Ammonium Hydroxide in Synthesis of Biodegradable Nanocarriers
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ammonium Hydroxide in Nanocarrier Synthesis: Background and Objectives
The use of ammonium hydroxide in the synthesis of biodegradable nanocarriers represents a significant advancement in the field of drug delivery and nanomedicine. This technology has evolved over the past few decades, driven by the increasing demand for targeted and controlled drug release systems. The journey began with the development of liposomes in the 1960s and has since progressed to include a wide array of nanocarrier types, including polymeric nanoparticles, dendrimers, and inorganic nanoparticles.
Ammonium hydroxide, a weak base, has emerged as a crucial component in the synthesis process of these nanocarriers. Its role is multifaceted, serving as a pH regulator, a catalyst for certain reactions, and a stabilizing agent. The use of ammonium hydroxide allows for better control over the size, shape, and surface properties of nanocarriers, which are critical factors in determining their efficacy and biodistribution.
The primary objective of incorporating ammonium hydroxide in nanocarrier synthesis is to enhance the biodegradability and biocompatibility of the resulting particles. This aligns with the broader goal of developing safer and more effective drug delivery systems that can minimize side effects and improve patient outcomes. Additionally, the use of ammonium hydroxide can potentially lead to more efficient encapsulation of therapeutic agents and improved release kinetics.
Recent technological advancements have focused on optimizing the use of ammonium hydroxide in nanocarrier synthesis. These efforts aim to achieve better control over particle size distribution, increase loading capacity, and improve the stability of nanocarriers in biological environments. Researchers are also exploring the potential of ammonium hydroxide to facilitate the development of stimuli-responsive nanocarriers that can release their payload under specific physiological conditions.
The evolution of this technology is closely tied to advancements in analytical techniques, such as dynamic light scattering, electron microscopy, and spectroscopic methods. These tools have enabled researchers to characterize nanocarriers with unprecedented precision, leading to a better understanding of the role of ammonium hydroxide in the synthesis process and its impact on the final product properties.
Looking ahead, the field is moving towards the development of more sophisticated, multifunctional nanocarriers that can simultaneously perform diagnostic and therapeutic functions. The use of ammonium hydroxide in these advanced systems is expected to play a crucial role in achieving the desired physicochemical properties and biological performance. As research progresses, the goal is to translate these technological advancements into clinically viable products that can revolutionize the treatment of various diseases, particularly in the areas of cancer therapy and gene delivery.
Ammonium hydroxide, a weak base, has emerged as a crucial component in the synthesis process of these nanocarriers. Its role is multifaceted, serving as a pH regulator, a catalyst for certain reactions, and a stabilizing agent. The use of ammonium hydroxide allows for better control over the size, shape, and surface properties of nanocarriers, which are critical factors in determining their efficacy and biodistribution.
The primary objective of incorporating ammonium hydroxide in nanocarrier synthesis is to enhance the biodegradability and biocompatibility of the resulting particles. This aligns with the broader goal of developing safer and more effective drug delivery systems that can minimize side effects and improve patient outcomes. Additionally, the use of ammonium hydroxide can potentially lead to more efficient encapsulation of therapeutic agents and improved release kinetics.
Recent technological advancements have focused on optimizing the use of ammonium hydroxide in nanocarrier synthesis. These efforts aim to achieve better control over particle size distribution, increase loading capacity, and improve the stability of nanocarriers in biological environments. Researchers are also exploring the potential of ammonium hydroxide to facilitate the development of stimuli-responsive nanocarriers that can release their payload under specific physiological conditions.
The evolution of this technology is closely tied to advancements in analytical techniques, such as dynamic light scattering, electron microscopy, and spectroscopic methods. These tools have enabled researchers to characterize nanocarriers with unprecedented precision, leading to a better understanding of the role of ammonium hydroxide in the synthesis process and its impact on the final product properties.
Looking ahead, the field is moving towards the development of more sophisticated, multifunctional nanocarriers that can simultaneously perform diagnostic and therapeutic functions. The use of ammonium hydroxide in these advanced systems is expected to play a crucial role in achieving the desired physicochemical properties and biological performance. As research progresses, the goal is to translate these technological advancements into clinically viable products that can revolutionize the treatment of various diseases, particularly in the areas of cancer therapy and gene delivery.
