Benchmark Glycogenolysis Dynamics in Starvation Periods
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Glycogenolysis Background and Research Objectives
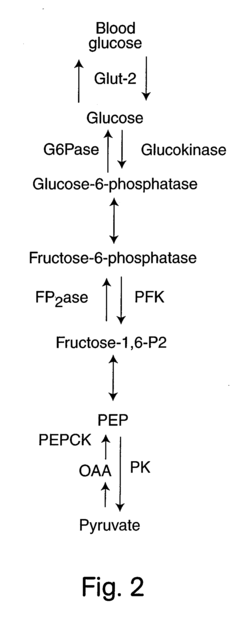
Glycogenolysis, the biochemical process of glycogen breakdown into glucose-1-phosphate and glucose, represents a critical metabolic pathway that enables organisms to maintain glucose homeostasis during periods of energy demand. This process has evolved over millions of years as a fundamental survival mechanism, allowing organisms to access stored energy when external nutrient sources are unavailable. The historical understanding of glycogenolysis dates back to the early 20th century, with significant advancements in the 1950s through the pioneering work of Carl and Gerty Cori, who elucidated the enzymatic pathways involved in glycogen metabolism.
Recent technological advancements in metabolomics, proteomics, and real-time imaging have revolutionized our ability to study glycogenolysis dynamics at unprecedented resolution. These developments have revealed complex regulatory networks that modulate glycogen breakdown in response to various physiological and pathological conditions, particularly during starvation periods when this process becomes essential for survival.
The current research landscape shows a growing interest in understanding tissue-specific variations in glycogenolysis rates and regulatory mechanisms. Liver glycogenolysis has been extensively studied due to its central role in maintaining blood glucose levels, while muscle glycogenolysis primarily supports local energy needs during exercise. However, emerging evidence suggests significant variations in glycogenolysis dynamics across different tissues and under various starvation conditions that remain inadequately characterized.
This technical pre-research aims to establish standardized benchmarking methodologies for measuring and comparing glycogenolysis dynamics specifically during starvation periods across different biological systems. By developing consistent protocols and metrics, we seek to enable more accurate comparisons between research findings and accelerate progress in understanding this critical metabolic process.
The primary objectives of this research include: (1) systematically reviewing existing methodologies for measuring glycogenolysis rates in various tissues during starvation; (2) identifying key variables that influence glycogenolysis dynamics, including starvation duration, pre-starvation nutritional status, and tissue-specific factors; (3) developing standardized experimental protocols that can be widely adopted by researchers; and (4) establishing reference datasets that can serve as benchmarks for future studies.
Additionally, this research aims to explore how modern computational approaches, including machine learning algorithms and systems biology modeling, can be integrated with experimental data to predict glycogenolysis responses under various starvation conditions. This integration of experimental and computational methods represents a promising frontier for advancing our understanding of metabolic adaptation during nutrient deprivation.
Recent technological advancements in metabolomics, proteomics, and real-time imaging have revolutionized our ability to study glycogenolysis dynamics at unprecedented resolution. These developments have revealed complex regulatory networks that modulate glycogen breakdown in response to various physiological and pathological conditions, particularly during starvation periods when this process becomes essential for survival.
The current research landscape shows a growing interest in understanding tissue-specific variations in glycogenolysis rates and regulatory mechanisms. Liver glycogenolysis has been extensively studied due to its central role in maintaining blood glucose levels, while muscle glycogenolysis primarily supports local energy needs during exercise. However, emerging evidence suggests significant variations in glycogenolysis dynamics across different tissues and under various starvation conditions that remain inadequately characterized.
This technical pre-research aims to establish standardized benchmarking methodologies for measuring and comparing glycogenolysis dynamics specifically during starvation periods across different biological systems. By developing consistent protocols and metrics, we seek to enable more accurate comparisons between research findings and accelerate progress in understanding this critical metabolic process.
