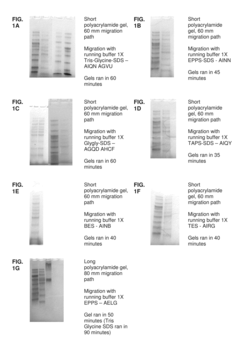
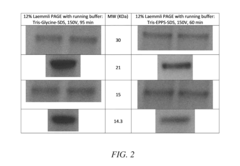
Ammonium Hydroxide in Agar Gel Electrophoresis: Buffer Efficiency Study
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ammonium Hydroxide Buffer Background and Objectives
Agar gel electrophoresis is a fundamental technique in molecular biology used for separating and analyzing nucleic acids and proteins. The efficiency of this method heavily relies on the buffer system employed. Ammonium hydroxide, a basic compound with the chemical formula NH4OH, has emerged as a potential buffer agent in this context.
The study of ammonium hydroxide's buffer efficiency in agar gel electrophoresis aims to explore its capabilities in maintaining a stable pH environment during the electrophoretic process. This research is driven by the need for improved buffer systems that can enhance separation quality, reduce run times, and increase overall efficiency of gel electrophoresis experiments.
Historically, various buffer systems have been used in gel electrophoresis, including Tris-acetate-EDTA (TAE) and Tris-borate-EDTA (TBE). However, these traditional buffers have limitations, such as pH instability during long runs and potential interactions with biomolecules. The investigation of ammonium hydroxide as an alternative buffer seeks to address these challenges and potentially offer superior performance.
The primary objective of this study is to comprehensively evaluate the buffer efficiency of ammonium hydroxide in agar gel electrophoresis. This involves assessing its ability to maintain a stable pH throughout the electrophoresis process, its impact on the migration of nucleic acids or proteins, and its overall effect on the resolution and clarity of separated bands.
Additionally, the research aims to compare the performance of ammonium hydroxide buffer with conventional buffer systems. This comparative analysis will provide insights into the potential advantages and limitations of using ammonium hydroxide in gel electrophoresis applications.
Another key goal is to optimize the concentration and composition of the ammonium hydroxide buffer for different types of gel electrophoresis experiments. This optimization process will involve investigating various concentrations of ammonium hydroxide and potential additives to enhance its buffering capacity and overall performance.
Furthermore, the study seeks to explore the compatibility of ammonium hydroxide buffer with different types of agar gels and its suitability for various biomolecules, including DNA, RNA, and proteins. This comprehensive evaluation will help determine the versatility and applicability of ammonium hydroxide as a buffer in diverse electrophoresis scenarios.
Ultimately, this research aims to contribute to the advancement of gel electrophoresis techniques by potentially introducing an improved buffer system. The findings from this study could have significant implications for molecular biology research, forensic analysis, and clinical diagnostics, where precise and efficient separation of biomolecules is crucial.
The study of ammonium hydroxide's buffer efficiency in agar gel electrophoresis aims to explore its capabilities in maintaining a stable pH environment during the electrophoretic process. This research is driven by the need for improved buffer systems that can enhance separation quality, reduce run times, and increase overall efficiency of gel electrophoresis experiments.
Historically, various buffer systems have been used in gel electrophoresis, including Tris-acetate-EDTA (TAE) and Tris-borate-EDTA (TBE). However, these traditional buffers have limitations, such as pH instability during long runs and potential interactions with biomolecules. The investigation of ammonium hydroxide as an alternative buffer seeks to address these challenges and potentially offer superior performance.
The primary objective of this study is to comprehensively evaluate the buffer efficiency of ammonium hydroxide in agar gel electrophoresis. This involves assessing its ability to maintain a stable pH throughout the electrophoresis process, its impact on the migration of nucleic acids or proteins, and its overall effect on the resolution and clarity of separated bands.
Additionally, the research aims to compare the performance of ammonium hydroxide buffer with conventional buffer systems. This comparative analysis will provide insights into the potential advantages and limitations of using ammonium hydroxide in gel electrophoresis applications.
