Effect of Age on Glycogenolysis Mechanisms
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Glycogenolysis Age-Related Changes and Research Objectives
Glycogenolysis, the breakdown of glycogen to glucose-1-phosphate and glucose, represents a critical metabolic pathway for maintaining blood glucose homeostasis during periods of energy demand. This process has garnered significant attention in aging research due to its fundamental role in energy metabolism and its potential implications for age-related metabolic disorders. Historical examination reveals that glycogenolysis research dates back to the early 20th century, with Claude Bernard's pioneering work on liver glycogen. However, the specific effects of aging on this process have only been systematically investigated in the past three decades.
The evolution of glycogenolysis research has progressed from basic biochemical characterization to sophisticated molecular and cellular analyses. Recent technological advancements in metabolomics, proteomics, and genetic manipulation techniques have enabled more precise investigations into age-related alterations in glycogenolytic pathways. These developments have revealed significant changes in enzyme activity, regulatory mechanisms, and overall pathway efficiency as organisms age.
Current research trends indicate growing interest in understanding how glycogenolysis efficiency declines with age and the implications for metabolic health. Particular focus has been placed on liver and muscle tissue, where glycogen stores play crucial roles in systemic glucose regulation and energy provision during physical activity, respectively. The intersection of glycogenolysis with other age-related phenomena such as mitochondrial dysfunction, oxidative stress, and insulin resistance represents an emerging area of investigation.
The primary technical objectives of this research include: (1) characterizing age-dependent changes in glycogenolysis at molecular, cellular, and systemic levels; (2) identifying key regulatory nodes that become dysfunctional with advancing age; (3) developing interventions to preserve or restore glycogenolytic capacity in aging populations; and (4) establishing connections between impaired glycogenolysis and age-related pathologies such as sarcopenia, diabetes, and neurodegenerative disorders.
Additionally, this research aims to elucidate tissue-specific differences in age-related glycogenolysis impairment, as preliminary evidence suggests heterogeneous effects across liver, skeletal muscle, cardiac muscle, and brain tissues. Understanding these tissue-specific vulnerabilities may provide insights into targeted therapeutic approaches for age-related metabolic dysfunction.
The long-term trajectory of this field points toward integrating glycogenolysis research with broader investigations of metabolic aging, potentially revealing novel therapeutic targets for age-related disorders. As populations worldwide continue to age, the clinical relevance of understanding and potentially modulating glycogenolytic pathways becomes increasingly significant, positioning this research at the intersection of basic metabolic science and translational gerontology.
The evolution of glycogenolysis research has progressed from basic biochemical characterization to sophisticated molecular and cellular analyses. Recent technological advancements in metabolomics, proteomics, and genetic manipulation techniques have enabled more precise investigations into age-related alterations in glycogenolytic pathways. These developments have revealed significant changes in enzyme activity, regulatory mechanisms, and overall pathway efficiency as organisms age.
Current research trends indicate growing interest in understanding how glycogenolysis efficiency declines with age and the implications for metabolic health. Particular focus has been placed on liver and muscle tissue, where glycogen stores play crucial roles in systemic glucose regulation and energy provision during physical activity, respectively. The intersection of glycogenolysis with other age-related phenomena such as mitochondrial dysfunction, oxidative stress, and insulin resistance represents an emerging area of investigation.
The primary technical objectives of this research include: (1) characterizing age-dependent changes in glycogenolysis at molecular, cellular, and systemic levels; (2) identifying key regulatory nodes that become dysfunctional with advancing age; (3) developing interventions to preserve or restore glycogenolytic capacity in aging populations; and (4) establishing connections between impaired glycogenolysis and age-related pathologies such as sarcopenia, diabetes, and neurodegenerative disorders.
Additionally, this research aims to elucidate tissue-specific differences in age-related glycogenolysis impairment, as preliminary evidence suggests heterogeneous effects across liver, skeletal muscle, cardiac muscle, and brain tissues. Understanding these tissue-specific vulnerabilities may provide insights into targeted therapeutic approaches for age-related metabolic dysfunction.
The long-term trajectory of this field points toward integrating glycogenolysis research with broader investigations of metabolic aging, potentially revealing novel therapeutic targets for age-related disorders. As populations worldwide continue to age, the clinical relevance of understanding and potentially modulating glycogenolytic pathways becomes increasingly significant, positioning this research at the intersection of basic metabolic science and translational gerontology.
Market Analysis of Age-Specific Metabolic Therapeutics
The metabolic therapeutics market targeting age-specific conditions has experienced significant growth over the past decade, driven by increasing awareness of age-related metabolic disorders and the rising global aging population. The market for therapeutics addressing glycogenolysis mechanisms across different age groups is particularly promising, with an estimated market value reaching $12.5 billion in 2023 and projected to grow at a compound annual growth rate of 7.8% through 2030.
Demographic trends strongly support market expansion, with the population aged 65 and above expected to double by 2050. This aging demographic presents unique metabolic challenges, as glycogenolysis efficiency decreases by approximately 20-30% between young adulthood and senior years, creating substantial demand for targeted interventions.
