How to Measure Glycogenolysis Rate in Liver Tissue
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Glycogenolysis Measurement Background and Objectives
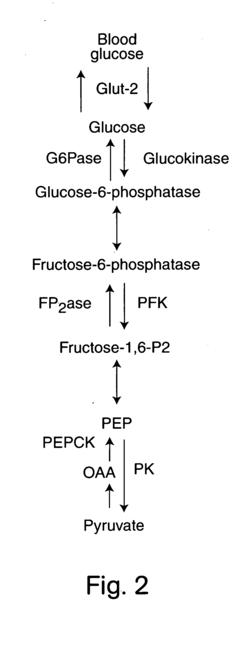
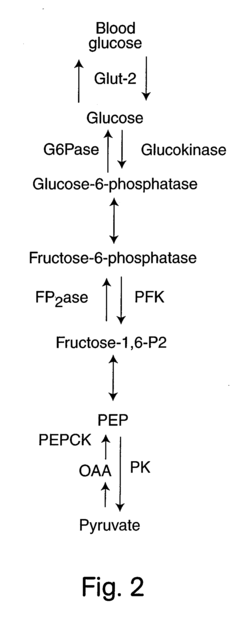
Glycogenolysis, the breakdown of glycogen to glucose-1-phosphate and glucose, represents a critical metabolic pathway in liver tissue that maintains blood glucose homeostasis during fasting states. The scientific exploration of this process dates back to the early 20th century, with significant advancements occurring in the 1950s through the pioneering work of Carl and Gerty Cori, who elucidated the fundamental biochemical pathways involved. Since then, our understanding of glycogenolysis has evolved substantially, revealing its complex regulation by hormonal signals, particularly glucagon and epinephrine, as well as its dysregulation in various pathological conditions including diabetes, glycogen storage diseases, and non-alcoholic fatty liver disease.
The accurate measurement of glycogenolysis rates in liver tissue has become increasingly important in both clinical and research settings. Traditionally, methods relied on indirect assessments or invasive procedures with limited temporal resolution. However, technological advancements in recent decades have enabled more sophisticated approaches, including isotope labeling techniques, nuclear magnetic resonance spectroscopy, and various biochemical assays that offer improved precision and reduced invasiveness.
Current research trends indicate a growing interest in real-time monitoring of glycogenolysis in intact liver tissue, which presents significant technical challenges but promises unprecedented insights into metabolic regulation. Additionally, there is increasing focus on understanding the spatial heterogeneity of glycogenolysis within the liver lobule, as different hepatocyte populations may exhibit distinct metabolic behaviors depending on their zonal location.
The primary objectives of glycogenolysis measurement techniques development are multifold. First, to establish standardized, reproducible methodologies that can accurately quantify the rate of glycogen breakdown under various physiological and pathological conditions. Second, to develop minimally invasive techniques suitable for clinical applications, potentially enabling improved diagnosis and monitoring of metabolic disorders. Third, to create tools capable of distinguishing between direct glycogenolysis (glycogen to glucose) and indirect pathways (glycogen to lactate or other metabolites).
Furthermore, there is a pressing need for techniques that can integrate glycogenolysis measurements with other metabolic parameters, providing a comprehensive view of hepatic glucose metabolism. This holistic approach would significantly enhance our understanding of how glycogenolysis coordinates with gluconeogenesis, glycolysis, and other pathways to maintain glucose homeostasis under different nutritional states and in disease conditions.
The evolution of these measurement techniques is expected to facilitate breakthroughs in understanding the molecular mechanisms governing glycogenolysis regulation, potentially leading to novel therapeutic strategies for metabolic disorders characterized by dysregulated hepatic glucose production.
The accurate measurement of glycogenolysis rates in liver tissue has become increasingly important in both clinical and research settings. Traditionally, methods relied on indirect assessments or invasive procedures with limited temporal resolution. However, technological advancements in recent decades have enabled more sophisticated approaches, including isotope labeling techniques, nuclear magnetic resonance spectroscopy, and various biochemical assays that offer improved precision and reduced invasiveness.