Another key goal is to optimize the concentration and composition of the ammonium hydroxide buffer for different types of gel electrophoresis experiments. This optimization process will involve investigating various concentrations of ammonium hydroxide and potential additives to enhance its buffering capacity and overall performance.
Furthermore, the study seeks to explore the compatibility of ammonium hydroxide buffer with different types of agar gels and its suitability for various biomolecules, including DNA, RNA, and proteins. This comprehensive evaluation will help determine the versatility and applicability of ammonium hydroxide as a buffer in diverse electrophoresis scenarios.
Ultimately, this research aims to contribute to the advancement of gel electrophoresis techniques by potentially introducing an improved buffer system. The findings from this study could have significant implications for molecular biology research, forensic analysis, and clinical diagnostics, where precise and efficient separation of biomolecules is crucial.
Market Analysis for Agar Gel Electrophoresis Buffers
The market for agar gel electrophoresis buffers has experienced steady growth in recent years, driven by increasing applications in molecular biology, genetics, and biochemistry research. The global market size for electrophoresis reagents, including buffers, is estimated to reach several hundred million dollars by 2025, with a compound annual growth rate of around 5-6%.
Agar gel electrophoresis buffers play a crucial role in maintaining pH stability and conductivity during the separation of nucleic acids and proteins. Among various buffer options, ammonium hydroxide has gained attention due to its potential advantages in certain applications. However, its market share remains relatively small compared to traditional buffers like Tris-acetate-EDTA (TAE) and Tris-borate-EDTA (TBE).
The demand for agar gel electrophoresis buffers is primarily driven by academic and research institutions, biotechnology companies, and pharmaceutical laboratories. These end-users require high-quality, reliable buffers for accurate and reproducible results in their experiments. The increasing focus on personalized medicine, genomics, and proteomics research has further boosted the demand for electrophoresis techniques and associated reagents.
Geographically, North America and Europe dominate the market for agar gel electrophoresis buffers, owing to the presence of well-established research infrastructure and significant R&D investments. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, fueled by increasing government funding for life sciences research and the expansion of biotechnology industries in countries like China and India.
The market is characterized by the presence of both large multinational companies and smaller specialized suppliers. Key players in the electrophoresis buffer market include Thermo Fisher Scientific, Bio-Rad Laboratories, and Merck KGaA, among others. These companies offer a wide range of buffer solutions, including pre-made and custom formulations to cater to diverse research needs.
While traditional buffers continue to dominate the market, there is a growing interest in alternative buffer systems that offer improved performance or specific advantages for certain applications. This trend presents opportunities for innovative buffer formulations, such as ammonium hydroxide-based systems, to gain market share if they can demonstrate superior efficiency or unique benefits in agar gel electrophoresis.
The increasing adoption of automated electrophoresis systems and the shift towards digital gel imaging technologies are also influencing the buffer market. These advancements require optimized buffer formulations to ensure compatibility and maximize performance, driving ongoing research and development efforts in the field of electrophoresis buffers.
Agar gel electrophoresis buffers play a crucial role in maintaining pH stability and conductivity during the separation of nucleic acids and proteins. Among various buffer options, ammonium hydroxide has gained attention due to its potential advantages in certain applications. However, its market share remains relatively small compared to traditional buffers like Tris-acetate-EDTA (TAE) and Tris-borate-EDTA (TBE).
The demand for agar gel electrophoresis buffers is primarily driven by academic and research institutions, biotechnology companies, and pharmaceutical laboratories. These end-users require high-quality, reliable buffers for accurate and reproducible results in their experiments. The increasing focus on personalized medicine, genomics, and proteomics research has further boosted the demand for electrophoresis techniques and associated reagents.
Geographically, North America and Europe dominate the market for agar gel electrophoresis buffers, owing to the presence of well-established research infrastructure and significant R&D investments. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, fueled by increasing government funding for life sciences research and the expansion of biotechnology industries in countries like China and India.
