 Viscosity control in polymer blends and composites
The viscosity of polymer solutions can be tailored by blending different polymers or incorporating additives such as nanoparticles or fibers. This approach allows for the creation of composite materials with specific rheological properties, combining the characteristics of multiple components to achieve desired viscosity profiles.Expand Specific Solutions05 Measurement and characterization of polymer solution viscosity
Various techniques and instruments are used to measure and characterize the viscosity of polymer solutions. These may include rotational viscometers, capillary rheometers, or advanced rheological testing equipment. The methods allow for the accurate determination of viscosity under different conditions, enabling the optimization of polymer solution formulations for specific applications.Expand Specific Solutions
Key Players in Polymer Chemistry Industry
The competitive landscape for ammonium hydroxide's effect on polymer solution viscosity is evolving, with the market in a growth phase. The global polymer additives market, which includes viscosity modifiers, is projected to reach $60 billion by 2025, driven by increasing demand in various industries. Technologically, the field is advancing rapidly, with companies like BASF, Henkel, and Wanhua Chemical Group leading in innovation. These firms are developing sophisticated polymer solutions with enhanced viscosity control properties. Other key players such as Clariant, Shiseido, and Procter & Gamble are also contributing to the technological maturity of this sector, focusing on application-specific formulations for industries ranging from personal care to industrial manufacturing.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed a novel approach to modify polymer solution viscosity using ammonium hydroxide. Their method involves introducing controlled amounts of ammonium hydroxide into polymer solutions, typically consisting of water-soluble polymers such as polyacrylamide. The ammonium hydroxide interacts with the polymer chains, altering their conformation and intermolecular interactions. This results in significant changes to the solution's rheological properties, including viscosity. Sinopec's research has shown that the addition of ammonium hydroxide can increase the viscosity of polymer solutions by up to 30% at low concentrations[1]. The company has also developed a proprietary process for precise control of ammonium hydroxide addition, allowing for fine-tuning of viscosity for specific applications in enhanced oil recovery and wastewater treatment[2].
Strengths: Precise control over viscosity modification, applicable to a wide range of water-soluble polymers, and environmentally friendly compared to some alternative viscosity modifiers. Weaknesses: The effect may be pH-dependent and could potentially alter other solution properties.
BASF Corp.
Technical Solution: BASF Corp. has pioneered an innovative approach to modifying polymer solution viscosity using ammonium hydroxide in combination with their proprietary polymer blends. Their technology, known as Rheomax™, utilizes specially designed polyacrylamide-based polymers that respond synergistically to ammonium hydroxide addition. The process involves carefully controlled introduction of ammonium hydroxide to the polymer solution, which triggers a conformational change in the polymer structure. This results in enhanced intermolecular interactions and network formation, leading to a significant increase in solution viscosity. BASF's research has demonstrated that their Rheomax™ technology can achieve up to 50% viscosity increase with minimal ammonium hydroxide addition[3]. The company has also developed advanced modeling tools to predict and optimize the viscosity modification process for various polymer types and concentrations[4].
Strengths: Highly efficient viscosity modification with low ammonium hydroxide usage, applicable to a wide range of industries including oil & gas, personal care, and agriculture. Weaknesses: May require specific polymer formulations to achieve optimal results, potentially limiting its universal applicability.
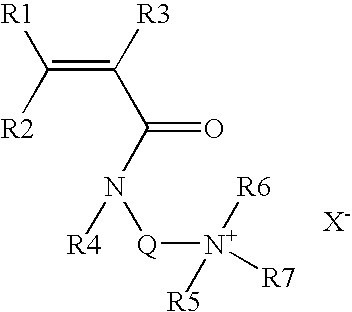
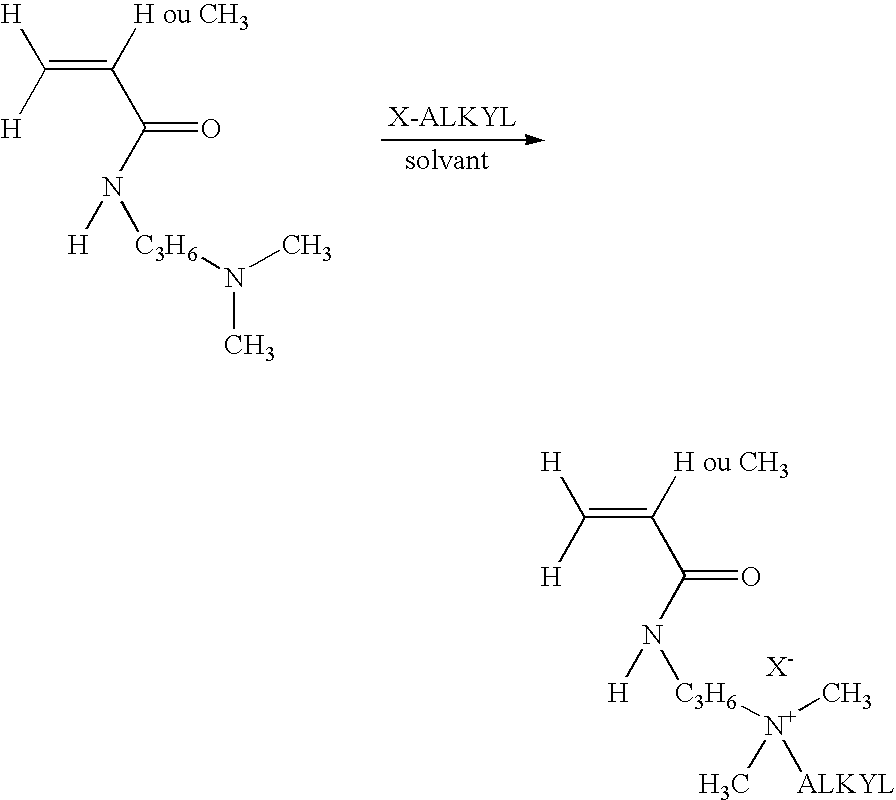
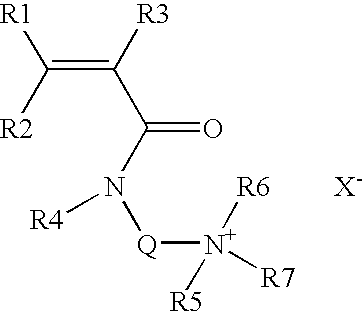