Current research trends indicate a growing interest in real-time monitoring of glycogenolysis in intact liver tissue, which presents significant technical challenges but promises unprecedented insights into metabolic regulation. Additionally, there is increasing focus on understanding the spatial heterogeneity of glycogenolysis within the liver lobule, as different hepatocyte populations may exhibit distinct metabolic behaviors depending on their zonal location.
The primary objectives of glycogenolysis measurement techniques development are multifold. First, to establish standardized, reproducible methodologies that can accurately quantify the rate of glycogen breakdown under various physiological and pathological conditions. Second, to develop minimally invasive techniques suitable for clinical applications, potentially enabling improved diagnosis and monitoring of metabolic disorders. Third, to create tools capable of distinguishing between direct glycogenolysis (glycogen to glucose) and indirect pathways (glycogen to lactate or other metabolites).
Furthermore, there is a pressing need for techniques that can integrate glycogenolysis measurements with other metabolic parameters, providing a comprehensive view of hepatic glucose metabolism. This holistic approach would significantly enhance our understanding of how glycogenolysis coordinates with gluconeogenesis, glycolysis, and other pathways to maintain glucose homeostasis under different nutritional states and in disease conditions.
The evolution of these measurement techniques is expected to facilitate breakthroughs in understanding the molecular mechanisms governing glycogenolysis regulation, potentially leading to novel therapeutic strategies for metabolic disorders characterized by dysregulated hepatic glucose production.
Clinical and Research Market Demand Analysis
The market for glycogenolysis rate measurement in liver tissue spans both clinical and research domains, with significant growth potential driven by increasing prevalence of liver-related disorders. The global liver disease diagnostics market was valued at $32.9 billion in 2021 and is projected to reach $50.4 billion by 2028, growing at a CAGR of 6.3%. Within this broader market, technologies specifically focused on measuring glycogen metabolism represent a specialized but expanding segment.
In clinical settings, the demand for precise glycogenolysis measurement techniques stems primarily from the rising incidence of metabolic disorders such as diabetes, non-alcoholic fatty liver disease (NAFLD), and glycogen storage diseases. NAFLD alone affects approximately 25% of the global population, creating substantial need for diagnostic tools that can assess liver metabolic function. Hospitals and specialized liver centers constitute the largest market segment, particularly in regions with high prevalence of metabolic syndrome.
The research market demonstrates equally robust demand, driven by pharmaceutical companies investing in drug development for metabolic disorders. The global market for liver disease therapeutics reached $20.3 billion in 2022, with companies allocating significant R&D budgets to develop treatments targeting glycogen metabolism pathways. Academic and research institutions form another key market segment, with increasing grant funding directed toward metabolic research.
Geographically, North America dominates the market due to advanced healthcare infrastructure and higher research spending. However, the Asia-Pacific region shows the fastest growth rate at 8.7% annually, attributed to rising diabetes prevalence and increasing healthcare expenditure in countries like China and India.
Market analysis reveals several unmet needs driving demand for improved glycogenolysis measurement techniques. Current methods often lack real-time capabilities, require invasive procedures, or provide insufficient sensitivity for early-stage metabolic alterations. Stakeholders consistently express demand for non-invasive alternatives that can provide quantitative, reproducible measurements suitable for both clinical monitoring and research applications.
The competitive landscape features established diagnostic companies expanding their liver assessment portfolios alongside specialized biotechnology firms developing novel metabolic assessment technologies. Recent market trends indicate growing interest in integrated platforms that combine glycogenolysis measurement with broader metabolic profiling, reflecting the clinical reality that liver metabolic dysfunction rarely occurs in isolation.
In clinical settings, the demand for precise glycogenolysis measurement techniques stems primarily from the rising incidence of metabolic disorders such as diabetes, non-alcoholic fatty liver disease (NAFLD), and glycogen storage diseases. NAFLD alone affects approximately 25% of the global population, creating substantial need for diagnostic tools that can assess liver metabolic function. Hospitals and specialized liver centers constitute the largest market segment, particularly in regions with high prevalence of metabolic syndrome.