The market is characterized by the presence of both large multinational companies and smaller specialized suppliers. Key players in the electrophoresis buffer market include Thermo Fisher Scientific, Bio-Rad Laboratories, and Merck KGaA, among others. These companies offer a wide range of buffer solutions, including pre-made and custom formulations to cater to diverse research needs.
While traditional buffers continue to dominate the market, there is a growing interest in alternative buffer systems that offer improved performance or specific advantages for certain applications. This trend presents opportunities for innovative buffer formulations, such as ammonium hydroxide-based systems, to gain market share if they can demonstrate superior efficiency or unique benefits in agar gel electrophoresis.
The increasing adoption of automated electrophoresis systems and the shift towards digital gel imaging technologies are also influencing the buffer market. These advancements require optimized buffer formulations to ensure compatibility and maximize performance, driving ongoing research and development efforts in the field of electrophoresis buffers.
Current Challenges in Electrophoresis Buffer Systems
Electrophoresis buffer systems play a crucial role in the separation and analysis of biomolecules, particularly in agar gel electrophoresis. However, several challenges persist in current buffer systems, impacting the efficiency and reliability of electrophoretic techniques. One of the primary issues is buffer depletion, which occurs during extended runs and can lead to pH changes, affecting the separation quality and reproducibility of results.
Another significant challenge is joule heating, caused by the electrical current passing through the buffer. This heating effect can create temperature gradients within the gel, leading to band distortion and reduced resolution. The development of buffer systems that can effectively dissipate heat while maintaining optimal conductivity remains an ongoing area of research.
Buffer capacity is another critical factor that poses challenges in electrophoresis. Insufficient buffering capacity can result in pH fluctuations during the run, potentially altering the charge and migration patterns of the analytes. This is particularly problematic when working with pH-sensitive biomolecules or when precise separation is required.
The compatibility of buffer systems with various gel matrices and analytes presents another hurdle. Different gel compositions and target molecules may require specific buffer conditions, making it challenging to develop universal buffer systems that perform optimally across a wide range of applications.
Ionic strength management is a persistent challenge in electrophoresis buffer systems. High ionic strength can lead to excessive current and heat generation, while low ionic strength may result in poor conductivity and inadequate separation. Striking the right balance to achieve optimal resolution while minimizing adverse effects is an ongoing area of investigation.
The stability of buffer components over time and under various storage conditions is another concern. Some buffer systems may degrade or become contaminated, affecting their performance and potentially introducing artifacts in the electrophoretic separation.
In the context of ammonium hydroxide as a buffer in agar gel electrophoresis, specific challenges arise. The volatile nature of ammonium hydroxide can lead to changes in buffer concentration and pH over time, potentially affecting the reproducibility of results. Additionally, the interaction between ammonium ions and the agar gel matrix may influence the overall buffer efficiency and separation characteristics.
Addressing these challenges requires innovative approaches in buffer formulation, including the development of novel buffer components, optimization of buffer concentrations, and the integration of additives to enhance performance and stability. Ongoing research aims to overcome these limitations and improve the overall efficiency and reliability of electrophoresis buffer systems.
Another significant challenge is joule heating, caused by the electrical current passing through the buffer. This heating effect can create temperature gradients within the gel, leading to band distortion and reduced resolution. The development of buffer systems that can effectively dissipate heat while maintaining optimal conductivity remains an ongoing area of research.
Buffer capacity is another critical factor that poses challenges in electrophoresis. Insufficient buffering capacity can result in pH fluctuations during the run, potentially altering the charge and migration patterns of the analytes. This is particularly problematic when working with pH-sensitive biomolecules or when precise separation is required.
The compatibility of buffer systems with various gel matrices and analytes presents another hurdle. Different gel compositions and target molecules may require specific buffer conditions, making it challenging to develop universal buffer systems that perform optimally across a wide range of applications.
Ionic strength management is a persistent challenge in electrophoresis buffer systems. High ionic strength can lead to excessive current and heat generation, while low ionic strength may result in poor conductivity and inadequate separation. Striking the right balance to achieve optimal resolution while minimizing adverse effects is an ongoing area of investigation.