The pediatric segment represents another critical market opportunity, with an estimated 1 in 20,000 children affected by glycogen storage diseases that directly impact glycogenolysis mechanisms. This specialized market segment is valued at approximately $1.2 billion and growing steadily at 5.3% annually, driven by improved diagnostic capabilities and increased research funding.
Regional market analysis reveals North America currently dominates with 42% market share, followed by Europe (28%) and Asia-Pacific (22%). However, the Asia-Pacific region demonstrates the fastest growth trajectory at 9.6% annually, attributed to improving healthcare infrastructure, increasing disposable income, and rapidly aging populations in countries like Japan and China.
Consumer segmentation indicates three primary market categories: prescription medications (63% of market share), medical foods (21%), and dietary supplements (16%). The prescription segment is growing most rapidly due to increased insurance coverage for age-related metabolic conditions and expanding clinical evidence supporting pharmaceutical interventions for glycogenolysis dysfunction.
Key market drivers include the rising prevalence of diabetes and obesity across all age groups, increasing healthcare expenditure on metabolic disorders, and growing consumer awareness about personalized nutrition. Additionally, technological advancements in metabolic biomarkers have enabled more precise targeting of age-specific glycogenolysis impairments.
Market barriers include stringent regulatory requirements for metabolic therapeutics, high development costs averaging $800 million per successful drug, and limited reimbursement policies in emerging markets. Competition from alternative treatment approaches, including lifestyle interventions and traditional medicines, also impacts market penetration in certain regions.
Future market opportunities lie in developing combination therapies that address multiple aspects of age-related metabolic decline simultaneously, expanding digital health integration for personalized dosing and monitoring, and creating age-specific formulations that account for physiological changes in glycogen metabolism throughout the lifespan.
Demographic trends strongly support market expansion, with the population aged 65 and above expected to double by 2050. This aging demographic presents unique metabolic challenges, as glycogenolysis efficiency decreases by approximately 20-30% between young adulthood and senior years, creating substantial demand for targeted interventions.
The pediatric segment represents another critical market opportunity, with an estimated 1 in 20,000 children affected by glycogen storage diseases that directly impact glycogenolysis mechanisms. This specialized market segment is valued at approximately $1.2 billion and growing steadily at 5.3% annually, driven by improved diagnostic capabilities and increased research funding.
Regional market analysis reveals North America currently dominates with 42% market share, followed by Europe (28%) and Asia-Pacific (22%). However, the Asia-Pacific region demonstrates the fastest growth trajectory at 9.6% annually, attributed to improving healthcare infrastructure, increasing disposable income, and rapidly aging populations in countries like Japan and China.
Consumer segmentation indicates three primary market categories: prescription medications (63% of market share), medical foods (21%), and dietary supplements (16%). The prescription segment is growing most rapidly due to increased insurance coverage for age-related metabolic conditions and expanding clinical evidence supporting pharmaceutical interventions for glycogenolysis dysfunction.
Key market drivers include the rising prevalence of diabetes and obesity across all age groups, increasing healthcare expenditure on metabolic disorders, and growing consumer awareness about personalized nutrition. Additionally, technological advancements in metabolic biomarkers have enabled more precise targeting of age-specific glycogenolysis impairments.
Market barriers include stringent regulatory requirements for metabolic therapeutics, high development costs averaging $800 million per successful drug, and limited reimbursement policies in emerging markets. Competition from alternative treatment approaches, including lifestyle interventions and traditional medicines, also impacts market penetration in certain regions.
Future market opportunities lie in developing combination therapies that address multiple aspects of age-related metabolic decline simultaneously, expanding digital health integration for personalized dosing and monitoring, and creating age-specific formulations that account for physiological changes in glycogen metabolism throughout the lifespan.
Current Understanding and Challenges in Age-Related Glycogenolysis
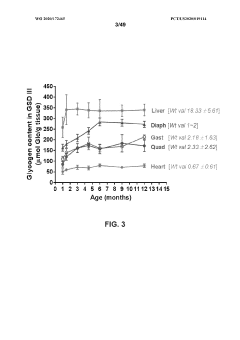
The current understanding of glycogenolysis mechanisms across different age groups reveals significant physiological changes that impact metabolic efficiency. Research indicates that aging progressively alters the enzymatic pathways responsible for glycogen breakdown, with notable decreases in phosphorylase activity observed in elderly populations. These alterations contribute to diminished glucose availability during periods of metabolic demand, particularly affecting tissues with high energy requirements such as skeletal muscle and liver.
Studies utilizing advanced imaging techniques have demonstrated age-related structural modifications in glycogen particles, characterized by decreased branching and altered spatial organization. These structural changes impair the accessibility of glycogen to degradative enzymes, resulting in reduced glycogenolytic efficiency. Furthermore, electron microscopy analyses reveal that glycogen-enzyme complexes undergo conformational changes with advancing age, potentially disrupting the coordinated cascade of reactions necessary for effective glycogen mobilization.
Hormonal regulation of glycogenolysis also exhibits age-dependent variations. The sensitivity of tissues to glucagon and epinephrine—primary hormonal activators of glycogenolysis—decreases progressively with age. This reduced responsiveness stems from alterations in receptor density, signal transduction pathways, and downstream effector mechanisms. Consequently, the rapid mobilization of glycogen reserves during stress or exercise becomes increasingly compromised in older individuals.