Market Analysis for Biodegradable Nanocarriers
The market for biodegradable nanocarriers is experiencing significant growth, driven by increasing demand for sustainable and environmentally friendly drug delivery systems. The global biodegradable nanocarriers market is expected to expand at a compound annual growth rate (CAGR) of over 10% in the coming years, reflecting the growing interest in these advanced materials across various industries.
The pharmaceutical and healthcare sectors are the primary drivers of this market, as biodegradable nanocarriers offer numerous advantages in drug delivery applications. These include improved drug solubility, enhanced bioavailability, targeted delivery, and reduced side effects. The rising prevalence of chronic diseases and the need for more effective treatments are fueling the adoption of nanocarrier-based drug delivery systems.
In addition to pharmaceuticals, the cosmetics and personal care industry is emerging as a significant market for biodegradable nanocarriers. The demand for advanced skincare products and novel formulations is driving the incorporation of nanocarriers in cosmetic applications. This trend is particularly evident in anti-aging products and sunscreens, where nanocarriers can enhance the efficacy and stability of active ingredients.
The food and beverage industry is also showing increasing interest in biodegradable nanocarriers for applications such as nutrient delivery, food preservation, and packaging. As consumers become more health-conscious and environmentally aware, the demand for functional foods and sustainable packaging solutions is expected to drive further growth in this sector.
Geographically, North America and Europe currently dominate the biodegradable nanocarriers market, owing to their advanced healthcare infrastructure and strong research and development capabilities. However, the Asia-Pacific region is anticipated to witness the fastest growth in the coming years, driven by increasing healthcare expenditure, growing awareness of nanotechnology, and rising investments in research and development.
The market landscape is characterized by intense competition and ongoing innovation. Key players in the biodegradable nanocarriers market are focusing on developing novel materials and expanding their product portfolios to gain a competitive edge. Collaborations between academic institutions, research organizations, and industry players are becoming increasingly common, fostering the development of new technologies and applications.
Despite the promising growth prospects, the biodegradable nanocarriers market faces several challenges. These include regulatory hurdles, concerns about the long-term safety of nanomaterials, and the high costs associated with research and development. Overcoming these obstacles will be crucial for the widespread adoption of biodegradable nanocarriers across various industries.
The pharmaceutical and healthcare sectors are the primary drivers of this market, as biodegradable nanocarriers offer numerous advantages in drug delivery applications. These include improved drug solubility, enhanced bioavailability, targeted delivery, and reduced side effects. The rising prevalence of chronic diseases and the need for more effective treatments are fueling the adoption of nanocarrier-based drug delivery systems.
In addition to pharmaceuticals, the cosmetics and personal care industry is emerging as a significant market for biodegradable nanocarriers. The demand for advanced skincare products and novel formulations is driving the incorporation of nanocarriers in cosmetic applications. This trend is particularly evident in anti-aging products and sunscreens, where nanocarriers can enhance the efficacy and stability of active ingredients.
The food and beverage industry is also showing increasing interest in biodegradable nanocarriers for applications such as nutrient delivery, food preservation, and packaging. As consumers become more health-conscious and environmentally aware, the demand for functional foods and sustainable packaging solutions is expected to drive further growth in this sector.
Geographically, North America and Europe currently dominate the biodegradable nanocarriers market, owing to their advanced healthcare infrastructure and strong research and development capabilities. However, the Asia-Pacific region is anticipated to witness the fastest growth in the coming years, driven by increasing healthcare expenditure, growing awareness of nanotechnology, and rising investments in research and development.
The market landscape is characterized by intense competition and ongoing innovation. Key players in the biodegradable nanocarriers market are focusing on developing novel materials and expanding their product portfolios to gain a competitive edge. Collaborations between academic institutions, research organizations, and industry players are becoming increasingly common, fostering the development of new technologies and applications.
Despite the promising growth prospects, the biodegradable nanocarriers market faces several challenges. These include regulatory hurdles, concerns about the long-term safety of nanomaterials, and the high costs associated with research and development. Overcoming these obstacles will be crucial for the widespread adoption of biodegradable nanocarriers across various industries.