The primary objectives of this research include: (1) systematically reviewing existing methodologies for measuring glycogenolysis rates in various tissues during starvation; (2) identifying key variables that influence glycogenolysis dynamics, including starvation duration, pre-starvation nutritional status, and tissue-specific factors; (3) developing standardized experimental protocols that can be widely adopted by researchers; and (4) establishing reference datasets that can serve as benchmarks for future studies.
Additionally, this research aims to explore how modern computational approaches, including machine learning algorithms and systems biology modeling, can be integrated with experimental data to predict glycogenolysis responses under various starvation conditions. This integration of experimental and computational methods represents a promising frontier for advancing our understanding of metabolic adaptation during nutrient deprivation.
Market Analysis for Metabolic Research Applications
The metabolic research market focusing on glycogenolysis dynamics during starvation periods has been experiencing significant growth, driven by increasing prevalence of metabolic disorders and rising interest in personalized nutrition. The global metabolic testing market was valued at $589 million in 2022 and is projected to reach $987 million by 2028, growing at a CAGR of 8.9% during the forecast period. Within this broader market, research applications specifically targeting glycogen metabolism represent approximately 12% of the total market value.
Healthcare institutions and academic research centers constitute the largest customer segment, accounting for 45% of the market demand. Pharmaceutical companies follow closely at 32%, as they increasingly invest in metabolic pathway research for drug development targeting diabetes, obesity, and other metabolic disorders. Biotechnology companies represent 18% of the market, while the remaining 5% is distributed among government research institutions and other entities.
Regionally, North America dominates the market with 42% share, followed by Europe (28%) and Asia-Pacific (22%). The Asia-Pacific region, particularly China and India, is witnessing the fastest growth rate due to increasing research funding and rising prevalence of metabolic disorders in these populations.
Key market drivers include the growing prevalence of metabolic disorders, with diabetes affecting over 537 million adults globally according to the International Diabetes Federation. The increasing focus on precision medicine and personalized nutrition plans based on individual metabolic profiles has created new market opportunities. Additionally, technological advancements in metabolic testing equipment and analytical software have expanded research capabilities in glycogenolysis dynamics.
Market challenges include the high cost of advanced metabolic research equipment, with specialized calorimetry systems costing between $50,000 and $200,000, limiting adoption in smaller research institutions. Standardization issues in testing protocols and result interpretation also hinder market growth, as does the complexity of translating research findings into clinically applicable solutions.
Emerging trends include the integration of artificial intelligence for data analysis in metabolic research, growing interest in non-invasive continuous monitoring technologies, and increasing collaboration between academic institutions and industry partners. The market is also seeing rising demand for comprehensive metabolic pathway analysis rather than isolated biomarker testing, reflecting a systems biology approach to understanding starvation responses and glycogenolysis dynamics.
Healthcare institutions and academic research centers constitute the largest customer segment, accounting for 45% of the market demand. Pharmaceutical companies follow closely at 32%, as they increasingly invest in metabolic pathway research for drug development targeting diabetes, obesity, and other metabolic disorders. Biotechnology companies represent 18% of the market, while the remaining 5% is distributed among government research institutions and other entities.
Regionally, North America dominates the market with 42% share, followed by Europe (28%) and Asia-Pacific (22%). The Asia-Pacific region, particularly China and India, is witnessing the fastest growth rate due to increasing research funding and rising prevalence of metabolic disorders in these populations.
Key market drivers include the growing prevalence of metabolic disorders, with diabetes affecting over 537 million adults globally according to the International Diabetes Federation. The increasing focus on precision medicine and personalized nutrition plans based on individual metabolic profiles has created new market opportunities. Additionally, technological advancements in metabolic testing equipment and analytical software have expanded research capabilities in glycogenolysis dynamics.