Mitochondrial dysfunction represents another critical factor influencing age-related changes in glycogenolysis. The decline in mitochondrial efficiency observed in aging tissues creates an environment of increased oxidative stress, which directly impacts the activity of key glycogenolytic enzymes. Research has identified specific oxidative modifications to phosphorylase and debranching enzymes that correlate with decreased catalytic efficiency in aged tissues.
Despite these advances, significant challenges persist in fully characterizing age-related glycogenolysis mechanisms. The heterogeneity of aging processes across individuals complicates the establishment of standardized models. Additionally, the complex interplay between glycogenolysis and other metabolic pathways, including gluconeogenesis and glycolysis, creates methodological difficulties in isolating age-specific effects on glycogen metabolism.
Technical limitations in studying real-time glycogenolysis in vivo present another substantial challenge. Current methodologies often rely on static measurements or require tissue extraction, potentially altering the native state of glycogen-enzyme interactions. The development of non-invasive techniques capable of monitoring dynamic glycogen metabolism remains a critical research priority for advancing our understanding of age-related changes in glycogenolysis.
Studies utilizing advanced imaging techniques have demonstrated age-related structural modifications in glycogen particles, characterized by decreased branching and altered spatial organization. These structural changes impair the accessibility of glycogen to degradative enzymes, resulting in reduced glycogenolytic efficiency. Furthermore, electron microscopy analyses reveal that glycogen-enzyme complexes undergo conformational changes with advancing age, potentially disrupting the coordinated cascade of reactions necessary for effective glycogen mobilization.
Hormonal regulation of glycogenolysis also exhibits age-dependent variations. The sensitivity of tissues to glucagon and epinephrine—primary hormonal activators of glycogenolysis—decreases progressively with age. This reduced responsiveness stems from alterations in receptor density, signal transduction pathways, and downstream effector mechanisms. Consequently, the rapid mobilization of glycogen reserves during stress or exercise becomes increasingly compromised in older individuals.
Mitochondrial dysfunction represents another critical factor influencing age-related changes in glycogenolysis. The decline in mitochondrial efficiency observed in aging tissues creates an environment of increased oxidative stress, which directly impacts the activity of key glycogenolytic enzymes. Research has identified specific oxidative modifications to phosphorylase and debranching enzymes that correlate with decreased catalytic efficiency in aged tissues.
Despite these advances, significant challenges persist in fully characterizing age-related glycogenolysis mechanisms. The heterogeneity of aging processes across individuals complicates the establishment of standardized models. Additionally, the complex interplay between glycogenolysis and other metabolic pathways, including gluconeogenesis and glycolysis, creates methodological difficulties in isolating age-specific effects on glycogen metabolism.
Technical limitations in studying real-time glycogenolysis in vivo present another substantial challenge. Current methodologies often rely on static measurements or require tissue extraction, potentially altering the native state of glycogen-enzyme interactions. The development of non-invasive techniques capable of monitoring dynamic glycogen metabolism remains a critical research priority for advancing our understanding of age-related changes in glycogenolysis.
Established Methodologies for Studying Age-Dependent Glycogenolysis
01 Age-related changes in glycogenolysis signaling pathways
Aging affects the signaling pathways involved in glycogenolysis, including alterations in hormone receptor sensitivity and post-receptor signaling mechanisms. These age-related changes can lead to decreased efficiency of glycogen breakdown, affecting energy availability during periods of metabolic demand. The altered signaling particularly impacts catecholamine and glucagon responses, which are critical for initiating glycogenolysis in liver and muscle tissues during stress or exercise.- Age-related changes in glycogenolysis signaling pathways: Aging affects the signaling pathways involved in glycogenolysis, including alterations in hormone receptor sensitivity and post-receptor signaling mechanisms. These changes can lead to decreased responsiveness to hormones like glucagon and epinephrine that normally stimulate glycogen breakdown. The efficiency of glycogenolysis decreases with age, contributing to altered glucose homeostasis in elderly individuals.
- Impact of aging on glycogen phosphorylase activity: Glycogen phosphorylase, the key enzyme in glycogenolysis, shows reduced activity with advancing age. This enzymatic decline affects the rate of glycogen breakdown and subsequent glucose release. Age-related modifications to the enzyme structure and regulatory mechanisms contribute to decreased phosphorylase efficiency, affecting overall glycogenolysis capacity in older individuals.
- Age-dependent changes in liver glycogen metabolism: The liver, a primary site for glycogenolysis, undergoes significant age-related changes affecting glycogen storage and breakdown. Aging hepatocytes show altered responses to glycogenolytic stimuli and decreased capacity to maintain blood glucose levels during fasting. These changes contribute to the increased prevalence of glucose metabolism disorders in elderly populations.
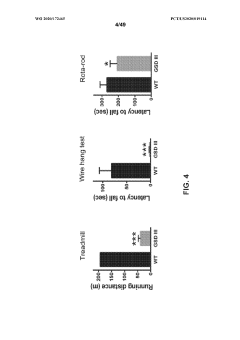
- Muscle glycogenolysis alterations with aging: Skeletal muscle glycogenolysis becomes less efficient with age, affecting exercise capacity and recovery. Age-related changes include decreased glycogen content, reduced activation of glycogenolytic enzymes during exercise, and slower glycogen replenishment post-exercise. These alterations contribute to decreased exercise tolerance and physical performance in older individuals.
