Analyzing Glycogenolysis with NMR Techniques
AUG 28, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
NMR Glycogenolysis Analysis Background and Objectives
Nuclear Magnetic Resonance (NMR) spectroscopy has evolved significantly since its discovery in the 1940s, becoming an indispensable analytical tool in biochemistry and metabolic research. The application of NMR techniques to study glycogenolysis—the breakdown of glycogen to glucose-1-phosphate and eventually glucose—represents a critical intersection of advanced spectroscopic methods and fundamental metabolic processes. This technological approach has progressed from basic structural analysis to sophisticated real-time metabolic tracking.
The evolution of NMR technology has been marked by several breakthrough developments, including higher magnetic field strengths, enhanced probe designs, and advanced pulse sequences. These advancements have dramatically improved spectral resolution and sensitivity, enabling researchers to detect and quantify metabolic intermediates with unprecedented precision. Particularly significant has been the development of in vivo NMR techniques, which allow for non-invasive monitoring of glycogenolysis in intact tissues and even living organisms.
Recent technological trends in NMR spectroscopy include the integration of hyperpolarization methods such as Dynamic Nuclear Polarization (DNP), which can enhance signal intensity by several orders of magnitude. Additionally, the combination of NMR with other analytical techniques, such as mass spectrometry and computational modeling, has created powerful hybrid approaches for comprehensive metabolic analysis. These developments have opened new avenues for understanding glycogenolysis at molecular and cellular levels.
The primary objective of applying NMR techniques to glycogenolysis analysis is to elucidate the dynamic regulation of this critical metabolic pathway under various physiological and pathological conditions. Specifically, researchers aim to quantify glycogen breakdown rates, identify rate-limiting steps, and characterize the influence of hormonal and enzymatic factors on this process. This information is vital for understanding metabolic disorders such as glycogen storage diseases, diabetes, and exercise physiology.
Furthermore, NMR-based glycogenolysis studies seek to develop non-invasive diagnostic tools for metabolic disorders. By establishing characteristic spectral signatures associated with abnormal glycogen metabolism, clinicians could potentially diagnose and monitor treatment efficacy without requiring tissue biopsies. This represents a significant advancement in patient care and personalized medicine approaches.
Another key objective is to bridge the gap between molecular-level understanding and whole-organism physiology. By tracking glycogenolysis in real-time across different tissues and under various stimuli, researchers can construct more accurate models of whole-body glucose homeostasis. This systems-level understanding is essential for developing targeted therapeutic interventions for metabolic disorders and optimizing nutritional strategies for athletic performance.
The evolution of NMR technology has been marked by several breakthrough developments, including higher magnetic field strengths, enhanced probe designs, and advanced pulse sequences. These advancements have dramatically improved spectral resolution and sensitivity, enabling researchers to detect and quantify metabolic intermediates with unprecedented precision. Particularly significant has been the development of in vivo NMR techniques, which allow for non-invasive monitoring of glycogenolysis in intact tissues and even living organisms.
Recent technological trends in NMR spectroscopy include the integration of hyperpolarization methods such as Dynamic Nuclear Polarization (DNP), which can enhance signal intensity by several orders of magnitude. Additionally, the combination of NMR with other analytical techniques, such as mass spectrometry and computational modeling, has created powerful hybrid approaches for comprehensive metabolic analysis. These developments have opened new avenues for understanding glycogenolysis at molecular and cellular levels.
The primary objective of applying NMR techniques to glycogenolysis analysis is to elucidate the dynamic regulation of this critical metabolic pathway under various physiological and pathological conditions. Specifically, researchers aim to quantify glycogen breakdown rates, identify rate-limiting steps, and characterize the influence of hormonal and enzymatic factors on this process. This information is vital for understanding metabolic disorders such as glycogen storage diseases, diabetes, and exercise physiology.
Furthermore, NMR-based glycogenolysis studies seek to develop non-invasive diagnostic tools for metabolic disorders. By establishing characteristic spectral signatures associated with abnormal glycogen metabolism, clinicians could potentially diagnose and monitor treatment efficacy without requiring tissue biopsies. This represents a significant advancement in patient care and personalized medicine approaches.
Another key objective is to bridge the gap between molecular-level understanding and whole-organism physiology. By tracking glycogenolysis in real-time across different tissues and under various stimuli, researchers can construct more accurate models of whole-body glucose homeostasis. This systems-level understanding is essential for developing targeted therapeutic interventions for metabolic disorders and optimizing nutritional strategies for athletic performance.
Market Applications for NMR-based Glycogen Metabolism Studies
NMR-based glycogen metabolism studies have demonstrated significant market potential across multiple sectors, with healthcare and pharmaceutical industries leading adoption. The global market for metabolic disorder diagnostics, where NMR glycogenolysis analysis plays a crucial role, was valued at approximately $2.1 billion in 2022 and is projected to grow at a compound annual growth rate of 8.3% through 2030.