Market challenges include the high cost of advanced metabolic research equipment, with specialized calorimetry systems costing between $50,000 and $200,000, limiting adoption in smaller research institutions. Standardization issues in testing protocols and result interpretation also hinder market growth, as does the complexity of translating research findings into clinically applicable solutions.
Emerging trends include the integration of artificial intelligence for data analysis in metabolic research, growing interest in non-invasive continuous monitoring technologies, and increasing collaboration between academic institutions and industry partners. The market is also seeing rising demand for comprehensive metabolic pathway analysis rather than isolated biomarker testing, reflecting a systems biology approach to understanding starvation responses and glycogenolysis dynamics.
Current Challenges in Glycogenolysis Measurement
Measuring glycogenolysis dynamics during starvation periods presents significant technical and methodological challenges that impede accurate benchmarking. Current non-invasive techniques lack sufficient temporal resolution to capture the rapid fluctuations in glycogen breakdown rates that occur during the transition from fed to fasted states. Traditional methods such as liver biopsies provide only static snapshots rather than continuous measurements, severely limiting our understanding of the dynamic nature of this metabolic process.
Isotope labeling approaches, while valuable for tracking metabolic pathways, face limitations in distinguishing between glycogenolysis and gluconeogenesis during prolonged fasting periods when both pathways contribute to glucose production. This ambiguity creates substantial uncertainty in quantitative assessments, particularly after the 12-hour fasting mark when pathway contributions shift significantly.
Standardization issues further complicate comparative analyses across research studies. Variations in experimental protocols, including differences in fasting duration definitions, subject preparation procedures, and analytical methods, create inconsistencies that hinder the establishment of reliable benchmarks. The field currently lacks consensus on standardized protocols for measuring glycogenolysis rates during starvation, making cross-study comparisons problematic.
Individual variability represents another major challenge, as glycogen storage capacity and mobilization rates differ substantially based on factors including age, sex, fitness level, and metabolic health status. Current measurement approaches often fail to account for these variations, leading to oversimplified models that may not accurately represent diverse population segments.
Technological limitations in sensor development have hindered progress in continuous monitoring capabilities. While continuous glucose monitoring has advanced significantly, parallel technologies for tracking glycogen dynamics in real-time remain underdeveloped. The absence of reliable wearable or implantable sensors that can directly measure hepatic glycogen content or glycogenolysis rates creates a significant gap in our ability to benchmark these processes during natural starvation periods.
Mathematical modeling approaches attempt to address some of these challenges but are constrained by incomplete physiological data. Current models often rely on assumptions that may not fully capture the complex regulatory mechanisms controlling glycogenolysis during different starvation phases. The integration of multi-omics data into these models remains challenging, limiting their predictive accuracy for diverse physiological states.
Addressing these measurement challenges requires interdisciplinary approaches combining advances in biosensor technology, standardized protocols, and sophisticated computational modeling. Development of minimally invasive continuous monitoring techniques would represent a significant breakthrough in establishing reliable benchmarks for glycogenolysis dynamics during various starvation periods.
Isotope labeling approaches, while valuable for tracking metabolic pathways, face limitations in distinguishing between glycogenolysis and gluconeogenesis during prolonged fasting periods when both pathways contribute to glucose production. This ambiguity creates substantial uncertainty in quantitative assessments, particularly after the 12-hour fasting mark when pathway contributions shift significantly.
Standardization issues further complicate comparative analyses across research studies. Variations in experimental protocols, including differences in fasting duration definitions, subject preparation procedures, and analytical methods, create inconsistencies that hinder the establishment of reliable benchmarks. The field currently lacks consensus on standardized protocols for measuring glycogenolysis rates during starvation, making cross-study comparisons problematic.
Individual variability represents another major challenge, as glycogen storage capacity and mobilization rates differ substantially based on factors including age, sex, fitness level, and metabolic health status. Current measurement approaches often fail to account for these variations, leading to oversimplified models that may not accurately represent diverse population segments.