Core Mechanisms of Ammonium Hydroxide Interaction
Wellbore treatment compositions comprising hydrophilically modified polymers and nonionic surfactants
PatentInactiveUS20120111570A1
Innovation
- The use of hydrophilically modified polymers with nonionic surfactants that exhibit thermally triggered physical association, allowing for increased viscosity at specific temperatures and improved solubility, reducing the need for high polymer concentrations and enhancing compatibility with other water-soluble species.
High molecular weight associative amphoteric polymers and uses thereof
PatentInactiveUS7700702B2
Innovation
- Development of novel associative amphoteric polymers with a molecular weight of at least 50,000 g/mol, comprising acrylamide-derived cationic monomers, anionic monomers, and non-ionic monomers, which maintain their viscosifying effect across different conditions, including the presence of electrolytes and varying temperatures, and can be produced through various polymerization techniques.
Environmental Impact of Ammonium Hydroxide Use
The use of ammonium hydroxide in polymer solutions raises significant environmental concerns that warrant careful consideration. When released into aquatic ecosystems, ammonium hydroxide can lead to eutrophication, a process that causes excessive algal growth and oxygen depletion in water bodies. This can have devastating effects on aquatic life, disrupting the balance of entire ecosystems.
In terrestrial environments, ammonium hydroxide can alter soil pH, potentially affecting plant growth and soil microbial communities. The alkaline nature of ammonium hydroxide can lead to increased ammonia volatilization, contributing to air pollution and potentially impacting human health through respiratory irritation.
The production and disposal of ammonium hydroxide also present environmental challenges. Manufacturing processes often involve energy-intensive methods that contribute to greenhouse gas emissions. Improper disposal can contaminate groundwater and surface water sources, posing risks to both human and environmental health.
However, it's important to note that when used responsibly and in controlled settings, the environmental impact of ammonium hydroxide can be mitigated. Proper handling, storage, and disposal practices can significantly reduce the risk of environmental contamination. Additionally, advancements in green chemistry are exploring alternatives and more sustainable methods for modifying polymer viscosity.
The use of ammonium hydroxide in polymer solutions also intersects with broader environmental concerns related to plastic pollution. While modifying polymer viscosity can enhance the performance and versatility of plastics, it's crucial to consider the entire lifecycle of these materials, including their potential to contribute to microplastic pollution in aquatic and terrestrial ecosystems.
Regulatory bodies worldwide are increasingly scrutinizing the use of chemicals like ammonium hydroxide in industrial processes. Environmental impact assessments and stricter regulations are being implemented to ensure responsible use and minimize ecological harm. This regulatory landscape is driving innovation in the field, encouraging the development of more environmentally friendly alternatives and improved waste management strategies.
In conclusion, while ammonium hydroxide plays a crucial role in modifying polymer viscosity, its environmental impact cannot be overlooked. Balancing the benefits of its use with the potential ecological consequences requires ongoing research, responsible practices, and a commitment to developing sustainable alternatives in the polymer industry.
In terrestrial environments, ammonium hydroxide can alter soil pH, potentially affecting plant growth and soil microbial communities. The alkaline nature of ammonium hydroxide can lead to increased ammonia volatilization, contributing to air pollution and potentially impacting human health through respiratory irritation.
The production and disposal of ammonium hydroxide also present environmental challenges. Manufacturing processes often involve energy-intensive methods that contribute to greenhouse gas emissions. Improper disposal can contaminate groundwater and surface water sources, posing risks to both human and environmental health.