In clinical diagnostics, NMR techniques for analyzing glycogenolysis provide valuable tools for monitoring metabolic disorders such as glycogen storage diseases, diabetes, and liver dysfunction. Hospitals and specialized diagnostic centers represent the primary market segment, with increasing integration of these advanced analytical methods into standard diagnostic protocols. The non-invasive nature of NMR spectroscopy makes it particularly valuable for pediatric patients with suspected metabolic disorders.
The pharmaceutical industry constitutes another significant market application, where NMR-based glycogen metabolism studies facilitate drug discovery and development processes. Companies developing therapeutics for metabolic disorders utilize these techniques to evaluate drug efficacy and mechanism of action. This application segment is experiencing rapid growth due to the increasing pipeline of drugs targeting glycogen metabolism pathways.
Sports medicine and nutrition represent an emerging market with substantial growth potential. Elite athletic training programs and sports nutrition companies are increasingly incorporating NMR-based glycogen metabolism analysis to optimize performance and recovery protocols. This segment is expected to expand as awareness of metabolic optimization in sports performance continues to grow.
Academic and research institutions form a stable market base, utilizing NMR techniques for fundamental glycogen metabolism research. This segment drives technological innovation and method development, often through collaborations with instrument manufacturers and clinical partners.
Geographically, North America dominates the market with approximately 42% share, followed by Europe at 31% and Asia-Pacific at 18%. The Asia-Pacific region is projected to experience the fastest growth rate due to increasing healthcare expenditure and expanding research infrastructure in countries like China, Japan, and South Korea.
Market barriers include the high cost of NMR equipment, technical expertise requirements, and competition from alternative technologies such as mass spectrometry. However, technological advancements reducing instrument size and cost, alongside increasing automation of analysis processes, are gradually addressing these limitations and expanding market accessibility.
The market is expected to benefit from growing personalized medicine approaches, where individual glycogen metabolism profiles can inform tailored therapeutic strategies. This trend aligns with broader healthcare shifts toward precision diagnostics and treatment optimization based on individual metabolic characteristics.
In clinical diagnostics, NMR techniques for analyzing glycogenolysis provide valuable tools for monitoring metabolic disorders such as glycogen storage diseases, diabetes, and liver dysfunction. Hospitals and specialized diagnostic centers represent the primary market segment, with increasing integration of these advanced analytical methods into standard diagnostic protocols. The non-invasive nature of NMR spectroscopy makes it particularly valuable for pediatric patients with suspected metabolic disorders.
The pharmaceutical industry constitutes another significant market application, where NMR-based glycogen metabolism studies facilitate drug discovery and development processes. Companies developing therapeutics for metabolic disorders utilize these techniques to evaluate drug efficacy and mechanism of action. This application segment is experiencing rapid growth due to the increasing pipeline of drugs targeting glycogen metabolism pathways.
Sports medicine and nutrition represent an emerging market with substantial growth potential. Elite athletic training programs and sports nutrition companies are increasingly incorporating NMR-based glycogen metabolism analysis to optimize performance and recovery protocols. This segment is expected to expand as awareness of metabolic optimization in sports performance continues to grow.
Academic and research institutions form a stable market base, utilizing NMR techniques for fundamental glycogen metabolism research. This segment drives technological innovation and method development, often through collaborations with instrument manufacturers and clinical partners.
Geographically, North America dominates the market with approximately 42% share, followed by Europe at 31% and Asia-Pacific at 18%. The Asia-Pacific region is projected to experience the fastest growth rate due to increasing healthcare expenditure and expanding research infrastructure in countries like China, Japan, and South Korea.
Market barriers include the high cost of NMR equipment, technical expertise requirements, and competition from alternative technologies such as mass spectrometry. However, technological advancements reducing instrument size and cost, alongside increasing automation of analysis processes, are gradually addressing these limitations and expanding market accessibility.
The market is expected to benefit from growing personalized medicine approaches, where individual glycogen metabolism profiles can inform tailored therapeutic strategies. This trend aligns with broader healthcare shifts toward precision diagnostics and treatment optimization based on individual metabolic characteristics.
Current NMR Techniques and Limitations in Glycogenolysis Research
Nuclear Magnetic Resonance (NMR) spectroscopy has emerged as a powerful analytical tool for studying glycogenolysis, the metabolic process of glycogen breakdown. Current NMR techniques employed in this field include 1H-NMR, 13C-NMR, and 31P-NMR spectroscopy, each offering unique insights into different aspects of the glycogenolytic pathway.
1H-NMR spectroscopy provides detailed information about hydrogen-containing metabolites involved in glycogenolysis, allowing researchers to track changes in glucose, lactate, and other intermediates. This technique offers excellent sensitivity and is particularly useful for quantifying metabolite concentrations in biological samples.