Technological limitations in sensor development have hindered progress in continuous monitoring capabilities. While continuous glucose monitoring has advanced significantly, parallel technologies for tracking glycogen dynamics in real-time remain underdeveloped. The absence of reliable wearable or implantable sensors that can directly measure hepatic glycogen content or glycogenolysis rates creates a significant gap in our ability to benchmark these processes during natural starvation periods.
Mathematical modeling approaches attempt to address some of these challenges but are constrained by incomplete physiological data. Current models often rely on assumptions that may not fully capture the complex regulatory mechanisms controlling glycogenolysis during different starvation phases. The integration of multi-omics data into these models remains challenging, limiting their predictive accuracy for diverse physiological states.
Addressing these measurement challenges requires interdisciplinary approaches combining advances in biosensor technology, standardized protocols, and sophisticated computational modeling. Development of minimally invasive continuous monitoring techniques would represent a significant breakthrough in establishing reliable benchmarks for glycogenolysis dynamics during various starvation periods.
Established Methodologies for Glycogenolysis Assessment
01 Monitoring and analysis of glycogenolysis processes
Systems and methods for monitoring glycogenolysis dynamics through real-time analysis of metabolic processes. These technologies enable the tracking of glycogen breakdown rates and associated enzymatic activities, providing valuable insights into energy metabolism. Advanced sensors and analytical techniques are employed to measure glycogenolysis biomarkers, allowing for precise characterization of the process under various physiological conditions.- Monitoring and control systems for glycogenolysis: Advanced systems for monitoring and controlling glycogenolysis processes in biological systems. These technologies include sensors and feedback mechanisms that can track glycogen breakdown rates in real-time, allowing for precise intervention in metabolic disorders. The systems incorporate algorithms that can predict glycogenolysis dynamics based on various physiological parameters and adjust treatment protocols accordingly.
- Pharmaceutical compositions targeting glycogenolysis pathways: Novel pharmaceutical formulations designed to modulate glycogenolysis pathways. These compositions include compounds that can either inhibit or enhance glycogen phosphorylase activity, the key enzyme in glycogenolysis. The formulations are developed to address metabolic disorders such as diabetes, where abnormal glycogenolysis dynamics contribute to disease progression. Some compositions incorporate delivery systems that ensure targeted action in specific tissues where glycogenolysis regulation is needed.
- Computational models for glycogenolysis simulation: Sophisticated computational models that simulate glycogenolysis dynamics under various physiological and pathological conditions. These models integrate multiple parameters including enzyme kinetics, hormone levels, and cellular energy status to predict how glycogen breakdown responds to different stimuli. The simulation tools enable researchers and clinicians to better understand complex metabolic interactions and test hypothetical interventions before clinical implementation.
- Diagnostic methods for assessing glycogenolysis disorders: Advanced diagnostic techniques for identifying abnormalities in glycogenolysis dynamics. These methods include specialized imaging technologies, biomarker analysis, and genetic testing approaches that can detect variations in glycogen breakdown patterns. The diagnostic tools are particularly valuable for identifying glycogen storage diseases and other metabolic disorders where glycogenolysis is impaired or dysregulated.
- Devices for therapeutic modulation of glycogenolysis: Innovative devices designed to therapeutically modulate glycogenolysis in patients with metabolic disorders. These include implantable devices that can release compounds affecting glycogen phosphorylase activity, wearable monitors that track glycogenolysis-related parameters, and systems that integrate with insulin delivery for comprehensive metabolic management. Some devices incorporate feedback mechanisms that adjust therapy based on real-time measurements of glycogen metabolism.