The research market demonstrates equally robust demand, driven by pharmaceutical companies investing in drug development for metabolic disorders. The global market for liver disease therapeutics reached $20.3 billion in 2022, with companies allocating significant R&D budgets to develop treatments targeting glycogen metabolism pathways. Academic and research institutions form another key market segment, with increasing grant funding directed toward metabolic research.
Geographically, North America dominates the market due to advanced healthcare infrastructure and higher research spending. However, the Asia-Pacific region shows the fastest growth rate at 8.7% annually, attributed to rising diabetes prevalence and increasing healthcare expenditure in countries like China and India.
Market analysis reveals several unmet needs driving demand for improved glycogenolysis measurement techniques. Current methods often lack real-time capabilities, require invasive procedures, or provide insufficient sensitivity for early-stage metabolic alterations. Stakeholders consistently express demand for non-invasive alternatives that can provide quantitative, reproducible measurements suitable for both clinical monitoring and research applications.
The competitive landscape features established diagnostic companies expanding their liver assessment portfolios alongside specialized biotechnology firms developing novel metabolic assessment technologies. Recent market trends indicate growing interest in integrated platforms that combine glycogenolysis measurement with broader metabolic profiling, reflecting the clinical reality that liver metabolic dysfunction rarely occurs in isolation.
Current Methodologies and Technical Limitations
The measurement of glycogenolysis rate in liver tissue currently employs several established methodologies, each with specific advantages and limitations. The gold standard remains isotopic labeling techniques, particularly using radioactive tracers such as 3H-glucose or 13C-glucose to track glycogen breakdown. This approach provides high sensitivity and specificity but requires specialized equipment, trained personnel, and presents radiation safety concerns that limit widespread clinical application.
Nuclear Magnetic Resonance (NMR) spectroscopy offers a non-invasive alternative for measuring glycogen dynamics in real-time. While 13C-NMR can directly quantify glycogen content changes, its relatively low sensitivity necessitates large sample sizes or extended acquisition times. Additionally, the high cost of NMR equipment and technical expertise required for data interpretation restrict its accessibility to specialized research centers.
Enzymatic assays represent the most accessible methodology, measuring either glycogen content at different time points or glucose release as an indicator of glycogenolysis. These biochemical approaches are cost-effective and widely available but suffer from potential interference from other metabolic pathways and limited temporal resolution, as they typically provide endpoint rather than continuous measurements.
Recent advances in fluorescence-based techniques have enabled more dynamic measurements using fluorescent glucose analogs or FRET-based biosensors. These methods offer improved spatial and temporal resolution but may not fully reflect physiological conditions due to the introduction of non-native molecules into the biological system.
A significant technical limitation across all methodologies is the challenge of distinguishing between glycogenolysis and concurrent metabolic processes such as gluconeogenesis and glycolysis in intact liver tissue. This metabolic complexity often necessitates complementary inhibitor studies or mathematical modeling to isolate the glycogenolysis component.
Sample preparation presents another critical challenge, as glycogen degradation can continue ex vivo, potentially confounding measurements. Rapid tissue fixation or freezing is essential but may introduce artifacts or damage cellular structures relevant to glycogen metabolism.
The heterogeneity of liver tissue further complicates accurate measurement, as glycogenolysis rates can vary significantly between periportal and perivenous regions. Current methods often provide averaged measurements across tissue samples, potentially masking important zone-specific differences in glycogen metabolism that may be physiologically or pathologically relevant.
Standardization remains problematic across laboratories, with variations in tissue handling, analytical procedures, and data interpretation limiting direct comparison between studies and hindering the establishment of reference ranges for normal and pathological conditions.
Nuclear Magnetic Resonance (NMR) spectroscopy offers a non-invasive alternative for measuring glycogen dynamics in real-time. While 13C-NMR can directly quantify glycogen content changes, its relatively low sensitivity necessitates large sample sizes or extended acquisition times. Additionally, the high cost of NMR equipment and technical expertise required for data interpretation restrict its accessibility to specialized research centers.