13C-NMR spectroscopy, especially when combined with isotope labeling approaches, enables the tracking of carbon flux through the glycogenolytic pathway. This method provides crucial information about the rate and directionality of metabolic processes, offering insights into the regulation of glycogen breakdown under various physiological conditions.
31P-NMR spectroscopy focuses on phosphorus-containing compounds, making it invaluable for monitoring phosphorylated intermediates in glycogenolysis, such as glucose-6-phosphate and glycogen phosphorylase activity. This technique allows for real-time assessment of energetic status during glycogen metabolism.
Despite these capabilities, current NMR techniques face significant limitations in glycogenolysis research. Sensitivity remains a major challenge, particularly when analyzing low-concentration metabolites in complex biological matrices. This often necessitates larger sample volumes or longer acquisition times, limiting applications in small tissue samples or time-sensitive experiments.
Spectral resolution presents another substantial hurdle, as the complex nature of biological samples often leads to signal overlap, complicating the identification and quantification of specific metabolites involved in glycogenolysis. This is particularly problematic when studying closely related carbohydrate species.
Sample preparation requirements for NMR analysis can be demanding, potentially altering the native state of glycogen metabolism. The need for tissue extraction or cell lysis means that dynamic, real-time measurements in intact systems remain challenging, limiting our understanding of the temporal aspects of glycogenolysis.
Additionally, the high cost and technical expertise required for advanced NMR instrumentation restrict widespread adoption in glycogenolysis research. Many laboratories lack access to high-field NMR spectrometers necessary for optimal resolution and sensitivity.
Recent methodological advances are addressing some of these limitations, including the development of hyperpolarization techniques like Dynamic Nuclear Polarization (DNP) to enhance sensitivity, and the implementation of advanced pulse sequences to improve spectral resolution. Integration with complementary techniques such as mass spectrometry is also providing more comprehensive insights into glycogenolysis pathways.
1H-NMR spectroscopy provides detailed information about hydrogen-containing metabolites involved in glycogenolysis, allowing researchers to track changes in glucose, lactate, and other intermediates. This technique offers excellent sensitivity and is particularly useful for quantifying metabolite concentrations in biological samples.
13C-NMR spectroscopy, especially when combined with isotope labeling approaches, enables the tracking of carbon flux through the glycogenolytic pathway. This method provides crucial information about the rate and directionality of metabolic processes, offering insights into the regulation of glycogen breakdown under various physiological conditions.
31P-NMR spectroscopy focuses on phosphorus-containing compounds, making it invaluable for monitoring phosphorylated intermediates in glycogenolysis, such as glucose-6-phosphate and glycogen phosphorylase activity. This technique allows for real-time assessment of energetic status during glycogen metabolism.
Despite these capabilities, current NMR techniques face significant limitations in glycogenolysis research. Sensitivity remains a major challenge, particularly when analyzing low-concentration metabolites in complex biological matrices. This often necessitates larger sample volumes or longer acquisition times, limiting applications in small tissue samples or time-sensitive experiments.
Spectral resolution presents another substantial hurdle, as the complex nature of biological samples often leads to signal overlap, complicating the identification and quantification of specific metabolites involved in glycogenolysis. This is particularly problematic when studying closely related carbohydrate species.
Sample preparation requirements for NMR analysis can be demanding, potentially altering the native state of glycogen metabolism. The need for tissue extraction or cell lysis means that dynamic, real-time measurements in intact systems remain challenging, limiting our understanding of the temporal aspects of glycogenolysis.
Additionally, the high cost and technical expertise required for advanced NMR instrumentation restrict widespread adoption in glycogenolysis research. Many laboratories lack access to high-field NMR spectrometers necessary for optimal resolution and sensitivity.
Recent methodological advances are addressing some of these limitations, including the development of hyperpolarization techniques like Dynamic Nuclear Polarization (DNP) to enhance sensitivity, and the implementation of advanced pulse sequences to improve spectral resolution. Integration with complementary techniques such as mass spectrometry is also providing more comprehensive insights into glycogenolysis pathways.
Established NMR Protocols for Glycogen Breakdown Assessment
01 Advanced NMR Pulse Sequence Techniques
Various pulse sequence techniques have been developed to enhance NMR analysis capabilities. These include specialized sequences for improved signal acquisition, noise reduction, and selective excitation of specific nuclei. Advanced pulse sequences allow for better discrimination between different molecular structures and can provide more detailed spectral information even in complex samples. These techniques have significantly improved the sensitivity and resolution of NMR spectroscopy.- Advanced NMR pulse sequence techniques: Various pulse sequence techniques have been developed to enhance NMR analysis capabilities. These include specialized sequences for improving signal-to-noise ratio, reducing artifacts, and enabling more precise measurements of molecular structures and dynamics. Advanced pulse sequences allow for better discrimination between different chemical environments and can be tailored for specific applications in chemistry, biology, and materials science.