Current Challenges in Ammonium Hydroxide-Based Synthesis
The synthesis of biodegradable nanocarriers using ammonium hydroxide faces several significant challenges that hinder its widespread adoption and optimization. One of the primary issues is the precise control of nanocarrier size and morphology. Ammonium hydroxide, while effective in facilitating the formation of nanoparticles, can lead to inconsistent particle sizes and shapes due to its volatile nature and sensitivity to environmental conditions.
Another major challenge lies in maintaining the stability of the nanocarriers during and after synthesis. The alkaline environment created by ammonium hydroxide can potentially degrade certain biodegradable materials, affecting the integrity and longevity of the nanocarriers. This instability may compromise the drug encapsulation efficiency and release kinetics, crucial factors in the performance of drug delivery systems.
The scalability of ammonium hydroxide-based synthesis processes presents a significant hurdle for industrial applications. While laboratory-scale production may yield satisfactory results, scaling up the process often leads to inconsistencies in nanocarrier properties and reduced batch-to-batch reproducibility. This challenge is particularly pronounced when attempting to maintain uniform conditions across larger reaction volumes.
Toxicity concerns associated with residual ammonium hydroxide in the final nanocarrier formulation pose another critical challenge. Ensuring complete removal of ammonia without compromising the nanocarrier structure is essential for biocompatibility and safety in medical applications. This necessitates the development of efficient purification techniques that do not adversely affect the nanocarrier properties.
The environmental impact of ammonium hydroxide usage in large-scale production is an emerging concern. As sustainability becomes increasingly important in industrial processes, finding eco-friendly alternatives or developing closed-loop systems for ammonia recovery and reuse is crucial. This challenge extends to waste management and the potential release of ammonia into the environment during manufacturing.
Lastly, the optimization of reaction parameters for different types of biodegradable materials remains a complex task. The interaction between ammonium hydroxide and various polymers or lipids used in nanocarrier synthesis can vary significantly, requiring extensive experimentation to determine optimal conditions for each material combination. This challenge is compounded by the need to tailor nanocarrier properties for specific drug delivery applications, necessitating a delicate balance between synthesis conditions and desired nanocarrier characteristics.
Another major challenge lies in maintaining the stability of the nanocarriers during and after synthesis. The alkaline environment created by ammonium hydroxide can potentially degrade certain biodegradable materials, affecting the integrity and longevity of the nanocarriers. This instability may compromise the drug encapsulation efficiency and release kinetics, crucial factors in the performance of drug delivery systems.
The scalability of ammonium hydroxide-based synthesis processes presents a significant hurdle for industrial applications. While laboratory-scale production may yield satisfactory results, scaling up the process often leads to inconsistencies in nanocarrier properties and reduced batch-to-batch reproducibility. This challenge is particularly pronounced when attempting to maintain uniform conditions across larger reaction volumes.
Toxicity concerns associated with residual ammonium hydroxide in the final nanocarrier formulation pose another critical challenge. Ensuring complete removal of ammonia without compromising the nanocarrier structure is essential for biocompatibility and safety in medical applications. This necessitates the development of efficient purification techniques that do not adversely affect the nanocarrier properties.
The environmental impact of ammonium hydroxide usage in large-scale production is an emerging concern. As sustainability becomes increasingly important in industrial processes, finding eco-friendly alternatives or developing closed-loop systems for ammonia recovery and reuse is crucial. This challenge extends to waste management and the potential release of ammonia into the environment during manufacturing.
Lastly, the optimization of reaction parameters for different types of biodegradable materials remains a complex task. The interaction between ammonium hydroxide and various polymers or lipids used in nanocarrier synthesis can vary significantly, requiring extensive experimentation to determine optimal conditions for each material combination. This challenge is compounded by the need to tailor nanocarrier properties for specific drug delivery applications, necessitating a delicate balance between synthesis conditions and desired nanocarrier characteristics.
Existing Ammonium Hydroxide-Based Synthesis Protocols
01 Biodegradability of ammonium hydroxide in wastewater treatment
Ammonium hydroxide is generally biodegradable in wastewater treatment processes. It can be broken down by microorganisms in activated sludge systems, contributing to the removal of nitrogen compounds from wastewater. The biodegradation process involves nitrification and denitrification steps, converting ammonium to nitrate and ultimately to nitrogen gas.- Biodegradability of ammonium hydroxide in wastewater treatment: Ammonium hydroxide is generally considered biodegradable in wastewater treatment processes. It can be broken down by microorganisms in activated sludge systems, contributing to the removal of nitrogen compounds from wastewater. The biodegradation process involves the conversion of ammonium to nitrite and then to nitrate through nitrification.