However, it's important to note that when used responsibly and in controlled settings, the environmental impact of ammonium hydroxide can be mitigated. Proper handling, storage, and disposal practices can significantly reduce the risk of environmental contamination. Additionally, advancements in green chemistry are exploring alternatives and more sustainable methods for modifying polymer viscosity.
The use of ammonium hydroxide in polymer solutions also intersects with broader environmental concerns related to plastic pollution. While modifying polymer viscosity can enhance the performance and versatility of plastics, it's crucial to consider the entire lifecycle of these materials, including their potential to contribute to microplastic pollution in aquatic and terrestrial ecosystems.
Regulatory bodies worldwide are increasingly scrutinizing the use of chemicals like ammonium hydroxide in industrial processes. Environmental impact assessments and stricter regulations are being implemented to ensure responsible use and minimize ecological harm. This regulatory landscape is driving innovation in the field, encouraging the development of more environmentally friendly alternatives and improved waste management strategies.
In conclusion, while ammonium hydroxide plays a crucial role in modifying polymer viscosity, its environmental impact cannot be overlooked. Balancing the benefits of its use with the potential ecological consequences requires ongoing research, responsible practices, and a commitment to developing sustainable alternatives in the polymer industry.
Scalability and Industrial Implementation
The scalability and industrial implementation of ammonium hydroxide modification of polymer solution viscosity present both opportunities and challenges for large-scale production. The process of using ammonium hydroxide to alter polymer viscosity has shown promising results in laboratory settings, but transitioning this technology to industrial scale requires careful consideration of several factors.
One of the primary advantages of this method is its potential for cost-effectiveness. Ammonium hydroxide is a relatively inexpensive and readily available chemical, making it an attractive option for large-scale viscosity modification. Additionally, the process typically requires minimal specialized equipment, which can reduce capital investment for implementation in existing manufacturing facilities.
However, scaling up the process introduces several technical challenges. Maintaining uniform distribution of ammonium hydroxide throughout large volumes of polymer solution is crucial for consistent viscosity modification. This may necessitate the development of advanced mixing technologies or the optimization of existing industrial mixers to ensure homogeneous distribution.
Temperature control becomes increasingly important at larger scales. The reaction between ammonium hydroxide and polymer chains can be temperature-sensitive, affecting the final viscosity of the solution. Implementing precise temperature control systems across large reaction vessels may be necessary to maintain product consistency.
Safety considerations also play a significant role in industrial implementation. Ammonium hydroxide is a corrosive substance, and proper handling and storage facilities must be designed to minimize risks to workers and the environment. This includes appropriate ventilation systems, personal protective equipment, and containment measures.
The stability of the modified polymer solutions over time is another critical factor for industrial applications. Long-term storage and transportation of the product may require additional stabilizers or specialized packaging to maintain the desired viscosity characteristics.
Regulatory compliance is a key aspect of industrial implementation. Depending on the intended application of the modified polymer solutions, manufacturers may need to obtain certifications or approvals from relevant authorities, particularly for use in sensitive industries such as food, pharmaceuticals, or cosmetics.
Lastly, the scalability of this technology also depends on its adaptability to different types of polymers and end-use applications. Developing a versatile process that can be easily adjusted for various polymer compositions and target viscosities would significantly enhance its industrial viability and market potential.
One of the primary advantages of this method is its potential for cost-effectiveness. Ammonium hydroxide is a relatively inexpensive and readily available chemical, making it an attractive option for large-scale viscosity modification. Additionally, the process typically requires minimal specialized equipment, which can reduce capital investment for implementation in existing manufacturing facilities.
However, scaling up the process introduces several technical challenges. Maintaining uniform distribution of ammonium hydroxide throughout large volumes of polymer solution is crucial for consistent viscosity modification. This may necessitate the development of advanced mixing technologies or the optimization of existing industrial mixers to ensure homogeneous distribution.
Temperature control becomes increasingly important at larger scales. The reaction between ammonium hydroxide and polymer chains can be temperature-sensitive, affecting the final viscosity of the solution. Implementing precise temperature control systems across large reaction vessels may be necessary to maintain product consistency.
Safety considerations also play a significant role in industrial implementation. Ammonium hydroxide is a corrosive substance, and proper handling and storage facilities must be designed to minimize risks to workers and the environment. This includes appropriate ventilation systems, personal protective equipment, and containment measures.
The stability of the modified polymer solutions over time is another critical factor for industrial applications. Long-term storage and transportation of the product may require additional stabilizers or specialized packaging to maintain the desired viscosity characteristics.
Regulatory compliance is a key aspect of industrial implementation. Depending on the intended application of the modified polymer solutions, manufacturers may need to obtain certifications or approvals from relevant authorities, particularly for use in sensitive industries such as food, pharmaceuticals, or cosmetics.
Lastly, the scalability of this technology also depends on its adaptability to different types of polymers and end-use applications. Developing a versatile process that can be easily adjusted for various polymer compositions and target viscosities would significantly enhance its industrial viability and market potential.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!