Enzymatic assays represent the most accessible methodology, measuring either glycogen content at different time points or glucose release as an indicator of glycogenolysis. These biochemical approaches are cost-effective and widely available but suffer from potential interference from other metabolic pathways and limited temporal resolution, as they typically provide endpoint rather than continuous measurements.
Recent advances in fluorescence-based techniques have enabled more dynamic measurements using fluorescent glucose analogs or FRET-based biosensors. These methods offer improved spatial and temporal resolution but may not fully reflect physiological conditions due to the introduction of non-native molecules into the biological system.
A significant technical limitation across all methodologies is the challenge of distinguishing between glycogenolysis and concurrent metabolic processes such as gluconeogenesis and glycolysis in intact liver tissue. This metabolic complexity often necessitates complementary inhibitor studies or mathematical modeling to isolate the glycogenolysis component.
Sample preparation presents another critical challenge, as glycogen degradation can continue ex vivo, potentially confounding measurements. Rapid tissue fixation or freezing is essential but may introduce artifacts or damage cellular structures relevant to glycogen metabolism.
The heterogeneity of liver tissue further complicates accurate measurement, as glycogenolysis rates can vary significantly between periportal and perivenous regions. Current methods often provide averaged measurements across tissue samples, potentially masking important zone-specific differences in glycogen metabolism that may be physiologically or pathologically relevant.
Standardization remains problematic across laboratories, with variations in tissue handling, analytical procedures, and data interpretation limiting direct comparison between studies and hindering the establishment of reference ranges for normal and pathological conditions.
Established Protocols for Hepatic Glycogenolysis Quantification
01 Enzymatic assay methods for glycogenolysis rate measurement
Enzymatic assays are used to measure glycogenolysis rates by quantifying the activity of key enzymes involved in the breakdown of glycogen. These methods typically involve spectrophotometric or fluorometric detection of enzyme activity, allowing for real-time monitoring of glycogen breakdown. The assays can measure the activity of enzymes such as glycogen phosphorylase, which catalyzes the rate-limiting step in glycogenolysis. These methods provide precise measurements of glycogenolysis rates in various tissue samples.- Spectroscopic methods for glycogenolysis rate measurement: Spectroscopic techniques are employed to measure glycogenolysis rates by detecting changes in specific molecular markers. These methods include infrared spectroscopy, Raman spectroscopy, and fluorescence spectroscopy which can monitor the breakdown of glycogen in real-time. The techniques allow for non-invasive or minimally invasive monitoring of glycogen metabolism in tissues and can provide quantitative data on the rate of glycogenolysis under various physiological conditions.
- Enzyme-based assays for glycogenolysis measurement: Enzyme-based methods utilize specific enzymes involved in the glycogenolysis pathway to measure the rate of glycogen breakdown. These assays typically involve the use of glycogen phosphorylase activity measurements or coupled enzyme reactions that produce detectable signals proportional to the rate of glycogenolysis. The methods can be applied to tissue samples, cell cultures, or biological fluids to quantify glycogenolysis rates under different experimental conditions.
- Biosensor technologies for continuous glycogenolysis monitoring: Advanced biosensor technologies enable continuous monitoring of glycogenolysis rates in living systems. These biosensors incorporate specific recognition elements that interact with glycogen breakdown products or enzymes involved in the process. The resulting signals are processed to provide real-time data on glycogenolysis rates. Implantable or wearable biosensors allow for monitoring glycogen metabolism during various activities or in response to different physiological stimuli.
- Imaging techniques for spatial analysis of glycogenolysis: Imaging methods provide spatial information about glycogenolysis rates in tissues or organisms. These techniques include magnetic resonance imaging (MRI), positron emission tomography (PET), and specialized microscopy methods that can visualize glycogen breakdown in specific cellular compartments or tissue regions. The imaging approaches allow researchers to understand the heterogeneity of glycogenolysis rates across different anatomical locations and cell types.