- Use of ammonium hydroxide in biodegradable cleaning compositions: Ammonium hydroxide is incorporated into biodegradable cleaning formulations due to its alkaline properties and ability to break down naturally. These compositions are designed to be environmentally friendly while maintaining effective cleaning performance. The biodegradability of ammonium hydroxide contributes to the overall eco-friendly profile of these products.
- Ammonium hydroxide as a biodegradable alternative in industrial processes: Ammonium hydroxide is utilized as a biodegradable alternative to more persistent chemicals in various industrial applications. Its ability to decompose naturally makes it suitable for processes where environmental impact is a concern. Industries such as textile manufacturing and paper production have explored the use of ammonium hydroxide to reduce their ecological footprint.
- Biodegradation of ammonium hydroxide in soil and aquatic environments: The biodegradability of ammonium hydroxide in natural environments such as soil and water bodies is an important consideration for its environmental impact. Microorganisms in these ecosystems can metabolize ammonium hydroxide, converting it into less harmful compounds. Factors such as pH, temperature, and microbial populations influence the rate of biodegradation in these settings.
- Enhancing biodegradability of ammonium hydroxide through formulation techniques: Research has been conducted on improving the biodegradability of ammonium hydroxide through various formulation techniques. These methods may involve combining ammonium hydroxide with other biodegradable substances or using specific additives to accelerate its breakdown in the environment. Such approaches aim to minimize the potential environmental impact of products containing ammonium hydroxide.
02 Environmental impact of ammonium hydroxide
While ammonium hydroxide is biodegradable, its release into the environment can have potential impacts. In aquatic ecosystems, it can contribute to eutrophication and ammonia toxicity. However, when properly managed and treated, the environmental risks can be minimized. The compound's relatively rapid biodegradation helps reduce its long-term environmental persistence.Expand Specific Solutions03 Use of ammonium hydroxide in biodegradable products
Ammonium hydroxide is utilized in the production of certain biodegradable materials and products. It can be incorporated into formulations for biodegradable plastics, cleaning agents, and agricultural products. The compound's biodegradability contributes to the overall environmental friendliness of these products, supporting their decomposition in natural environments.Expand Specific Solutions04 Biodegradation mechanisms of ammonium hydroxide
The biodegradation of ammonium hydroxide primarily occurs through microbial action. Specific bacteria, such as Nitrosomonas and Nitrobacter, play key roles in the nitrification process. These microorganisms oxidize ammonium to nitrite and then to nitrate. Understanding these mechanisms is crucial for optimizing wastewater treatment processes and managing environmental applications involving ammonium hydroxide.Expand Specific Solutions05 Factors affecting ammonium hydroxide biodegradability
Several factors influence the biodegradability of ammonium hydroxide, including pH, temperature, dissolved oxygen levels, and the presence of other compounds. Optimal conditions for biodegradation typically involve neutral to slightly alkaline pH, moderate temperatures, and adequate oxygen supply. These factors are important considerations in wastewater treatment design and environmental management strategies involving ammonium hydroxide.Expand Specific Solutions
Key Players in Nanocarrier Development and Production
The research on using Ammonium Hydroxide in biodegradable nanocarrier synthesis is in an early development stage, with a growing market potential due to increasing demand for sustainable drug delivery systems. The technology is still evolving, with varying levels of maturity among key players. Leading institutions like Harvard, MIT, and Northwestern University are at the forefront, conducting advanced research. Pharmaceutical companies such as Vertex Pharmaceuticals are also investing in this field, indicating its commercial potential. However, the technology requires further refinement and validation before widespread adoption in the pharmaceutical industry.