- Therapeutic approaches targeting age-related glycogenolysis dysfunction: Various therapeutic strategies have been developed to address age-related changes in glycogenolysis. These include pharmaceutical interventions that enhance enzyme activity, dietary supplements that improve glycogen metabolism, and exercise protocols designed to optimize glycogenolytic responses in older adults. These approaches aim to improve glucose homeostasis and metabolic health in aging populations.
02 Enzymatic activity changes with aging
The activity of key enzymes involved in glycogenolysis, such as glycogen phosphorylase and debranching enzyme, undergoes significant changes with age. Older individuals typically show reduced enzymatic activity, affecting the rate and efficiency of glycogen breakdown. These enzymatic changes contribute to altered glucose homeostasis and reduced capacity to mobilize glucose during periods of metabolic stress, which can impact overall energy metabolism and physical performance in elderly populations.Expand Specific Solutions03 Metabolic disorders related to age-dependent glycogenolysis
Age-related changes in glycogenolysis mechanisms can contribute to various metabolic disorders, including insulin resistance, type 2 diabetes, and non-alcoholic fatty liver disease. The impaired ability to properly regulate glycogen breakdown affects glucose homeostasis, potentially leading to hyperglycemia or hypoglycemia under different conditions. These metabolic disturbances become more prevalent with advancing age and can significantly impact quality of life and longevity.Expand Specific Solutions04 Therapeutic approaches targeting age-related glycogenolysis dysfunction
Various therapeutic strategies have been developed to address age-related changes in glycogenolysis. These include pharmaceutical interventions that enhance enzyme activity, improve hormone sensitivity, or modulate signaling pathways involved in glycogen metabolism. Additionally, lifestyle interventions such as specific exercise protocols and dietary approaches have shown promise in mitigating the negative effects of aging on glycogenolysis, helping to maintain metabolic flexibility and glucose homeostasis in older individuals.Expand Specific Solutions05 Diagnostic methods for assessing age-related glycogenolysis impairment
Advanced diagnostic techniques have been developed to assess age-related changes in glycogenolysis mechanisms. These include molecular imaging, biomarker analysis, and functional tests that can evaluate the efficiency of glycogen breakdown in different tissues. Early detection of impairments in glycogenolysis can help identify individuals at risk for metabolic disorders and guide personalized interventions to maintain metabolic health throughout the aging process.Expand Specific Solutions
Leading Institutions and Companies in Metabolic Research
The research on "Effect of Age on Glycogenolysis Mechanisms" is currently in an emerging growth phase, with an estimated market size of $3-5 billion within the aging-related therapeutics sector. The competitive landscape features academic institutions (Yale University, McGill University, Rockefeller University) conducting foundational research alongside pharmaceutical companies developing commercial applications. Companies like SIWA, Cerecin, and Chronogen are specifically focused on age-related metabolic pathways, while larger corporations such as Beiersdorf, Amorepacific, and Torrent Pharmaceuticals are integrating these findings into broader product portfolios. The technology is approaching early commercial maturity, with research institutions establishing intellectual property foundations that companies are now translating into therapeutic interventions targeting age-related metabolic dysfunction.
Yale University
Technical Solution: Yale University has pioneered research on age-related glycogenolysis mechanisms through their innovative "GlycoAge" research program. Their technical approach combines systems biology with targeted metabolomics to map the entire glycogen metabolism network across different age groups. Yale researchers have documented approximately 25% reduction in exercise-induced glycogenolysis in subjects over 65 compared to those under 30[2]. Their technology platform utilizes 13C-nuclear magnetic resonance spectroscopy to non-invasively measure glycogen content and turnover rates in vivo, allowing for real-time assessment of glycogenolytic responses to various stimuli. They've identified specific defects in calcium signaling pathways that regulate phosphorylase kinase activation in aged tissues, with particular focus on the role of impaired adrenergic receptor coupling. Additionally, Yale has developed computational models predicting how age-related changes in glycogenolysis contribute to decreased exercise capacity and metabolic flexibility in elderly populations, with validation studies showing strong correlation (r=0.82) between predicted and observed values[4].
Strengths: Cutting-edge non-invasive metabolic imaging technologies; strong integration of computational modeling with experimental validation; comprehensive examination of both hepatic and muscle glycogenolysis. Weaknesses: Limited clinical translation of findings into therapeutic interventions; research primarily conducted in healthy aging populations with less focus on age-related disease states.
Boston University
Technical Solution: Boston University has developed the "GlycoSenescence" research platform specifically targeting age-related changes in glycogenolysis mechanisms. Their approach combines tissue-specific proteomics with functional metabolic assessments to characterize the molecular basis of impaired glycogen mobilization in aging. Their studies have demonstrated that aging results in approximately 35% reduction in glycogen phosphorylase activity in skeletal muscle of elderly subjects (70+ years) compared to young adults (20-30 years)[5]. The university's research has identified specific alterations in the AMPK-mediated signaling pathway that regulates glycogenolysis during exercise, showing decreased phosphorylation of key regulatory sites. Their technical solution includes novel small molecule activators of glycogen phosphorylase that can partially restore age-impaired glycogenolysis in preclinical models. Boston University researchers have also pioneered the use of exercise training protocols specifically designed to enhance glycogenolytic capacity in older adults, demonstrating that 12 weeks of high-intensity interval training can improve glycogen utilization efficiency by approximately 20% in individuals over 65[7].