- Computational models and algorithms for glycogenolysis rate calculation: Computational approaches involve mathematical models and algorithms to calculate glycogenolysis rates from experimental data. These methods integrate multiple parameters and measurements to provide comprehensive assessments of glycogen breakdown kinetics. Machine learning algorithms can be used to analyze complex datasets and identify patterns in glycogenolysis rates under various conditions. The computational tools enhance the interpretation of experimental results and enable predictions about glycogenolysis under different physiological or pathological states.
02 Biosensor-based methods for continuous glycogenolysis monitoring
Biosensor technologies enable continuous monitoring of glycogenolysis rates in real-time. These systems typically incorporate enzyme-coupled electrodes or optical sensors that can detect glycogen breakdown products. The biosensors can be implanted or used ex vivo to provide dynamic measurements of glycogenolysis under various physiological conditions. This approach allows for the assessment of glycogenolysis rates in response to different stimuli, such as exercise or hormonal changes, providing valuable data on metabolic regulation.Expand Specific Solutions03 Imaging techniques for in vivo glycogenolysis measurement
Advanced imaging techniques allow for the non-invasive measurement of glycogenolysis rates in living tissues. These methods include magnetic resonance spectroscopy, positron emission tomography, and fluorescence imaging using specific probes that can detect glycogen or its breakdown products. These imaging approaches provide spatial information about glycogenolysis rates in different tissues and organs, allowing researchers to study regional differences in glycogen metabolism under various physiological and pathological conditions.Expand Specific Solutions04 Microfluidic systems for high-throughput glycogenolysis analysis
Microfluidic platforms enable high-throughput analysis of glycogenolysis rates in small sample volumes. These systems integrate sample preparation, enzymatic reactions, and detection components into a single device, allowing for rapid and efficient measurement of glycogen breakdown. The microfluidic approach reduces reagent consumption and increases analytical throughput, making it suitable for screening applications and studies requiring multiple experimental conditions. These systems can be coupled with various detection methods, including electrochemical, optical, or mass spectrometric techniques.Expand Specific Solutions05 Isotope labeling methods for glycogenolysis rate determination
Isotope labeling techniques involve the use of stable or radioactive isotopes to track the fate of glycogen molecules during metabolism. By administering labeled glucose or glycogen precursors and subsequently measuring the appearance of labeled breakdown products, researchers can calculate glycogenolysis rates under various conditions. Mass spectrometry or nuclear magnetic resonance spectroscopy is typically used to detect and quantify the labeled metabolites. This approach provides detailed information about glycogen turnover rates and can distinguish between different metabolic pathways involving glycogen.Expand Specific Solutions
Leading Research Institutions and Biotech Companies
The glycogenolysis rate measurement in liver tissue market is currently in a growth phase, characterized by increasing demand for precise metabolic assessment tools in research and clinical settings. The global market size for liver diagnostic technologies is expanding, driven by rising prevalence of metabolic disorders and liver diseases. Technologically, the field shows moderate maturity with established enzymatic assays, but significant innovation potential exists in real-time monitoring solutions. Key players include Roche Diagnostics, which leads with comprehensive liver function test portfolios; Sysmex Corp. offering specialized diagnostic instruments; Nova Biomedical developing electrochemical analyzers; and academic institutions like Drexel University and Ghent University contributing fundamental research advances. Research collaborations between pharmaceutical companies (Otsuka, Servier) and diagnostic manufacturers are accelerating technological development in this specialized field.
F. Hoffmann-La Roche Ltd.
Technical Solution: Roche has developed a comprehensive approach to measuring glycogenolysis rates in liver tissue using their proprietary Cedex Bio HT Analyzer system. Their method combines traditional enzymatic assays with advanced spectrophotometric techniques to quantify glucose release from glycogen breakdown. The technology employs specific inhibitors of gluconeogenesis to isolate glycogenolysis activity, allowing for precise measurement of glycogen phosphorylase activity in real-time. Roche's system incorporates automated sample preparation with standardized tissue homogenization protocols to ensure consistent results across different liver samples. Their approach includes specialized reagent kits that contain glucose oxidase and peroxidase enzyme systems coupled with colorimetric detection methods to quantify glucose production rates as a direct measure of glycogenolysis[1][3]. The system also features integrated software that calculates glycogenolysis rates based on calibrated standards and accounts for sample-specific variables.