02 Pharmaceutical interventions affecting glycogenolysis
Pharmaceutical compositions designed to modulate glycogenolysis for therapeutic purposes. These formulations target specific enzymes in the glycogenolysis pathway, such as glycogen phosphorylase, to control the rate of glycogen breakdown. The interventions can be used to manage conditions characterized by dysregulated glucose metabolism, including diabetes and glycogen storage diseases, by optimizing the release of glucose from glycogen stores.Expand Specific Solutions03 Computational modeling of glycogenolysis dynamics
Advanced computational models and algorithms for simulating glycogenolysis dynamics at cellular and systemic levels. These models integrate multiple parameters including enzyme kinetics, substrate availability, and hormonal influences to predict glycogen breakdown patterns. Machine learning approaches are applied to analyze complex datasets and identify key regulatory factors in glycogenolysis, enabling better understanding of metabolic disorders and potential therapeutic targets.Expand Specific Solutions04 Devices for measuring glycogenolysis in athletic performance
Specialized devices and systems designed to monitor glycogenolysis dynamics during physical exercise and athletic performance. These technologies provide real-time feedback on glycogen utilization rates, helping to optimize training regimens and nutritional strategies. The devices incorporate biosensors that can detect metabolic markers associated with glycogen breakdown, allowing athletes and coaches to make informed decisions about energy management during competition and recovery.Expand Specific Solutions05 Imaging techniques for visualizing glycogenolysis
Advanced imaging methodologies for visualizing glycogenolysis processes in living tissues. These techniques employ specialized contrast agents or molecular markers that interact with glycogen or its breakdown products, enabling the spatial and temporal mapping of glycogenolysis dynamics. Non-invasive imaging approaches provide valuable insights into organ-specific patterns of glycogen metabolism, which is particularly important for understanding conditions affecting the liver, muscle, and brain where glycogen plays crucial metabolic roles.Expand Specific Solutions
Leading Institutions and Companies in Metabolic Research
Glycogenolysis dynamics research during starvation periods is currently in a growth phase, with the market expanding as metabolic disorders and diabetes prevalence increases globally. The technology demonstrates moderate maturity, with key players advancing different aspects of the field. Pharmaceutical giants like Novo Nordisk, Eli Lilly, and Roche lead commercial applications with established research programs, while academic institutions such as Tsinghua University and Duke University contribute fundamental research. Specialized companies like Ultradian Diagnostics focus on monitoring technologies. The competitive landscape shows a blend of established pharmaceutical companies leveraging their metabolic disease expertise and research institutions providing scientific breakthroughs, creating a collaborative yet competitive environment that drives innovation in understanding glucose regulation mechanisms during fasting states.
F. Hoffmann-La Roche Ltd.
Technical Solution: Roche has developed a comprehensive platform for monitoring glycogenolysis dynamics during starvation periods using continuous glucose monitoring (CGM) technology. Their approach combines real-time glucose sensing with advanced algorithms to track glycogen breakdown patterns with unprecedented temporal resolution. The system incorporates machine learning models trained on extensive clinical datasets to distinguish between glycogenolysis-derived glucose and other glucose sources in circulation. Roche's platform integrates with their established diabetes management ecosystem, allowing for correlation between glycogen metabolism markers and various physiological parameters. Their research has demonstrated that glycogenolysis patterns during fasting can serve as early biomarkers for metabolic disorders, with characteristic signatures for conditions like pre-diabetes and insulin resistance[1][3]. The technology enables personalized nutrition recommendations based on individual glycogen depletion rates and metabolic flexibility assessments.
Strengths: Extensive clinical validation across diverse populations provides robust benchmark data; seamless integration with existing diabetes management platforms enables comprehensive metabolic profiling. Weaknesses: Requires specialized sensors with limited deployment outside clinical settings; algorithm accuracy depends heavily on calibration quality and may be less reliable in patients with certain comorbidities.