President & Fellows of Harvard College
Technical Solution: Harvard College has developed a novel approach for synthesizing biodegradable nanocarriers using ammonium hydroxide. Their method involves a controlled precipitation process where ammonium hydroxide acts as a pH regulator, facilitating the formation of stable nanoparticles[1]. The researchers have optimized the concentration of ammonium hydroxide to achieve desired particle size and surface properties. This technique allows for the encapsulation of various therapeutic agents, including hydrophobic drugs and nucleic acids[2]. The nanocarriers produced exhibit enhanced cellular uptake and controlled release profiles, making them suitable for targeted drug delivery applications[3]. Harvard's research also focuses on improving the biodegradability of these nanocarriers by incorporating enzyme-sensitive linkages in the polymer structure, which can be triggered by specific physiological conditions[4].
Strengths: High degree of control over nanocarrier properties, versatility in drug encapsulation, and enhanced cellular uptake. Weaknesses: Potential toxicity concerns related to residual ammonium hydroxide, scalability challenges for industrial production.
The Brigham & Women's Hospital, Inc.
Technical Solution: The Brigham & Women's Hospital has developed an innovative approach to synthesizing biodegradable nanocarriers using ammonium hydroxide as a key component. Their method involves a two-step process: first, creating a core-shell structure using a biodegradable polymer, and then using ammonium hydroxide to modify the surface properties[1]. This technique allows for precise control over the nanocarrier's size, shape, and surface charge, which are crucial factors in determining its biodistribution and cellular uptake[2]. The researchers have successfully incorporated various therapeutic agents, including small molecule drugs and proteins, into these nanocarriers[3]. Additionally, they have demonstrated the ability to functionalize the nanocarrier surface with targeting ligands, enhancing their specificity for diseased tissues[4]. The use of ammonium hydroxide in this process has been shown to improve the stability of the nanocarriers and increase their drug loading capacity compared to conventional methods[5].
Strengths: Precise control over nanocarrier properties, improved drug loading capacity, and potential for targeted delivery. Weaknesses: Possible toxicity issues related to residual ammonium hydroxide, complexity of the two-step synthesis process.
Innovations in Ammonium Hydroxide Utilization for Nanocarriers
Nanocarrier compositions and methods
PatentWO2010107831A1
Innovation
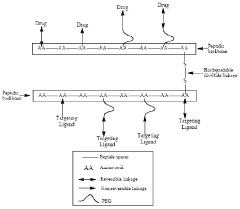
- A biodegradable multimeric nanocarrier system comprising covalently linked peptide monomers with functional groups and PEG linkers, designed to enhance the bioavailability of bioactive agents by facilitating their absorption and targeted delivery through reversible or irreversible bonds, suitable for various administration routes including oral, transmucosal, and systemic.
Nanocarriers having surface conjugated peptides and uses thereof for sustained local release of drugs
PatentInactiveUS20220096656A1
Innovation
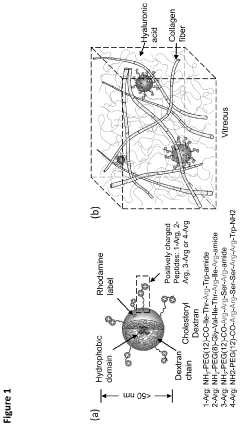
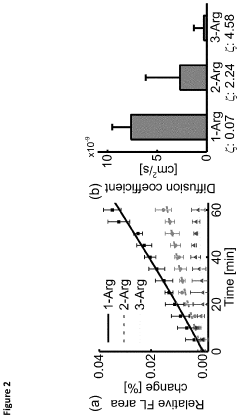
- Biodegradable nanocarriers with a net positive surface charge and zeta potential between +2 to +20 mV, anchored by peptides that adhere to poly-anionic carbohydrates, allowing slow diffusion and controlled release of therapeutic agents, extending the time between injections and reducing toxicity.
Environmental Impact Assessment of Synthesis Process
The synthesis of biodegradable nanocarriers using ammonium hydroxide presents both opportunities and challenges from an environmental perspective. This process, while innovative in its approach to creating sustainable drug delivery systems, requires careful consideration of its ecological footprint.
The use of ammonium hydroxide in the synthesis process introduces potential environmental concerns. As a strong base, ammonium hydroxide can alter the pH of aquatic environments if released untreated. This pH change can have cascading effects on local ecosystems, potentially impacting aquatic life and water quality. However, the biodegradable nature of the nanocarriers themselves offers a significant environmental advantage over traditional, non-degradable alternatives.