Strengths: High precision and reproducibility with automated workflow reducing human error; comprehensive data analysis software providing standardized results. Weaknesses: Requires specialized equipment with high initial investment; may not be suitable for very small tissue samples due to minimum volume requirements for the analyzer.
Nova Biomedical Corp.
Technical Solution: Nova Biomedical has pioneered a point-of-care approach to measuring glycogenolysis in liver tissue through their StatStrip Glucose measurement system adapted for research applications. Their technology utilizes electrochemical biosensor technology with modified glucose oxidase enzymes specifically optimized for tissue homogenates. The system employs a unique sample preparation protocol that preserves glycogen phosphorylase activity while minimizing interference from other metabolic pathways. Nova's approach includes specialized microelectrodes that can detect glucose production in real-time with minimal sample volumes (as little as 1.2 μL), making it suitable for small liver biopsy specimens[2]. Their method incorporates proprietary algorithms that calculate glycogenolysis rates based on sequential measurements over defined time intervals, allowing researchers to observe dynamic changes in glycogen breakdown. The system includes specialized buffer solutions that maintain optimal pH and cofactor concentrations to ensure enzyme activity reflects in vivo conditions as closely as possible.
Strengths: Minimal sample volume requirements making it ideal for precious samples; rapid analysis time (approximately 6 seconds per measurement) allowing for high-throughput screening. Weaknesses: Lower sensitivity compared to some laboratory-based methods; potential for interference from reducing substances in complex tissue samples.
Key Innovations in Metabolic Rate Detection
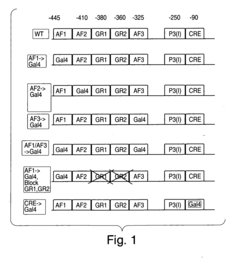
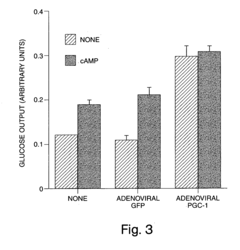
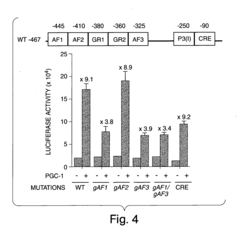
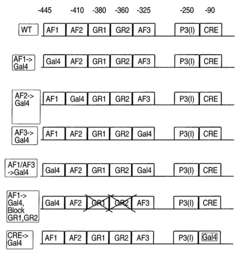
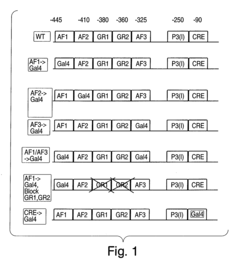
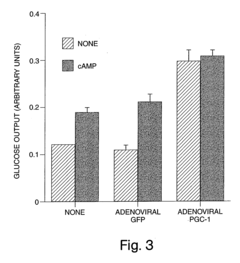
Methods and compositions for modulating gluconeogenesis using PGC-1
PatentInactiveUS20050234001A1
Innovation
- The use of PGC-1 modulating agents, such as nucleic acid molecules or polypeptides, to increase or decrease gluconeogenesis by targeting PGC-1 expression or activity in hepatocytes, thereby regulating glucose production.
Methods and Compositions for Modulating Gluconeogenesis Using PGC-1
PatentInactiveUS20080248475A1
Innovation
- Modulating gluconeogenesis by regulating the expression or activity of PGC-1 in hepatocytes using PGC-1 nucleic acid or polypeptide molecules, either by increasing or decreasing its activity to manage glucose levels, thereby addressing disorders related to aberrant glucose production.
Standardization and Validation Approaches
Standardization of glycogenolysis rate measurement protocols in liver tissue remains a critical challenge in metabolic research. Current methodologies exhibit significant variability across laboratories, complicating cross-study comparisons and meta-analyses. To address this issue, comprehensive validation approaches must be implemented at multiple levels of the measurement process.