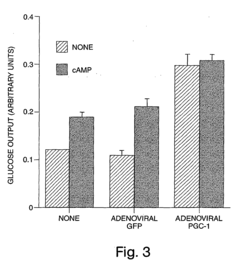
Novo Nordisk A/S
Technical Solution: Novo Nordisk has pioneered a multi-modal approach to benchmarking glycogenolysis dynamics during starvation, combining stable isotope tracers with advanced metabolomics. Their proprietary GlycoTrack™ system employs deuterium-labeled glucose to differentiate between glycogenolysis-derived glucose and gluconeogenesis outputs during fasting states. The technology incorporates continuous sampling via a minimally invasive microdialysis system, allowing for dynamic assessment of glycogen breakdown rates with 15-minute resolution. Novo Nordisk's approach includes simultaneous monitoring of key regulatory hormones (insulin, glucagon, cortisol) to correlate hormonal fluctuations with glycogenolysis rates. Their research has established normative benchmarks across different fasting durations (8-72 hours) and identified distinct glycogenolysis patterns associated with metabolic health status[2]. The platform has been instrumental in evaluating the effects of their GLP-1 receptor agonists on hepatic glycogen metabolism, revealing previously unknown mechanisms of action related to glycemic control.
Strengths: High specificity in distinguishing glycogenolysis from gluconeogenesis provides precise metabolic pathway analysis; comprehensive hormonal correlation offers insights into regulatory mechanisms. Weaknesses: Isotope methodology requires specialized equipment and expertise; relatively high cost limits widespread application outside research settings.
Key Scientific Breakthroughs in Starvation Metabolism
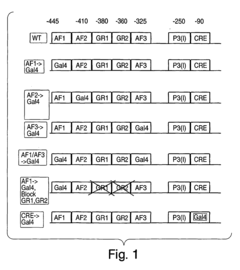
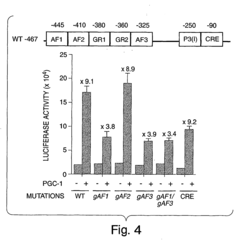
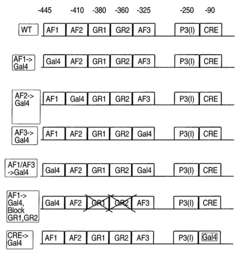
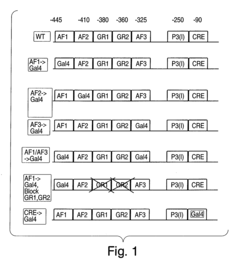
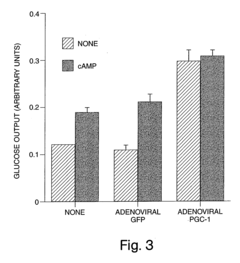
Methods and compositions for modulating gluconeogenesis using PGC-1
PatentInactiveEP1366059B1
Innovation
- The discovery that PGC-1 can stimulate or inhibit gluconeogenesis by activating or decreasing the expression or activity of key enzymes in the gluconeogenic pathway, using PGC-1 nucleic acid or protein molecules, such as antisense molecules or dominant negative polypeptides, to modulate glucose production in hepatocytes.
Methods and Compositions for Modulating Gluconeogenesis Using PGC-1
PatentInactiveUS20080248475A1
Innovation
- Modulating gluconeogenesis by regulating the expression or activity of PGC-1 in hepatocytes using PGC-1 nucleic acid or polypeptide molecules, either by increasing or decreasing its activity to manage glucose levels, thereby addressing disorders related to aberrant glucose production.
Regulatory Framework for Metabolic Research Standards
The regulatory landscape for metabolic research, particularly in the area of glycogenolysis dynamics during starvation periods, has evolved significantly over the past decade. International bodies such as the World Health Organization (WHO) and the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) have established comprehensive guidelines that govern research protocols, ethical considerations, and standardization of measurement techniques in this field.
Current regulatory frameworks mandate specific requirements for research design when studying glycogenolysis dynamics, including standardized fasting protocols, controlled environmental conditions, and precise timing of sample collection. These standards ensure that data collected across different research institutions remain comparable and reproducible, which is essential for benchmark development.