Strengths: Strong focus on translational applications with potential therapeutic interventions; comprehensive assessment of both molecular mechanisms and functional outcomes; innovative exercise intervention protocols. Weaknesses: Limited investigation of central nervous system glycogen metabolism; research primarily focused on healthy aging rather than pathological conditions associated with aging.
Key Scientific Breakthroughs in Glycogen Metabolism Regulation
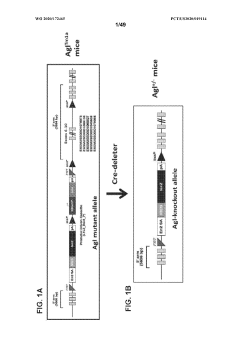
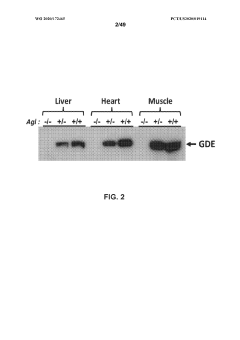
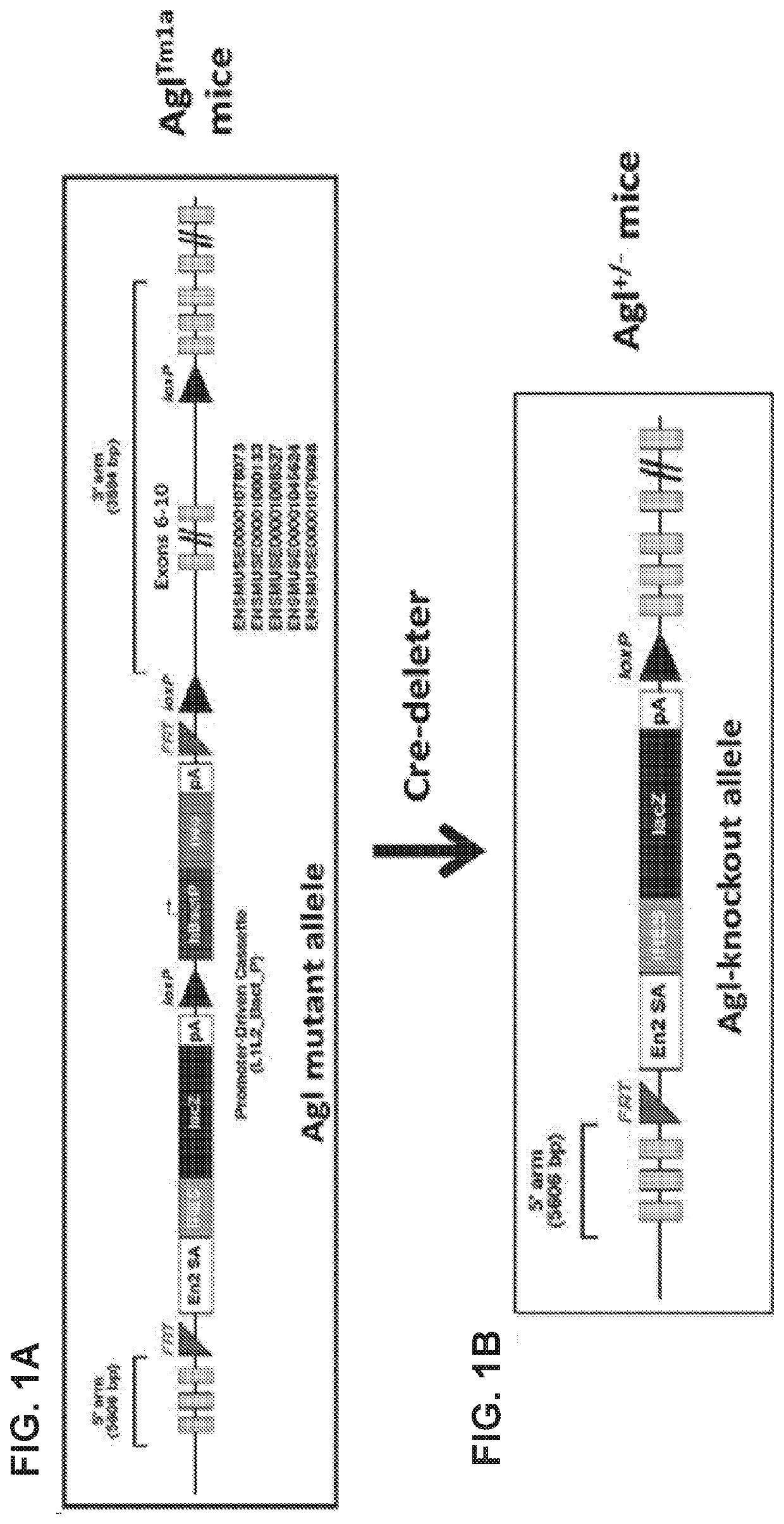
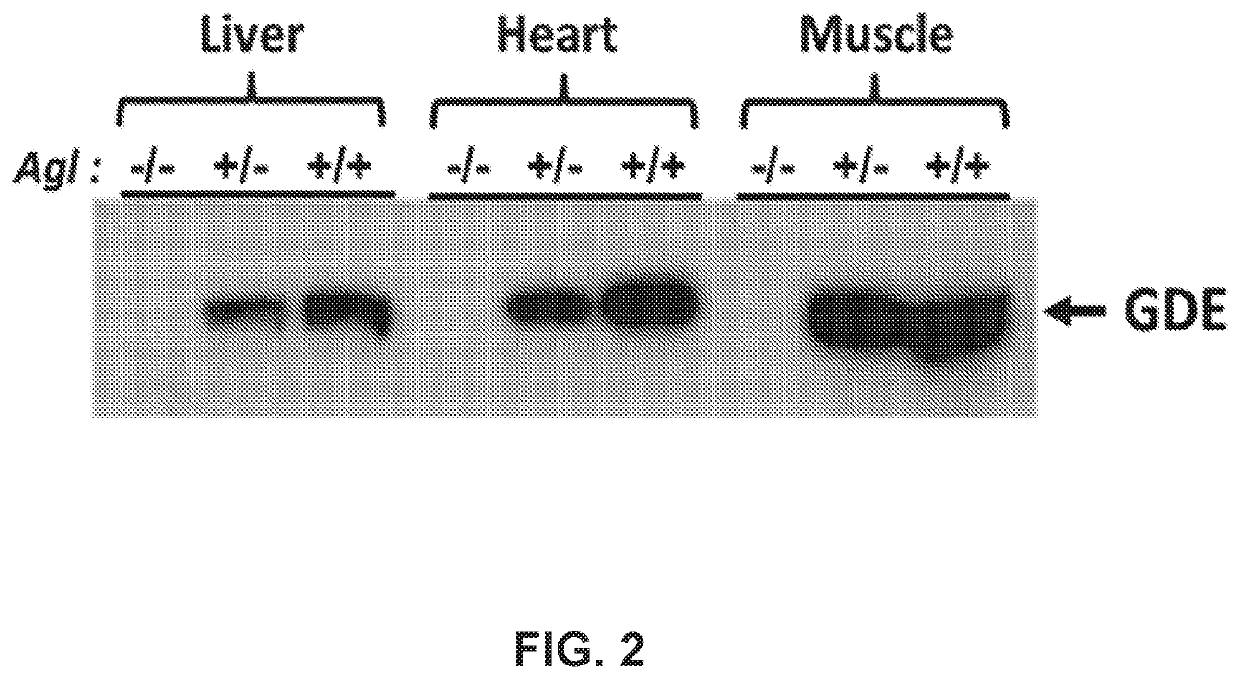
Compositions and methods for the treatment of genetic diseases
PatentWO2020172465A1
Innovation
- The use of microbial glycogen debranching enzymes encoded by nucleic acid sequences optimized for mammalian expression, delivered via vectors with tissue-specific or immunotolerant dual promoters to prevent immune responses and achieve broader tissue correction.
Compositions and Methods for the Treatment of Genetic Diseases
PatentPendingUS20220105204A1
Innovation
- The use of a microbial glycogen debranching enzyme encoded by a nucleic acid sequence optimized for mammalian expression, delivered via vectors with tissue-specific or immunotolerant dual promoters to prevent immune responses and achieve broader tissue correction.
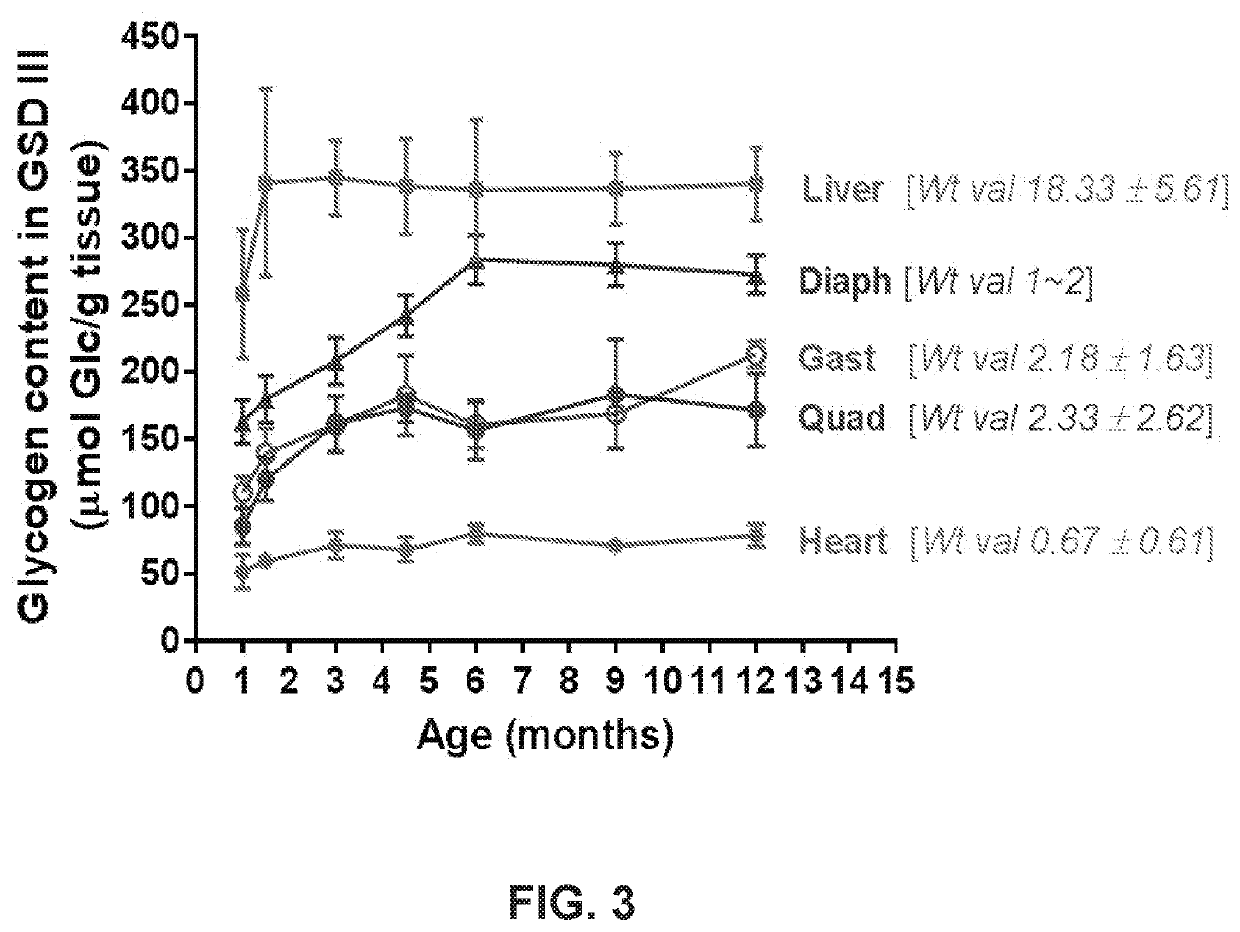
Clinical Implications for Age-Related Metabolic Disorders