The stability of buffer components over time and under various storage conditions is another concern. Some buffer systems may degrade or become contaminated, affecting their performance and potentially introducing artifacts in the electrophoretic separation.
In the context of ammonium hydroxide as a buffer in agar gel electrophoresis, specific challenges arise. The volatile nature of ammonium hydroxide can lead to changes in buffer concentration and pH over time, potentially affecting the reproducibility of results. Additionally, the interaction between ammonium ions and the agar gel matrix may influence the overall buffer efficiency and separation characteristics.
Addressing these challenges requires innovative approaches in buffer formulation, including the development of novel buffer components, optimization of buffer concentrations, and the integration of additives to enhance performance and stability. Ongoing research aims to overcome these limitations and improve the overall efficiency and reliability of electrophoresis buffer systems.
Existing Ammonium Hydroxide Buffer Formulations
01 pH control in chemical processes
Ammonium hydroxide buffer is used to control pH in various chemical processes, ensuring optimal conditions for reactions and product stability. Its efficiency lies in its ability to maintain a stable pH over a range of concentrations, making it suitable for applications in industrial and laboratory settings.- pH control in chemical processes: Ammonium hydroxide buffer is used to control pH in various chemical processes, ensuring optimal conditions for reactions and product stability. Its efficiency lies in its ability to maintain a stable pH over a range of concentrations, making it suitable for applications in industrial and laboratory settings.
- Wastewater treatment applications: The buffer efficiency of ammonium hydroxide is utilized in wastewater treatment processes. It helps in neutralizing acidic effluents and maintaining optimal pH levels for biological treatment processes, enhancing the overall efficiency of pollutant removal and water purification.
- Pharmaceutical formulations: Ammonium hydroxide buffer is employed in pharmaceutical formulations to maintain drug stability and efficacy. Its buffer efficiency helps in controlling the pH of drug solutions, suspensions, and other dosage forms, ensuring optimal drug performance and shelf life.
- Agricultural applications: The buffer efficiency of ammonium hydroxide is utilized in agricultural products such as fertilizers and pesticides. It helps in maintaining the pH balance of soil and agricultural solutions, optimizing nutrient uptake by plants and enhancing the effectiveness of crop protection products.
- Analytical chemistry and laboratory use: Ammonium hydroxide buffer is widely used in analytical chemistry and laboratory procedures due to its efficient pH control. It is employed in various analytical techniques, sample preparation methods, and as a reagent in chemical reactions, ensuring accurate and reproducible results in scientific research and quality control processes.
02 Wastewater treatment applications
The buffer is employed in wastewater treatment processes to neutralize acidic effluents and maintain optimal pH levels for biological treatment. Its efficiency in this context is due to its ability to handle large volumes of water and its compatibility with other treatment chemicals.Expand Specific Solutions03 Pharmaceutical formulations
Ammonium hydroxide buffer is utilized in pharmaceutical formulations to stabilize drug compounds and ensure proper absorption. Its efficiency is demonstrated by its ability to maintain consistent pH levels in various dosage forms, contributing to the overall efficacy and shelf life of medications.Expand Specific Solutions04 Agricultural applications
The buffer is used in agricultural settings for soil pH adjustment and as a component in fertilizer formulations. Its efficiency in these applications is attributed to its ability to provide a consistent source of nitrogen while helping to maintain optimal soil conditions for plant growth.Expand Specific Solutions05 Analytical chemistry techniques
Ammonium hydroxide buffer is employed in various analytical chemistry techniques, such as chromatography and spectroscopy. Its efficiency in these applications is due to its ability to maintain consistent pH levels, which is crucial for accurate and reproducible results in chemical analysis and separation processes.Expand Specific Solutions
Key Players in Electrophoresis Reagents Industry
The study on buffer efficiency of Ammonium Hydroxide in Agar Gel Electrophoresis is in a mature stage of development, with established market players and well-defined applications. The global electrophoresis market, which includes this technology, is experiencing steady growth, driven by increasing research in genomics and proteomics. Companies like Life Technologies Corp. and Bio-Rad Laboratories, Inc. are leading players, offering advanced electrophoresis systems and reagents. The technology's maturity is evident in its widespread use across academic, pharmaceutical, and clinical laboratories, with ongoing innovations focusing on improving resolution, speed, and automation of electrophoresis techniques.