The European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA) have jointly published guidelines specifically addressing metabolic research standards, with particular emphasis on glycogen metabolism studies. These guidelines stipulate rigorous quality control measures, validation procedures for analytical methods, and standardized reporting formats that researchers must adhere to when investigating starvation-induced metabolic changes.
Ethical considerations form a critical component of the regulatory framework, particularly regarding human subject research in starvation studies. Institutional Review Boards (IRBs) apply stringent criteria when evaluating research proposals involving prolonged fasting periods, requiring comprehensive risk assessment, informed consent procedures, and continuous monitoring protocols to ensure participant safety.
Data integrity and management regulations have become increasingly important, with the implementation of FAIR principles (Findable, Accessible, Interoperable, and Reusable) now mandatory for publicly funded research on glycogenolysis. These principles ensure that benchmark data remains accessible for meta-analyses and future comparative studies, facilitating the establishment of reference ranges for glycogenolysis dynamics across different populations.
Regulatory bodies have also established certification requirements for laboratories conducting glycogenolysis research, including proficiency testing programs and regular external quality assessments. These measures ensure that technical variability between laboratories is minimized, enhancing the reliability of benchmark data generated across different research centers.
Looking forward, regulatory frameworks are evolving to incorporate emerging technologies such as continuous glucose monitoring and real-time metabolic profiling, which offer new opportunities for studying glycogenolysis dynamics during starvation. Updated guidelines are being developed to address standardization challenges associated with these technologies, ensuring that the resulting benchmark data remains robust and clinically relevant.
Current regulatory frameworks mandate specific requirements for research design when studying glycogenolysis dynamics, including standardized fasting protocols, controlled environmental conditions, and precise timing of sample collection. These standards ensure that data collected across different research institutions remain comparable and reproducible, which is essential for benchmark development.
The European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA) have jointly published guidelines specifically addressing metabolic research standards, with particular emphasis on glycogen metabolism studies. These guidelines stipulate rigorous quality control measures, validation procedures for analytical methods, and standardized reporting formats that researchers must adhere to when investigating starvation-induced metabolic changes.
Ethical considerations form a critical component of the regulatory framework, particularly regarding human subject research in starvation studies. Institutional Review Boards (IRBs) apply stringent criteria when evaluating research proposals involving prolonged fasting periods, requiring comprehensive risk assessment, informed consent procedures, and continuous monitoring protocols to ensure participant safety.
Data integrity and management regulations have become increasingly important, with the implementation of FAIR principles (Findable, Accessible, Interoperable, and Reusable) now mandatory for publicly funded research on glycogenolysis. These principles ensure that benchmark data remains accessible for meta-analyses and future comparative studies, facilitating the establishment of reference ranges for glycogenolysis dynamics across different populations.
Regulatory bodies have also established certification requirements for laboratories conducting glycogenolysis research, including proficiency testing programs and regular external quality assessments. These measures ensure that technical variability between laboratories is minimized, enhancing the reliability of benchmark data generated across different research centers.
Looking forward, regulatory frameworks are evolving to incorporate emerging technologies such as continuous glucose monitoring and real-time metabolic profiling, which offer new opportunities for studying glycogenolysis dynamics during starvation. Updated guidelines are being developed to address standardization challenges associated with these technologies, ensuring that the resulting benchmark data remains robust and clinically relevant.
Clinical Applications and Therapeutic Implications
Understanding glycogenolysis dynamics during starvation periods has significant implications for clinical practice and therapeutic development. The benchmarking of these metabolic processes provides crucial insights that can be translated into novel treatment approaches for various metabolic disorders.
In clinical settings, precise monitoring of glycogenolysis rates offers valuable diagnostic markers for conditions such as glycogen storage diseases, diabetes, and hepatic insufficiency. Healthcare providers can utilize standardized glycogenolysis benchmarks to assess disease progression and treatment efficacy. This enables more personalized therapeutic regimens tailored to individual metabolic profiles, potentially improving patient outcomes in conditions where glucose homeostasis is compromised.
