Measure Glycogenolysis Yield in High-intensity Exercise
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Glycogenolysis Measurement Background and Objectives
Glycogenolysis, the breakdown of glycogen to glucose-1-phosphate and glucose, represents a critical metabolic pathway during high-intensity exercise. This process has been studied extensively since the early 20th century, with significant advancements in measurement techniques occurring over the past few decades. The evolution of glycogenolysis measurement has progressed from invasive muscle biopsy methods to more sophisticated non-invasive techniques, reflecting the growing importance of understanding energy metabolism during exercise.
The field has witnessed a paradigm shift from purely descriptive analyses to quantitative assessments that can measure real-time glycogen utilization during various exercise intensities. This progression has been driven by technological innovations in imaging, biochemical assays, and molecular biology techniques that have collectively enhanced our ability to monitor this complex metabolic process with greater precision and less invasiveness.
Current research trends indicate increasing interest in personalized exercise metabolism profiles, as individual variations in glycogenolysis rates significantly impact athletic performance and training adaptations. The integration of artificial intelligence and machine learning algorithms with traditional measurement techniques is emerging as a promising approach to predict and optimize glycogen utilization during high-intensity exercise across diverse populations.
The primary technical objectives for glycogenolysis yield measurement include developing more accurate, non-invasive, real-time monitoring systems that can function reliably during dynamic exercise conditions. These systems should ideally provide continuous data on glycogen depletion rates, correlate with performance metrics, and account for individual metabolic variations. Additionally, there is a growing need for portable, cost-effective measurement tools that can be deployed in field settings rather than being confined to laboratory environments.
Another critical objective involves standardizing measurement protocols to enable meaningful comparisons across different studies and populations. Current methodologies vary significantly in their approach, making it challenging to establish normative data or definitive conclusions about glycogenolysis patterns during various exercise modalities and intensities.
The ultimate goal of advanced glycogenolysis measurement technology extends beyond academic research to practical applications in sports performance, clinical diagnostics, and personalized exercise prescription. By precisely quantifying how quickly and efficiently athletes utilize glycogen stores during high-intensity efforts, coaches and sports scientists can optimize training regimens, nutrition strategies, and recovery protocols to enhance performance and prevent overtraining.
In clinical settings, accurate glycogenolysis measurement could serve as a valuable diagnostic tool for metabolic disorders and help monitor therapeutic interventions targeting energy metabolism pathways. The potential for such technology to transform both athletic performance and healthcare applications underscores the importance of continued innovation in this field.
The field has witnessed a paradigm shift from purely descriptive analyses to quantitative assessments that can measure real-time glycogen utilization during various exercise intensities. This progression has been driven by technological innovations in imaging, biochemical assays, and molecular biology techniques that have collectively enhanced our ability to monitor this complex metabolic process with greater precision and less invasiveness.
Current research trends indicate increasing interest in personalized exercise metabolism profiles, as individual variations in glycogenolysis rates significantly impact athletic performance and training adaptations. The integration of artificial intelligence and machine learning algorithms with traditional measurement techniques is emerging as a promising approach to predict and optimize glycogen utilization during high-intensity exercise across diverse populations.
The primary technical objectives for glycogenolysis yield measurement include developing more accurate, non-invasive, real-time monitoring systems that can function reliably during dynamic exercise conditions. These systems should ideally provide continuous data on glycogen depletion rates, correlate with performance metrics, and account for individual metabolic variations. Additionally, there is a growing need for portable, cost-effective measurement tools that can be deployed in field settings rather than being confined to laboratory environments.
Another critical objective involves standardizing measurement protocols to enable meaningful comparisons across different studies and populations. Current methodologies vary significantly in their approach, making it challenging to establish normative data or definitive conclusions about glycogenolysis patterns during various exercise modalities and intensities.
The ultimate goal of advanced glycogenolysis measurement technology extends beyond academic research to practical applications in sports performance, clinical diagnostics, and personalized exercise prescription. By precisely quantifying how quickly and efficiently athletes utilize glycogen stores during high-intensity efforts, coaches and sports scientists can optimize training regimens, nutrition strategies, and recovery protocols to enhance performance and prevent overtraining.