The synthesis process may also generate waste products that require proper management. These could include unreacted precursors, byproducts of the reaction, and excess solvents. Implementing efficient recycling and waste treatment protocols is crucial to minimize the environmental impact of these residuals. Additionally, the energy consumption associated with the synthesis process should be evaluated, as it contributes to the overall carbon footprint of nanocarrier production.
On a positive note, the biodegradability of the nanocarriers reduces the long-term environmental burden typically associated with nanomaterials. As these carriers break down naturally, they are less likely to accumulate in the environment, potentially mitigating concerns about nanoparticle pollution in water bodies and soil.
The production scale is a critical factor in assessing environmental impact. Laboratory-scale synthesis may have minimal direct environmental consequences, but scaling up to industrial production levels could amplify potential risks. This necessitates the development of robust environmental management strategies and the implementation of green chemistry principles throughout the manufacturing process.
Life cycle assessment (LCA) methodologies should be applied to comprehensively evaluate the environmental impact from raw material extraction to final disposal. This holistic approach can identify hotspots in the production chain where environmental interventions would be most effective. It may also reveal unexpected environmental benefits or drawbacks that are not immediately apparent when focusing solely on the synthesis step.
Regulatory compliance is another crucial aspect of environmental impact assessment. Ensuring that the synthesis process meets or exceeds local and international environmental standards is essential for sustainable production. This may involve investing in advanced emission control technologies, implementing closed-loop systems to minimize waste, and continuously monitoring environmental parameters throughout the production cycle.
In conclusion, while the use of ammonium hydroxide in biodegradable nanocarrier synthesis offers promising advancements in sustainable drug delivery, it is imperative to conduct thorough environmental impact assessments. By addressing potential risks and optimizing the process for environmental sustainability, researchers and manufacturers can harness the benefits of this technology while minimizing its ecological footprint.
The use of ammonium hydroxide in the synthesis process introduces potential environmental concerns. As a strong base, ammonium hydroxide can alter the pH of aquatic environments if released untreated. This pH change can have cascading effects on local ecosystems, potentially impacting aquatic life and water quality. However, the biodegradable nature of the nanocarriers themselves offers a significant environmental advantage over traditional, non-degradable alternatives.
The synthesis process may also generate waste products that require proper management. These could include unreacted precursors, byproducts of the reaction, and excess solvents. Implementing efficient recycling and waste treatment protocols is crucial to minimize the environmental impact of these residuals. Additionally, the energy consumption associated with the synthesis process should be evaluated, as it contributes to the overall carbon footprint of nanocarrier production.
On a positive note, the biodegradability of the nanocarriers reduces the long-term environmental burden typically associated with nanomaterials. As these carriers break down naturally, they are less likely to accumulate in the environment, potentially mitigating concerns about nanoparticle pollution in water bodies and soil.
The production scale is a critical factor in assessing environmental impact. Laboratory-scale synthesis may have minimal direct environmental consequences, but scaling up to industrial production levels could amplify potential risks. This necessitates the development of robust environmental management strategies and the implementation of green chemistry principles throughout the manufacturing process.
Life cycle assessment (LCA) methodologies should be applied to comprehensively evaluate the environmental impact from raw material extraction to final disposal. This holistic approach can identify hotspots in the production chain where environmental interventions would be most effective. It may also reveal unexpected environmental benefits or drawbacks that are not immediately apparent when focusing solely on the synthesis step.
Regulatory compliance is another crucial aspect of environmental impact assessment. Ensuring that the synthesis process meets or exceeds local and international environmental standards is essential for sustainable production. This may involve investing in advanced emission control technologies, implementing closed-loop systems to minimize waste, and continuously monitoring environmental parameters throughout the production cycle.
In conclusion, while the use of ammonium hydroxide in biodegradable nanocarrier synthesis offers promising advancements in sustainable drug delivery, it is imperative to conduct thorough environmental impact assessments. By addressing potential risks and optimizing the process for environmental sustainability, researchers and manufacturers can harness the benefits of this technology while minimizing its ecological footprint.