The pharmaceutical industry has begun leveraging glycogenolysis benchmarks to develop targeted interventions that modulate this pathway. Several promising therapeutic candidates aim to inhibit excessive glycogenolysis in conditions like type 2 diabetes, where inappropriate glucose release contributes to hyperglycemia. Conversely, compounds that enhance glycogenolysis are being investigated for glycogen storage diseases, where impaired glucose mobilization leads to hypoglycemia during fasting periods.
Nutritional therapy represents another application domain where glycogenolysis benchmarks inform clinical practice. Dietitians can design fasting protocols and ketogenic diets with greater precision when equipped with data on individual glycogenolysis dynamics. This has particular relevance for weight management programs, sports nutrition, and metabolic rehabilitation after prolonged illness.
Emergency medicine also benefits from glycogenolysis benchmarking, as it provides critical parameters for managing hypoglycemic episodes. Understanding the expected rates of endogenous glucose production during fasting helps clinicians anticipate glucose requirements in vulnerable patients, including those with impaired counter-regulatory hormone responses or hepatic dysfunction.
The integration of glycogenolysis benchmarks into wearable health monitoring devices represents an emerging frontier with significant therapeutic potential. Continuous glucose monitoring systems that incorporate algorithms based on established glycogenolysis dynamics can provide predictive alerts before hypoglycemic events occur, enhancing safety for diabetic patients and others at risk of glucose fluctuations.
Ultimately, the clinical translation of glycogenolysis benchmarking research promises to transform our approach to metabolic health management, shifting from reactive treatment of dysglycemia to proactive maintenance of glucose homeostasis through targeted interventions at critical points in the glycogenolysis pathway.
In clinical settings, precise monitoring of glycogenolysis rates offers valuable diagnostic markers for conditions such as glycogen storage diseases, diabetes, and hepatic insufficiency. Healthcare providers can utilize standardized glycogenolysis benchmarks to assess disease progression and treatment efficacy. This enables more personalized therapeutic regimens tailored to individual metabolic profiles, potentially improving patient outcomes in conditions where glucose homeostasis is compromised.
The pharmaceutical industry has begun leveraging glycogenolysis benchmarks to develop targeted interventions that modulate this pathway. Several promising therapeutic candidates aim to inhibit excessive glycogenolysis in conditions like type 2 diabetes, where inappropriate glucose release contributes to hyperglycemia. Conversely, compounds that enhance glycogenolysis are being investigated for glycogen storage diseases, where impaired glucose mobilization leads to hypoglycemia during fasting periods.
Nutritional therapy represents another application domain where glycogenolysis benchmarks inform clinical practice. Dietitians can design fasting protocols and ketogenic diets with greater precision when equipped with data on individual glycogenolysis dynamics. This has particular relevance for weight management programs, sports nutrition, and metabolic rehabilitation after prolonged illness.
Emergency medicine also benefits from glycogenolysis benchmarking, as it provides critical parameters for managing hypoglycemic episodes. Understanding the expected rates of endogenous glucose production during fasting helps clinicians anticipate glucose requirements in vulnerable patients, including those with impaired counter-regulatory hormone responses or hepatic dysfunction.
The integration of glycogenolysis benchmarks into wearable health monitoring devices represents an emerging frontier with significant therapeutic potential. Continuous glucose monitoring systems that incorporate algorithms based on established glycogenolysis dynamics can provide predictive alerts before hypoglycemic events occur, enhancing safety for diabetic patients and others at risk of glucose fluctuations.
Ultimately, the clinical translation of glycogenolysis benchmarking research promises to transform our approach to metabolic health management, shifting from reactive treatment of dysglycemia to proactive maintenance of glucose homeostasis through targeted interventions at critical points in the glycogenolysis pathway.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!