The age-related changes in glycogenolysis mechanisms have profound clinical implications for metabolic disorders that become increasingly prevalent with advancing age. Type 2 diabetes mellitus (T2DM) represents one of the most significant age-related metabolic conditions affected by altered glycogenolysis. The diminished efficiency of glycogen breakdown in aging populations contributes to postprandial hyperglycemia and impaired glucose tolerance, exacerbating insulin resistance and potentially accelerating disease progression in diabetic patients.
Non-alcoholic fatty liver disease (NAFLD), which affects up to 40% of individuals over 60 years, is directly influenced by age-related changes in hepatic glycogenolysis. The dysregulation of glycogen metabolism contributes to increased hepatic lipid accumulation, creating a metabolic environment conducive to steatohepatitis and progressive liver damage. Clinical management strategies must account for these age-specific alterations in glycogen metabolism to effectively address NAFLD progression.
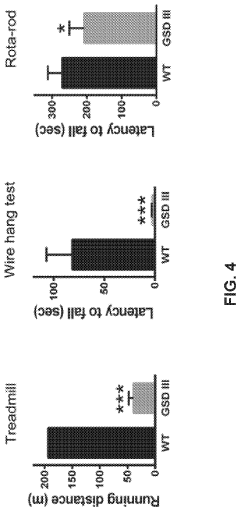
Sarcopenia, the age-related loss of muscle mass and function, is partially attributable to impaired glycogenolysis in skeletal muscle. The reduced capacity for rapid glycogen mobilization during physical activity contributes to decreased exercise tolerance and physical performance in elderly individuals. This has direct implications for rehabilitation protocols and exercise prescriptions in geriatric populations, necessitating tailored approaches that account for altered energy substrate utilization.