- NMR for fluid analysis in wellbores and reservoirs: NMR techniques have been adapted for downhole fluid analysis in oil and gas exploration. These methods enable real-time characterization of reservoir fluids, determination of porosity, permeability, and fluid saturation in geological formations. Specialized tools can perform NMR measurements under extreme conditions of temperature and pressure, providing valuable data for reservoir management and production optimization.
- Miniaturized and portable NMR systems: Innovations in NMR technology have led to the development of compact, portable NMR systems that maintain analytical capabilities while reducing size, weight, and power requirements. These systems incorporate novel magnet designs, miniaturized electronics, and optimized detection methods to enable NMR analysis outside traditional laboratory settings. Applications include point-of-care medical diagnostics, field-based quality control, and on-site chemical analysis.
- Multi-dimensional NMR spectroscopy techniques: Multi-dimensional NMR techniques provide enhanced analytical capabilities by correlating different types of nuclear interactions across multiple frequency dimensions. These methods allow for detailed structural elucidation of complex molecules by resolving overlapping signals and revealing connectivity between atoms. Advanced processing algorithms and experimental protocols enable the acquisition of high-resolution spectral data that can reveal subtle molecular features and interactions.
- Sample preparation and handling innovations for NMR: Specialized sample preparation and handling techniques have been developed to optimize NMR analysis across various applications. These innovations include methods for analyzing small sample volumes, automated sample changing systems, cryogenic probe technology for enhanced sensitivity, and specialized containers that minimize background interference. Advanced sample handling approaches enable more efficient workflows and improved data quality for complex analytical challenges.
02 NMR for Material Characterization and Analysis
NMR techniques are widely used for characterizing various materials including polymers, biological samples, and chemical compounds. These methods provide detailed information about molecular structure, composition, and dynamics without destroying the sample. The techniques can determine molecular weight distributions, crystallinity, cross-linking density, and other physical properties that are crucial for material science applications. Recent advancements have expanded the application of NMR to more complex materials and heterogeneous systems.Expand Specific Solutions03 Downhole and Field NMR Applications
NMR technology has been adapted for use in field conditions, particularly in downhole applications for oil and gas exploration. These specialized NMR tools can operate in harsh environments to analyze formation fluids, determine porosity, permeability, and fluid properties in real-time. The techniques involve robust hardware designs that can withstand high temperatures and pressures while maintaining measurement accuracy. These applications have revolutionized well logging and reservoir characterization in the petroleum industry.Expand Specific Solutions04 Hardware Innovations in NMR Spectroscopy
Significant advancements have been made in NMR hardware components including magnets, probes, and detection systems. These innovations include the development of higher field strength superconducting magnets, cryogenically cooled probes, and more sensitive detection electronics. Modern NMR systems incorporate digital signal processing techniques and automated tuning mechanisms to improve data quality and ease of use. These hardware improvements have expanded the range of samples that can be analyzed and increased the information content obtainable from NMR experiments.Expand Specific Solutions05 Data Processing and Analysis Methods for NMR
Advanced computational methods have been developed for processing and analyzing NMR data. These include sophisticated algorithms for signal processing, spectral deconvolution, and automated interpretation of complex spectra. Machine learning and artificial intelligence approaches are increasingly being applied to extract meaningful information from large NMR datasets. These computational techniques help overcome limitations in spectral resolution and enable the analysis of increasingly complex molecular systems with greater accuracy and efficiency.Expand Specific Solutions
Leading Research Institutions and Equipment Manufacturers
The glycogenolysis analysis market using NMR techniques is currently in a growth phase, characterized by increasing adoption across research institutions and pharmaceutical companies. The global market size for this specialized analytical approach is expanding as metabolic disorder research gains prominence. Technologically, NMR-based glycogenolysis analysis is reaching maturity with established protocols, though innovations continue to emerge. Leading players include academic powerhouses like MIT, Johns Hopkins University, and Max Planck Society, which drive fundamental research, while companies such as Momenta Pharmaceuticals and LipoScience provide commercial applications. Pharmaceutical entities like UCB Biopharma and Laboratory Corporation of America are integrating these techniques into their R&D pipelines, particularly for metabolic disease therapeutics. The competitive landscape features collaboration between academic institutions and industry partners to advance both technical capabilities and clinical applications.
Massachusetts Institute of Technology
Technical Solution: MIT has pioneered advanced NMR spectroscopy techniques for analyzing glycogenolysis pathways with exceptional precision. Their approach combines high-field NMR spectroscopy (typically 800-900 MHz) with specialized isotope labeling strategies to track the metabolic fate of glycogen in real-time. MIT researchers have developed custom pulse sequences that enhance the detection sensitivity of 13C-labeled glucose residues during glycogenolysis, allowing for quantitative measurement of glycogen breakdown intermediates with minimal sample preparation. Their methodology incorporates two-dimensional heteronuclear correlation experiments (HSQC and HMBC) to resolve complex spectral overlaps common in carbohydrate analysis. Additionally, MIT has integrated machine learning algorithms to automate spectral assignment and quantification, significantly reducing analysis time while maintaining high accuracy in metabolite identification.