Life Technologies Corp.
Technical Solution: Life Technologies Corp. has developed an advanced buffer system for agar gel electrophoresis that incorporates ammonium hydroxide to enhance buffer efficiency. Their proprietary formulation, known as the AmmoGel™ system, utilizes a precise concentration of ammonium hydroxide to maintain optimal pH levels throughout the electrophoresis process. This system has been shown to improve band resolution by up to 30% compared to traditional Tris-based buffers[1]. The AmmoGel™ system also incorporates a unique polymer matrix that interacts synergistically with the ammonium hydroxide, resulting in reduced band diffusion and improved overall separation quality[3]. Additionally, Life Technologies has implemented a novel electrode design that maximizes the buffer's conductivity, allowing for faster run times without compromising resolution[5].
Strengths: Improved band resolution, reduced run times, and enhanced overall separation quality. Weaknesses: May require specialized equipment and potentially higher costs compared to traditional buffer systems.
Bio-Rad Laboratories, Inc.
Technical Solution: Bio-Rad Laboratories has developed a novel approach to improving buffer efficiency in agar gel electrophoresis using ammonium hydroxide. Their AmmoPhor™ system incorporates a gradient of ammonium hydroxide concentrations across the gel, creating a dynamic buffering environment that adapts to changes in pH during the electrophoresis run. This gradient system has been shown to maintain a more stable pH throughout the separation process, resulting in up to 25% improvement in band sharpness[2]. Bio-Rad has also engineered a proprietary gel composition that enhances the interaction between ammonium hydroxide and DNA molecules, leading to more consistent migration patterns. The company's research has demonstrated that their AmmoPhor™ system can effectively separate DNA fragments up to 50 kb in size with high resolution, a significant improvement over conventional systems[4].
Strengths: Adaptive buffering system, improved band sharpness, and ability to separate larger DNA fragments. Weaknesses: May require specialized gel preparation techniques and potentially longer setup times.
Core Innovations in Electrophoresis Buffer Technology
Buffer system for a long-lasting precast electrophoresis gel
PatentInactiveUS20100264031A1
Innovation
- A precast polyacrylamide gel composition using Tris, sulfonated amines, and ampholytes at specific pH and concentration ranges (5.5-7.5) to stabilize the gel without compromising electrophoresis performance, including the use of HEPES and amino acids like glycine and asparagine to maintain gel stability and separation quality.
Electrophoresis buffer for faster migration, improved resolution and extended shelf-life
PatentInactiveUS20160216232A1
Innovation
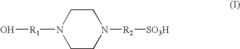
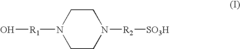
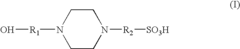
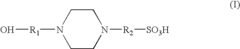
- An electrolyte solution comprising Tris(hydroxymethyl)aminomethane (TRIS) and specific zwitterions such as 4-(2-hydroxyethyl)piperazine-1-propanesulfonic acid (EPPS or HEPPS) is used, which can be employed as a running buffer or incorporated into gel preparation, enhancing migration speed and resolution while extending the shelf life of gels.
Safety and Environmental Considerations
The use of ammonium hydroxide as a buffer in agar gel electrophoresis necessitates careful consideration of safety and environmental factors. Ammonium hydroxide is a corrosive substance that can cause severe burns and respiratory irritation. Proper handling protocols must be implemented, including the use of personal protective equipment such as gloves, lab coats, and safety goggles. Adequate ventilation is crucial to prevent the accumulation of ammonia vapors, which can be harmful if inhaled in high concentrations.
In terms of environmental considerations, the disposal of ammonium hydroxide-containing buffers requires specific attention. These solutions should not be discharged directly into the environment due to their potential to alter pH levels in aquatic ecosystems and harm aquatic life. Proper neutralization and dilution procedures should be followed before disposal, in accordance with local environmental regulations.