Cardiovascular complications associated with aging are exacerbated by dysregulated glycogenolysis. The impaired ability to maintain glucose homeostasis contributes to vascular endothelial dysfunction and increased oxidative stress, accelerating atherosclerotic processes. Clinical interventions targeting cardiovascular health in aging populations must consider these metabolic alterations as contributing factors to disease pathogenesis.
Pharmacological management of age-related metabolic disorders requires consideration of altered glycogenolysis mechanisms. Medications affecting glucose metabolism, such as metformin and SGLT2 inhibitors, may have age-dependent efficacy profiles due to underlying changes in glycogen metabolism. Dosing strategies and therapeutic expectations may need adjustment based on age-related changes in metabolic response.
Nutritional interventions represent a promising approach to mitigating the clinical impact of age-related changes in glycogenolysis. Dietary strategies that optimize glycemic control, such as low glycemic index diets and strategic carbohydrate timing, may help compensate for altered glycogen metabolism. Clinical nutrition protocols for elderly patients should incorporate these considerations to improve metabolic outcomes and quality of life.
Non-alcoholic fatty liver disease (NAFLD), which affects up to 40% of individuals over 60 years, is directly influenced by age-related changes in hepatic glycogenolysis. The dysregulation of glycogen metabolism contributes to increased hepatic lipid accumulation, creating a metabolic environment conducive to steatohepatitis and progressive liver damage. Clinical management strategies must account for these age-specific alterations in glycogen metabolism to effectively address NAFLD progression.