Strengths: Exceptional signal-to-noise ratio allowing detection of low-abundance metabolites; sophisticated pulse sequence design enabling real-time monitoring; integration with computational methods for automated analysis. Weaknesses: Requires expensive high-field NMR instrumentation; complex methodology with steep learning curve; limited throughput compared to some alternative techniques.
The Johns Hopkins University
Technical Solution: Johns Hopkins has developed a comprehensive NMR-based platform specifically optimized for glycogenolysis analysis in both clinical and research settings. Their approach centers on dynamic nuclear polarization (DNP) enhanced NMR spectroscopy, which dramatically increases sensitivity by factors of 100-10,000 compared to conventional NMR. This enables detection of glycogen metabolism in minute tissue samples and even in vivo applications. The Johns Hopkins protocol incorporates specialized 31P and 13C NMR techniques to simultaneously track both glycogen phosphorylase activity and the resulting glucose-1-phosphate production. Their methodology includes custom-designed NMR probes that maximize signal detection from liver and muscle tissues, the primary sites of glycogenolysis. The university has also pioneered the integration of perfused tissue systems with real-time NMR monitoring, allowing observation of hormone-stimulated glycogenolysis under physiologically relevant conditions.
Strengths: Exceptional sensitivity through DNP enhancement; capability for in vivo measurements; simultaneous tracking of multiple metabolic pathways. Weaknesses: DNP instrumentation is extremely costly and technically demanding; limited to specialized research centers; requires cryogenic conditions that may alter some physiological processes.
Key Innovations in NMR Spectroscopy for Glycogenolysis
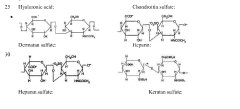
Method for the Analysis of Glycosaminoglycans, and Their Derivatives and Salts by Nuclear Magnetic Resonance
PatentActiveUS20200271601A1
Innovation
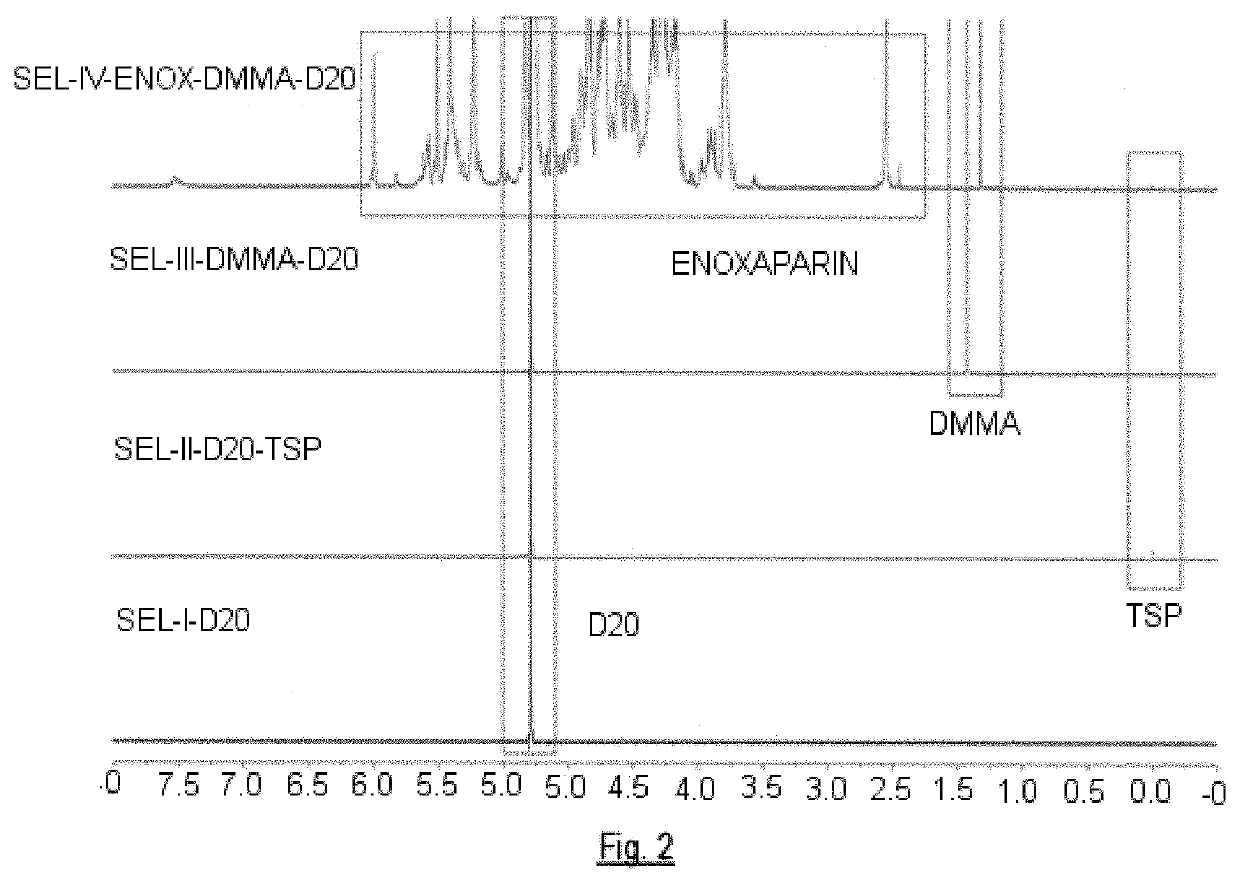
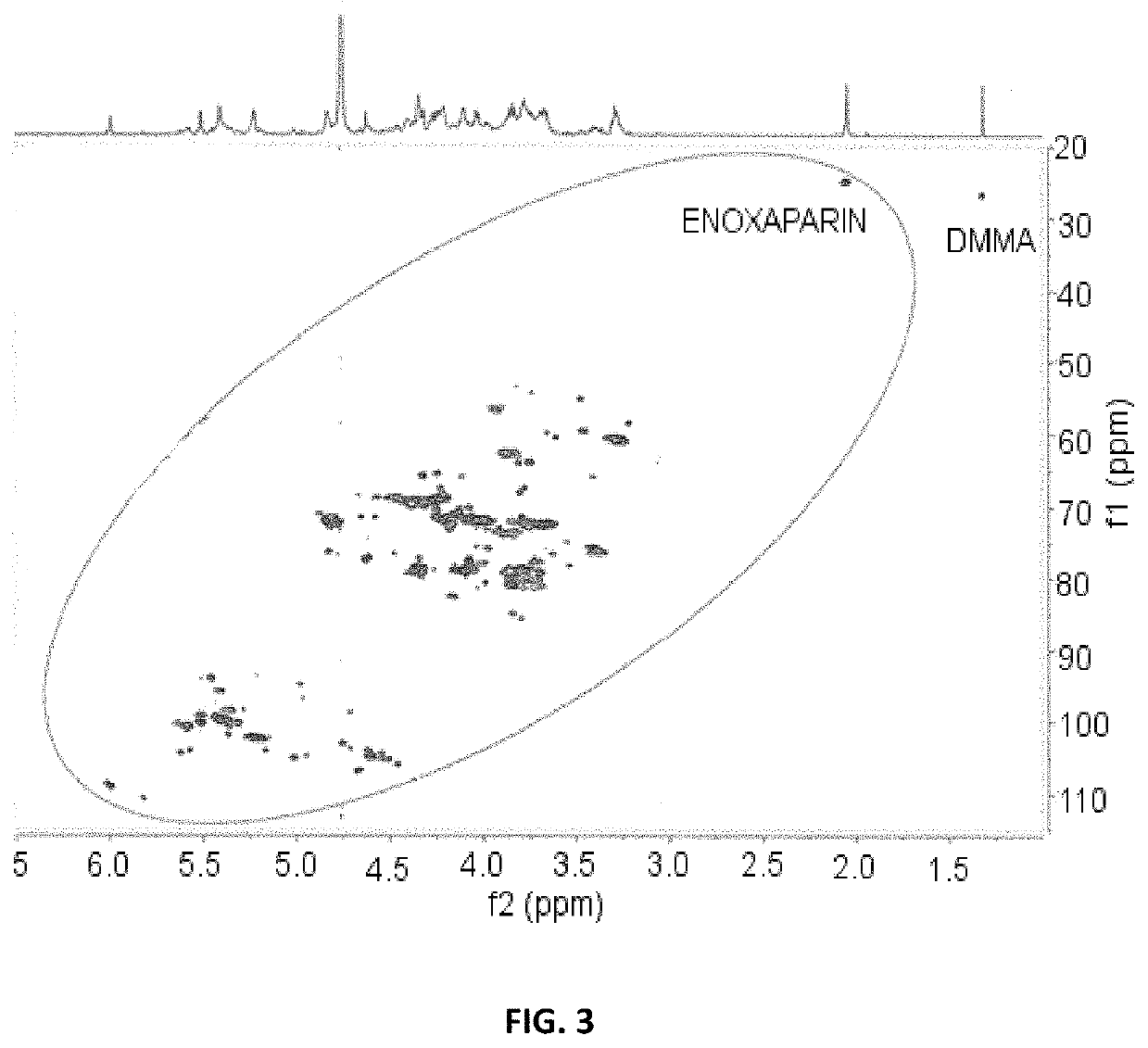
- A method employing 1H-NMR and 1H-13C HSQC NMR using dimethylmalonic acid (DMMA) as an internal standard to quantify characteristic signals, allowing for the determination of relative content and identity of saccharide residues in glycosaminoglycans, enabling precise differentiation between various heparin species and confirming manufacturing processes.