Primary standardization efforts should focus on tissue sampling procedures, including consistent liver lobe selection and rapid freezing techniques to prevent post-extraction glycogen degradation. The establishment of reference materials with certified glycogen content would provide crucial calibration standards for both enzymatic and isotopic measurement methods. These materials should represent various physiological states (fed, fasted, diseased) to validate measurement accuracy across the full range of expected glycogenolysis rates.
Method validation requires systematic assessment of analytical performance characteristics. Precision should be evaluated through intra-assay and inter-assay coefficient of variation studies, with acceptable thresholds of <5% and <10% respectively. Accuracy validation necessitates recovery experiments using spiked samples and comparison with established reference methods such as NMR spectroscopy. Sensitivity and detection limits must be determined specifically for each analytical platform, with clear reporting of the minimum quantifiable glycogenolysis rate.
Interlaboratory comparison studies represent a particularly valuable validation approach. Ring trials involving multiple research centers analyzing identical liver samples can identify systematic biases and establish consensus measurement protocols. Such collaborative efforts have successfully standardized other metabolic measurements but remain underutilized for glycogenolysis quantification.
Statistical validation frameworks must address the complex kinetics of glycogenolysis. Time-course experiments with multiple sampling points are essential to validate mathematical models used for rate calculations. Bayesian approaches to parameter estimation can incorporate physiological constraints and prior knowledge, improving the robustness of rate determinations from limited experimental data points.
Emerging technologies, particularly stable isotope techniques and real-time imaging methods, require specialized validation protocols. These should include assessment of isotope equilibration times, potential isotope effects on enzyme kinetics, and spatial resolution validation for imaging approaches. As these technologies transition from research to clinical applications, additional validation against patient outcomes will become increasingly important.
Implementation of these standardization and validation approaches would significantly enhance the reliability and comparability of glycogenolysis measurements, accelerating research progress in hepatic metabolism and related disorders.
Primary standardization efforts should focus on tissue sampling procedures, including consistent liver lobe selection and rapid freezing techniques to prevent post-extraction glycogen degradation. The establishment of reference materials with certified glycogen content would provide crucial calibration standards for both enzymatic and isotopic measurement methods. These materials should represent various physiological states (fed, fasted, diseased) to validate measurement accuracy across the full range of expected glycogenolysis rates.
Method validation requires systematic assessment of analytical performance characteristics. Precision should be evaluated through intra-assay and inter-assay coefficient of variation studies, with acceptable thresholds of <5% and <10% respectively. Accuracy validation necessitates recovery experiments using spiked samples and comparison with established reference methods such as NMR spectroscopy. Sensitivity and detection limits must be determined specifically for each analytical platform, with clear reporting of the minimum quantifiable glycogenolysis rate.
Interlaboratory comparison studies represent a particularly valuable validation approach. Ring trials involving multiple research centers analyzing identical liver samples can identify systematic biases and establish consensus measurement protocols. Such collaborative efforts have successfully standardized other metabolic measurements but remain underutilized for glycogenolysis quantification.
Statistical validation frameworks must address the complex kinetics of glycogenolysis. Time-course experiments with multiple sampling points are essential to validate mathematical models used for rate calculations. Bayesian approaches to parameter estimation can incorporate physiological constraints and prior knowledge, improving the robustness of rate determinations from limited experimental data points.
Emerging technologies, particularly stable isotope techniques and real-time imaging methods, require specialized validation protocols. These should include assessment of isotope equilibration times, potential isotope effects on enzyme kinetics, and spatial resolution validation for imaging approaches. As these technologies transition from research to clinical applications, additional validation against patient outcomes will become increasingly important.
Implementation of these standardization and validation approaches would significantly enhance the reliability and comparability of glycogenolysis measurements, accelerating research progress in hepatic metabolism and related disorders.