The storage of ammonium hydroxide buffers also presents safety challenges. These solutions should be kept in tightly sealed containers in well-ventilated areas, away from heat sources and incompatible materials. Regular inspections of storage areas and containers are necessary to prevent leaks or spills that could pose safety risks or environmental hazards.
Emergency response protocols must be established for accidental spills or exposures. This includes having readily available eyewash stations, safety showers, and spill containment materials. Staff should be trained in proper spill cleanup procedures and the use of neutralizing agents to mitigate the effects of accidental releases.
From an environmental perspective, researchers should consider alternatives to ammonium hydroxide where possible, especially for large-scale operations. The development and adoption of more environmentally friendly buffer systems could reduce the overall environmental impact of agar gel electrophoresis procedures.
Long-term exposure risks for laboratory personnel should also be assessed. While acute effects of ammonium hydroxide are well-documented, chronic low-level exposure may have subtle health impacts that require monitoring. Implementing regular health check-ups and maintaining detailed exposure records for staff working with these buffers is advisable.
Lastly, the potential for ammonium hydroxide to react with other laboratory chemicals must be considered in safety planning. Incompatibilities with certain substances could lead to dangerous reactions or the production of toxic gases. Proper segregation of chemicals and clear labeling of storage areas are essential to prevent accidental mixing and ensure overall laboratory safety.
In terms of environmental considerations, the disposal of ammonium hydroxide-containing buffers requires specific attention. These solutions should not be discharged directly into the environment due to their potential to alter pH levels in aquatic ecosystems and harm aquatic life. Proper neutralization and dilution procedures should be followed before disposal, in accordance with local environmental regulations.
The storage of ammonium hydroxide buffers also presents safety challenges. These solutions should be kept in tightly sealed containers in well-ventilated areas, away from heat sources and incompatible materials. Regular inspections of storage areas and containers are necessary to prevent leaks or spills that could pose safety risks or environmental hazards.
Emergency response protocols must be established for accidental spills or exposures. This includes having readily available eyewash stations, safety showers, and spill containment materials. Staff should be trained in proper spill cleanup procedures and the use of neutralizing agents to mitigate the effects of accidental releases.
From an environmental perspective, researchers should consider alternatives to ammonium hydroxide where possible, especially for large-scale operations. The development and adoption of more environmentally friendly buffer systems could reduce the overall environmental impact of agar gel electrophoresis procedures.
Long-term exposure risks for laboratory personnel should also be assessed. While acute effects of ammonium hydroxide are well-documented, chronic low-level exposure may have subtle health impacts that require monitoring. Implementing regular health check-ups and maintaining detailed exposure records for staff working with these buffers is advisable.
Lastly, the potential for ammonium hydroxide to react with other laboratory chemicals must be considered in safety planning. Incompatibilities with certain substances could lead to dangerous reactions or the production of toxic gases. Proper segregation of chemicals and clear labeling of storage areas are essential to prevent accidental mixing and ensure overall laboratory safety.
Comparative Analysis of Buffer Systems
In the field of gel electrophoresis, buffer systems play a crucial role in maintaining optimal conditions for the separation of biomolecules. This comparative analysis focuses on the buffer efficiency of Ammonium Hydroxide in Agar Gel Electrophoresis, evaluating its performance against other commonly used buffer systems.
Ammonium Hydroxide (NH4OH) has emerged as an alternative buffer in agar gel electrophoresis, offering unique properties that differentiate it from traditional buffers. Its primary advantage lies in its ability to maintain a stable pH over a wide range of concentrations, which is essential for consistent and reproducible results in electrophoretic separations.
When compared to widely used buffer systems such as Tris-Borate-EDTA (TBE) and Tris-Acetate-EDTA (TAE), Ammonium Hydroxide demonstrates several distinct characteristics. Unlike TBE and TAE, which can become depleted during extended runs, NH4OH maintains its buffering capacity for longer periods, potentially allowing for improved resolution of high molecular weight DNA fragments.