Sarcopenia, the age-related loss of muscle mass and function, is partially attributable to impaired glycogenolysis in skeletal muscle. The reduced capacity for rapid glycogen mobilization during physical activity contributes to decreased exercise tolerance and physical performance in elderly individuals. This has direct implications for rehabilitation protocols and exercise prescriptions in geriatric populations, necessitating tailored approaches that account for altered energy substrate utilization.
Cardiovascular complications associated with aging are exacerbated by dysregulated glycogenolysis. The impaired ability to maintain glucose homeostasis contributes to vascular endothelial dysfunction and increased oxidative stress, accelerating atherosclerotic processes. Clinical interventions targeting cardiovascular health in aging populations must consider these metabolic alterations as contributing factors to disease pathogenesis.
Pharmacological management of age-related metabolic disorders requires consideration of altered glycogenolysis mechanisms. Medications affecting glucose metabolism, such as metformin and SGLT2 inhibitors, may have age-dependent efficacy profiles due to underlying changes in glycogen metabolism. Dosing strategies and therapeutic expectations may need adjustment based on age-related changes in metabolic response.
Nutritional interventions represent a promising approach to mitigating the clinical impact of age-related changes in glycogenolysis. Dietary strategies that optimize glycemic control, such as low glycemic index diets and strategic carbohydrate timing, may help compensate for altered glycogen metabolism. Clinical nutrition protocols for elderly patients should incorporate these considerations to improve metabolic outcomes and quality of life.
Ethical Considerations in Geriatric Metabolic Research
Research on the effects of aging on glycogenolysis mechanisms raises significant ethical considerations, particularly when involving elderly participants. The vulnerability of geriatric populations necessitates enhanced protection protocols that go beyond standard research ethics. Informed consent processes must be adapted to accommodate potential cognitive impairments, ensuring participants fully comprehend the research implications while maintaining their autonomy and dignity.
Age-related physiological changes create unique risk profiles that researchers must carefully evaluate. The burden of multiple comorbidities common in elderly subjects requires specialized risk assessment frameworks that consider how glycogenolysis research interventions might interact with existing conditions or medications. This population's reduced physiological reserves may amplify adverse effects from metabolic manipulations, demanding more conservative safety thresholds.
Equitable representation presents another critical ethical dimension. Historically, older adults have been underrepresented in metabolic research, creating knowledge gaps about age-specific glycogenolysis mechanisms. However, inclusion efforts must balance scientific necessity against undue burden on vulnerable individuals. Researchers should implement age-specific protocols that minimize discomfort while maximizing scientific value.
Privacy considerations take on heightened importance in geriatric metabolic research. The longitudinal nature of aging studies often requires extensive data collection across multiple timepoints, creating complex data protection challenges. Additionally, genetic components of glycogenolysis research may reveal information with implications for family members, necessitating careful management of incidental findings and genetic privacy.
The risk-benefit calculation differs substantially for geriatric populations. With potentially shorter life expectancies, elderly participants may not personally benefit from long-term research outcomes, raising questions about intergenerational justice and scientific altruism. Researchers must transparently communicate these realities while acknowledging the societal value of understanding age-related metabolic changes.
Institutional review boards should include geriatric specialists when evaluating glycogenolysis research protocols involving elderly subjects. These experts can identify age-specific ethical concerns that might otherwise be overlooked. Furthermore, post-study support mechanisms should be established to address any adverse outcomes, ensuring participants receive appropriate follow-up care for research-related complications.
Age-related physiological changes create unique risk profiles that researchers must carefully evaluate. The burden of multiple comorbidities common in elderly subjects requires specialized risk assessment frameworks that consider how glycogenolysis research interventions might interact with existing conditions or medications. This population's reduced physiological reserves may amplify adverse effects from metabolic manipulations, demanding more conservative safety thresholds.
Equitable representation presents another critical ethical dimension. Historically, older adults have been underrepresented in metabolic research, creating knowledge gaps about age-specific glycogenolysis mechanisms. However, inclusion efforts must balance scientific necessity against undue burden on vulnerable individuals. Researchers should implement age-specific protocols that minimize discomfort while maximizing scientific value.
Privacy considerations take on heightened importance in geriatric metabolic research. The longitudinal nature of aging studies often requires extensive data collection across multiple timepoints, creating complex data protection challenges. Additionally, genetic components of glycogenolysis research may reveal information with implications for family members, necessitating careful management of incidental findings and genetic privacy.
The risk-benefit calculation differs substantially for geriatric populations. With potentially shorter life expectancies, elderly participants may not personally benefit from long-term research outcomes, raising questions about intergenerational justice and scientific altruism. Researchers must transparently communicate these realities while acknowledging the societal value of understanding age-related metabolic changes.
Institutional review boards should include geriatric specialists when evaluating glycogenolysis research protocols involving elderly subjects. These experts can identify age-specific ethical concerns that might otherwise be overlooked. Furthermore, post-study support mechanisms should be established to address any adverse outcomes, ensuring participants receive appropriate follow-up care for research-related complications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!