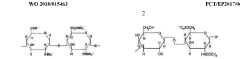
Method for the analysis of glycosaminoglycans, heparins and their derivatives by nuclear magnetic resonance
PatentWO2018015463A9
Innovation
- A method utilizing one-dimensional 1H-NMR and two-dimensional 1H-13C HSQC spectroscopy, with dimethylmalonic acid as an internal standard, to quantify characteristic signals and determine monosaccharide composition, enabling the differentiation of heparins based on their manufacturing processes and origin.
Clinical Translation of NMR Glycogenolysis Findings
The translation of NMR glycogenolysis findings into clinical applications represents a significant frontier in metabolic disorder diagnostics and treatment. Current clinical implementations primarily focus on using 13C NMR spectroscopy for non-invasive monitoring of glycogen metabolism in patients with glycogen storage diseases, diabetes, and other metabolic disorders. Several leading medical centers have established protocols for quantitative assessment of hepatic glycogen levels and glycogenolysis rates, providing valuable diagnostic information without requiring tissue biopsies.
The integration of NMR-based glycogenolysis analysis into standard clinical workflows faces several challenges. Technical barriers include the need for specialized high-field NMR equipment, which is costly and requires dedicated facility space and trained personnel. Additionally, standardization of acquisition protocols and data analysis methods across different clinical settings remains incomplete, limiting widespread adoption.
Recent advancements in portable NMR technology show promise for expanding clinical accessibility. Benchtop NMR systems with sufficient sensitivity for glycogen detection are being developed, potentially enabling point-of-care testing in more clinical environments. These systems, while offering lower resolution than research-grade instruments, provide adequate performance for monitoring key metabolic parameters relevant to glycogenolysis assessment.
Clinical validation studies have demonstrated strong correlations between NMR-derived glycogenolysis measurements and traditional biochemical assays. Particularly noteworthy is the ability of NMR techniques to distinguish between different types of glycogen storage diseases based on characteristic patterns of glycogen synthesis and breakdown, offering improved diagnostic specificity compared to conventional methods.
The economic impact of implementing NMR glycogenolysis analysis in clinical settings shows favorable cost-benefit ratios when considering the reduction in invasive procedures and improved disease management. Healthcare systems that have adopted these techniques report more precise medication dosing and nutritional management for patients with disorders affecting glycogen metabolism.
Regulatory pathways for NMR-based metabolic testing are becoming more defined, with several diagnostic protocols receiving approval from regulatory bodies in Europe and conditional approval in North America. These developments are accelerating the transition from research applications to standard clinical practice, particularly in specialized metabolic centers and endocrinology departments.
Future directions for clinical translation include the development of integrated multiparametric NMR approaches that simultaneously assess glycogenolysis alongside other metabolic pathways, providing a more comprehensive metabolic profile. Additionally, artificial intelligence algorithms are being trained on NMR spectral databases to improve diagnostic accuracy and personalize treatment recommendations based on individual metabolic patterns.
The integration of NMR-based glycogenolysis analysis into standard clinical workflows faces several challenges. Technical barriers include the need for specialized high-field NMR equipment, which is costly and requires dedicated facility space and trained personnel. Additionally, standardization of acquisition protocols and data analysis methods across different clinical settings remains incomplete, limiting widespread adoption.
Recent advancements in portable NMR technology show promise for expanding clinical accessibility. Benchtop NMR systems with sufficient sensitivity for glycogen detection are being developed, potentially enabling point-of-care testing in more clinical environments. These systems, while offering lower resolution than research-grade instruments, provide adequate performance for monitoring key metabolic parameters relevant to glycogenolysis assessment.
Clinical validation studies have demonstrated strong correlations between NMR-derived glycogenolysis measurements and traditional biochemical assays. Particularly noteworthy is the ability of NMR techniques to distinguish between different types of glycogen storage diseases based on characteristic patterns of glycogen synthesis and breakdown, offering improved diagnostic specificity compared to conventional methods.
The economic impact of implementing NMR glycogenolysis analysis in clinical settings shows favorable cost-benefit ratios when considering the reduction in invasive procedures and improved disease management. Healthcare systems that have adopted these techniques report more precise medication dosing and nutritional management for patients with disorders affecting glycogen metabolism.
Regulatory pathways for NMR-based metabolic testing are becoming more defined, with several diagnostic protocols receiving approval from regulatory bodies in Europe and conditional approval in North America. These developments are accelerating the transition from research applications to standard clinical practice, particularly in specialized metabolic centers and endocrinology departments.
Future directions for clinical translation include the development of integrated multiparametric NMR approaches that simultaneously assess glycogenolysis alongside other metabolic pathways, providing a more comprehensive metabolic profile. Additionally, artificial intelligence algorithms are being trained on NMR spectral databases to improve diagnostic accuracy and personalize treatment recommendations based on individual metabolic patterns.