Regulatory Landscape for Nanocarrier-Based Drug Delivery Systems
The regulatory landscape for nanocarrier-based drug delivery systems is complex and evolving, reflecting the unique challenges posed by these innovative technologies. Regulatory agencies worldwide are working to establish comprehensive frameworks that ensure the safety and efficacy of nanocarrier-based therapeutics while fostering innovation in this rapidly advancing field.
In the United States, the Food and Drug Administration (FDA) has taken a leading role in developing guidelines for nanomedicine. The FDA's approach is product-specific, considering the unique characteristics of each nanocarrier system. They have established the Nanotechnology Task Force to address the regulatory challenges posed by nanomaterials in drug delivery.
The European Medicines Agency (EMA) has also been proactive in developing regulatory guidance for nanomedicines. Their focus includes the characterization of nanomaterials, safety assessment, and quality control measures specific to nanocarrier-based drug delivery systems. The EMA's guidelines emphasize the importance of thorough physicochemical characterization and biological evaluation of nanocarriers.
In Asia, regulatory bodies such as Japan's Pharmaceuticals and Medical Devices Agency (PMDA) and China's National Medical Products Administration (NMPA) are developing their own frameworks for regulating nanomedicines. These agencies are often in dialogue with their Western counterparts to harmonize regulatory approaches and facilitate global development of nanocarrier-based therapies.
Key regulatory considerations for nanocarrier-based drug delivery systems include particle size distribution, surface properties, drug loading efficiency, and release kinetics. Regulatory bodies are particularly concerned with the potential for nanocarriers to alter the pharmacokinetics and biodistribution of drugs, which may impact safety and efficacy profiles.
Environmental impact and biodegradability are emerging areas of regulatory focus, particularly relevant to the use of ammonium hydroxide in the synthesis of biodegradable nanocarriers. Regulators are increasingly requiring data on the environmental fate of nanocarriers and their potential ecological impacts.
Standardization of characterization methods for nanocarriers remains a challenge in the regulatory landscape. Efforts are underway to develop internationally recognized standards for physicochemical characterization and biological evaluation of nanocarrier-based drug delivery systems.
As the field of nanomedicine continues to advance, regulatory frameworks are expected to evolve. Ongoing dialogue between researchers, industry, and regulatory agencies will be crucial in shaping policies that balance innovation with patient safety and environmental considerations in the development of nanocarrier-based drug delivery systems.
In the United States, the Food and Drug Administration (FDA) has taken a leading role in developing guidelines for nanomedicine. The FDA's approach is product-specific, considering the unique characteristics of each nanocarrier system. They have established the Nanotechnology Task Force to address the regulatory challenges posed by nanomaterials in drug delivery.
The European Medicines Agency (EMA) has also been proactive in developing regulatory guidance for nanomedicines. Their focus includes the characterization of nanomaterials, safety assessment, and quality control measures specific to nanocarrier-based drug delivery systems. The EMA's guidelines emphasize the importance of thorough physicochemical characterization and biological evaluation of nanocarriers.
In Asia, regulatory bodies such as Japan's Pharmaceuticals and Medical Devices Agency (PMDA) and China's National Medical Products Administration (NMPA) are developing their own frameworks for regulating nanomedicines. These agencies are often in dialogue with their Western counterparts to harmonize regulatory approaches and facilitate global development of nanocarrier-based therapies.
Key regulatory considerations for nanocarrier-based drug delivery systems include particle size distribution, surface properties, drug loading efficiency, and release kinetics. Regulatory bodies are particularly concerned with the potential for nanocarriers to alter the pharmacokinetics and biodistribution of drugs, which may impact safety and efficacy profiles.
Environmental impact and biodegradability are emerging areas of regulatory focus, particularly relevant to the use of ammonium hydroxide in the synthesis of biodegradable nanocarriers. Regulators are increasingly requiring data on the environmental fate of nanocarriers and their potential ecological impacts.
Standardization of characterization methods for nanocarriers remains a challenge in the regulatory landscape. Efforts are underway to develop internationally recognized standards for physicochemical characterization and biological evaluation of nanocarrier-based drug delivery systems.
As the field of nanomedicine continues to advance, regulatory frameworks are expected to evolve. Ongoing dialogue between researchers, industry, and regulatory agencies will be crucial in shaping policies that balance innovation with patient safety and environmental considerations in the development of nanocarrier-based drug delivery systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!