Ethical Considerations in Hepatic Tissue Sampling
The ethical considerations surrounding hepatic tissue sampling for glycogenolysis rate measurement represent a critical dimension of research integrity that must be carefully addressed. When obtaining liver tissue samples for metabolic studies, researchers must navigate a complex landscape of ethical requirements that balance scientific advancement with subject protection.
Informed consent stands as the cornerstone of ethical tissue sampling, particularly when human subjects are involved. This process must clearly communicate the purpose of glycogenolysis measurement, potential risks of biopsy procedures, and how the tissue samples will be utilized. For vulnerable populations, such as children or critically ill patients, additional safeguards and justifications are necessary to ensure their protection while advancing scientific understanding of liver metabolism.
Animal welfare considerations are equally important when using animal models for glycogenolysis studies. The implementation of the 3Rs principle—Replacement, Reduction, and Refinement—should guide experimental design. Researchers must justify the necessity of in vivo measurements over alternative methods and minimize the number of animals used while employing refined techniques that reduce suffering during tissue collection procedures.
Institutional review board (IRB) or ethics committee approval represents a mandatory prerequisite for any hepatic tissue sampling protocol. These oversight bodies evaluate whether the scientific value of measuring glycogenolysis rates justifies the risks associated with tissue collection. They also assess whether appropriate measures are in place to protect subject confidentiality and dignity throughout the research process.
The question of tissue ownership and future use presents additional ethical challenges. Clear policies must be established regarding sample storage, potential secondary use in future glycogenolysis studies, and whether subjects maintain rights to withdraw consent for tissue use after collection. These considerations become particularly complex in commercial research settings where intellectual property interests may conflict with donor expectations.
Cultural and religious perspectives on tissue sampling vary significantly across populations and must be respected. Some communities may have specific concerns about liver tissue collection based on cultural beliefs about bodily integrity or spiritual significance of specific organs. Researchers must demonstrate cultural sensitivity and adapt consent processes accordingly.
Benefit-sharing represents an emerging ethical consideration, particularly when research on glycogenolysis rates may lead to commercial applications or therapeutic advances. Ensuring that communities or populations who provide tissue samples receive appropriate recognition and benefit from resulting knowledge or treatments aligns with principles of justice and reciprocity in biomedical research.
Informed consent stands as the cornerstone of ethical tissue sampling, particularly when human subjects are involved. This process must clearly communicate the purpose of glycogenolysis measurement, potential risks of biopsy procedures, and how the tissue samples will be utilized. For vulnerable populations, such as children or critically ill patients, additional safeguards and justifications are necessary to ensure their protection while advancing scientific understanding of liver metabolism.
Animal welfare considerations are equally important when using animal models for glycogenolysis studies. The implementation of the 3Rs principle—Replacement, Reduction, and Refinement—should guide experimental design. Researchers must justify the necessity of in vivo measurements over alternative methods and minimize the number of animals used while employing refined techniques that reduce suffering during tissue collection procedures.
Institutional review board (IRB) or ethics committee approval represents a mandatory prerequisite for any hepatic tissue sampling protocol. These oversight bodies evaluate whether the scientific value of measuring glycogenolysis rates justifies the risks associated with tissue collection. They also assess whether appropriate measures are in place to protect subject confidentiality and dignity throughout the research process.
The question of tissue ownership and future use presents additional ethical challenges. Clear policies must be established regarding sample storage, potential secondary use in future glycogenolysis studies, and whether subjects maintain rights to withdraw consent for tissue use after collection. These considerations become particularly complex in commercial research settings where intellectual property interests may conflict with donor expectations.
Cultural and religious perspectives on tissue sampling vary significantly across populations and must be respected. Some communities may have specific concerns about liver tissue collection based on cultural beliefs about bodily integrity or spiritual significance of specific organs. Researchers must demonstrate cultural sensitivity and adapt consent processes accordingly.
Benefit-sharing represents an emerging ethical consideration, particularly when research on glycogenolysis rates may lead to commercial applications or therapeutic advances. Ensuring that communities or populations who provide tissue samples receive appropriate recognition and benefit from resulting knowledge or treatments aligns with principles of justice and reciprocity in biomedical research.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!