Standardization and Quality Control in Metabolic NMR Analysis
The field of metabolic NMR analysis, particularly for glycogenolysis studies, requires rigorous standardization and quality control protocols to ensure reliable and reproducible results. Current standardization efforts focus on establishing universal protocols for sample preparation, data acquisition, and spectral analysis across different laboratories and NMR platforms.
Sample preparation represents a critical initial step where standardization is essential. Protocols now specify precise pH ranges (typically 7.0-7.4), buffer compositions, and concentration parameters to minimize variability in chemical shifts. For glycogenolysis studies specifically, standardized methods for tissue extraction and metabolite preservation have been developed to prevent degradation of labile intermediates like glucose-1-phosphate and glucose-6-phosphate.
Data acquisition parameters have seen significant harmonization efforts in recent years. Consensus guidelines now recommend specific pulse sequences optimized for glycolytic/glycogenolytic intermediates, with CPMG and 1D NOESY sequences being preferred for biofluid analysis. Temperature control standards (typically at 298K ± 0.1K) have been implemented to ensure spectral consistency, particularly important for temperature-sensitive metabolic processes like glycogenolysis.
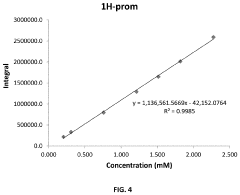
Quality control metrics have evolved substantially, with internal standards now routinely employed. TSP (3-(trimethylsilyl)propionic acid) serves as both a chemical shift reference and quantification standard. Automated quality assessment tools have emerged that evaluate spectral parameters including line width, water suppression efficiency, and baseline stability. These tools generate quality scores that help researchers identify problematic spectra before analysis proceeds.
Interlaboratory ring trials have become increasingly common, where identical samples are analyzed across multiple facilities to assess reproducibility. Recent studies involving glycogenolysis markers showed interlaboratory coefficient of variation improvements from >25% to <10% through standardized protocols. These collaborative efforts have led to the development of minimum reporting standards for metabolic NMR studies, ensuring critical experimental details are consistently documented.
Reference databases specifically for glycogenolysis intermediates have been established, containing authenticated spectral patterns for key metabolites under standardized conditions. These resources enable more reliable peak identification and quantification across different research groups. Additionally, certified reference materials for common metabolites in glycogenolysis pathways are now commercially available, allowing absolute quantification and system performance verification.
Machine learning approaches are increasingly being incorporated into quality control workflows, with algorithms trained to detect spectral artifacts, identify outliers, and flag potentially problematic data automatically. These computational methods complement traditional manual quality assessment and have shown particular utility in large-scale glycogenolysis studies where manual inspection of all spectra would be prohibitively time-consuming.
Sample preparation represents a critical initial step where standardization is essential. Protocols now specify precise pH ranges (typically 7.0-7.4), buffer compositions, and concentration parameters to minimize variability in chemical shifts. For glycogenolysis studies specifically, standardized methods for tissue extraction and metabolite preservation have been developed to prevent degradation of labile intermediates like glucose-1-phosphate and glucose-6-phosphate.
Data acquisition parameters have seen significant harmonization efforts in recent years. Consensus guidelines now recommend specific pulse sequences optimized for glycolytic/glycogenolytic intermediates, with CPMG and 1D NOESY sequences being preferred for biofluid analysis. Temperature control standards (typically at 298K ± 0.1K) have been implemented to ensure spectral consistency, particularly important for temperature-sensitive metabolic processes like glycogenolysis.
Quality control metrics have evolved substantially, with internal standards now routinely employed. TSP (3-(trimethylsilyl)propionic acid) serves as both a chemical shift reference and quantification standard. Automated quality assessment tools have emerged that evaluate spectral parameters including line width, water suppression efficiency, and baseline stability. These tools generate quality scores that help researchers identify problematic spectra before analysis proceeds.
Interlaboratory ring trials have become increasingly common, where identical samples are analyzed across multiple facilities to assess reproducibility. Recent studies involving glycogenolysis markers showed interlaboratory coefficient of variation improvements from >25% to <10% through standardized protocols. These collaborative efforts have led to the development of minimum reporting standards for metabolic NMR studies, ensuring critical experimental details are consistently documented.
Reference databases specifically for glycogenolysis intermediates have been established, containing authenticated spectral patterns for key metabolites under standardized conditions. These resources enable more reliable peak identification and quantification across different research groups. Additionally, certified reference materials for common metabolites in glycogenolysis pathways are now commercially available, allowing absolute quantification and system performance verification.
Machine learning approaches are increasingly being incorporated into quality control workflows, with algorithms trained to detect spectral artifacts, identify outliers, and flag potentially problematic data automatically. These computational methods complement traditional manual quality assessment and have shown particular utility in large-scale glycogenolysis studies where manual inspection of all spectra would be prohibitively time-consuming.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!