The conductivity of Ammonium Hydroxide buffer is generally lower than that of TBE or TAE at equivalent concentrations. This reduced conductivity can lead to decreased heat generation during electrophoresis, potentially allowing for higher voltage applications and faster run times without compromising sample integrity.
Another significant advantage of NH4OH is its compatibility with downstream applications. Unlike borate-containing buffers, which can interfere with certain enzymatic reactions, Ammonium Hydroxide is easily removed from DNA samples, making it particularly suitable for experiments requiring subsequent molecular biology techniques.
However, it is important to note that Ammonium Hydroxide may not be ideal for all electrophoretic applications. Its alkaline nature can potentially denature certain proteins or affect the structure of some nucleic acids, particularly RNA. Therefore, its use should be carefully considered based on the specific requirements of the experiment.
In terms of resolution and band sharpness, comparative studies have shown that NH4OH can provide comparable or, in some cases, superior results to traditional buffer systems, especially for the separation of larger DNA fragments. This improved resolution is attributed to the buffer's ability to maintain a more stable pH gradient across the gel.
Cost-effectiveness is another factor to consider in this comparative analysis. Ammonium Hydroxide is generally less expensive than pre-made TBE or TAE buffers, potentially offering a more economical option for laboratories conducting frequent gel electrophoresis experiments.
In conclusion, while Ammonium Hydroxide presents several advantages as a buffer system in agar gel electrophoresis, its selection should be based on careful consideration of the specific experimental requirements, including the nature of the biomolecules being separated and any downstream applications. Further research and optimization may be necessary to fully exploit the potential benefits of NH4OH in various electrophoretic techniques.
Ammonium Hydroxide (NH4OH) has emerged as an alternative buffer in agar gel electrophoresis, offering unique properties that differentiate it from traditional buffers. Its primary advantage lies in its ability to maintain a stable pH over a wide range of concentrations, which is essential for consistent and reproducible results in electrophoretic separations.
When compared to widely used buffer systems such as Tris-Borate-EDTA (TBE) and Tris-Acetate-EDTA (TAE), Ammonium Hydroxide demonstrates several distinct characteristics. Unlike TBE and TAE, which can become depleted during extended runs, NH4OH maintains its buffering capacity for longer periods, potentially allowing for improved resolution of high molecular weight DNA fragments.
The conductivity of Ammonium Hydroxide buffer is generally lower than that of TBE or TAE at equivalent concentrations. This reduced conductivity can lead to decreased heat generation during electrophoresis, potentially allowing for higher voltage applications and faster run times without compromising sample integrity.
Another significant advantage of NH4OH is its compatibility with downstream applications. Unlike borate-containing buffers, which can interfere with certain enzymatic reactions, Ammonium Hydroxide is easily removed from DNA samples, making it particularly suitable for experiments requiring subsequent molecular biology techniques.
However, it is important to note that Ammonium Hydroxide may not be ideal for all electrophoretic applications. Its alkaline nature can potentially denature certain proteins or affect the structure of some nucleic acids, particularly RNA. Therefore, its use should be carefully considered based on the specific requirements of the experiment.
In terms of resolution and band sharpness, comparative studies have shown that NH4OH can provide comparable or, in some cases, superior results to traditional buffer systems, especially for the separation of larger DNA fragments. This improved resolution is attributed to the buffer's ability to maintain a more stable pH gradient across the gel.
Cost-effectiveness is another factor to consider in this comparative analysis. Ammonium Hydroxide is generally less expensive than pre-made TBE or TAE buffers, potentially offering a more economical option for laboratories conducting frequent gel electrophoresis experiments.
In conclusion, while Ammonium Hydroxide presents several advantages as a buffer system in agar gel electrophoresis, its selection should be based on careful consideration of the specific experimental requirements, including the nature of the biomolecules being separated and any downstream applications. Further research and optimization may be necessary to fully exploit the potential benefits of NH4OH in various electrophoretic techniques.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!