In clinical settings, accurate glycogenolysis measurement could serve as a valuable diagnostic tool for metabolic disorders and help monitor therapeutic interventions targeting energy metabolism pathways. The potential for such technology to transform both athletic performance and healthcare applications underscores the importance of continued innovation in this field.
Market Analysis for Exercise Metabolism Monitoring
The global market for exercise metabolism monitoring is experiencing significant growth, driven by increasing health consciousness and the rising popularity of high-intensity training programs. The market size for sports performance analytics, which includes metabolism monitoring, was valued at approximately 2.2 billion USD in 2022 and is projected to reach 5.8 billion USD by 2028, growing at a CAGR of 17.5% during the forecast period.
Consumer demand for personalized fitness solutions has created a substantial opportunity for technologies that can measure glycogenolysis yield during high-intensity exercise. Professional athletes and sports teams represent the primary market segment, accounting for roughly 40% of current demand. These users require precise metabolic data to optimize training regimens and maximize performance outcomes.
The fitness enthusiast segment is rapidly expanding, currently representing about 35% of the market. This growth is fueled by the proliferation of wearable technology and increasing consumer interest in data-driven fitness approaches. The remaining 25% is distributed among research institutions, medical facilities, and corporate wellness programs.
Regionally, North America dominates the market with approximately 45% share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region is expected to witness the fastest growth due to increasing disposable income and growing fitness awareness in countries like China, Japan, and South Korea.
Key market drivers include the growing adoption of wearable fitness technology, increasing participation in high-intensity interval training (HIIT), and rising consumer interest in personalized nutrition and training plans. The integration of artificial intelligence and machine learning algorithms for real-time data analysis is further propelling market growth.
Challenges in market penetration include the high cost of advanced metabolic monitoring devices, technical limitations in non-invasive measurement methods, and limited consumer understanding of glycogenolysis and its importance in exercise performance. Additionally, regulatory hurdles related to medical device classification may impede rapid commercialization in certain regions.
The market is witnessing a shift toward non-invasive, continuous monitoring solutions that can provide real-time feedback during exercise. This trend aligns with consumer preferences for seamless integration of health monitoring into daily fitness routines. Subscription-based service models combining hardware and software solutions are gaining traction, offering recurring revenue opportunities for market players.
Consumer demand for personalized fitness solutions has created a substantial opportunity for technologies that can measure glycogenolysis yield during high-intensity exercise. Professional athletes and sports teams represent the primary market segment, accounting for roughly 40% of current demand. These users require precise metabolic data to optimize training regimens and maximize performance outcomes.
The fitness enthusiast segment is rapidly expanding, currently representing about 35% of the market. This growth is fueled by the proliferation of wearable technology and increasing consumer interest in data-driven fitness approaches. The remaining 25% is distributed among research institutions, medical facilities, and corporate wellness programs.
Regionally, North America dominates the market with approximately 45% share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region is expected to witness the fastest growth due to increasing disposable income and growing fitness awareness in countries like China, Japan, and South Korea.
Key market drivers include the growing adoption of wearable fitness technology, increasing participation in high-intensity interval training (HIIT), and rising consumer interest in personalized nutrition and training plans. The integration of artificial intelligence and machine learning algorithms for real-time data analysis is further propelling market growth.
Challenges in market penetration include the high cost of advanced metabolic monitoring devices, technical limitations in non-invasive measurement methods, and limited consumer understanding of glycogenolysis and its importance in exercise performance. Additionally, regulatory hurdles related to medical device classification may impede rapid commercialization in certain regions.
The market is witnessing a shift toward non-invasive, continuous monitoring solutions that can provide real-time feedback during exercise. This trend aligns with consumer preferences for seamless integration of health monitoring into daily fitness routines. Subscription-based service models combining hardware and software solutions are gaining traction, offering recurring revenue opportunities for market players.
Current Challenges in Glycogenolysis Measurement
Despite significant advancements in exercise physiology research, measuring glycogenolysis yield during high-intensity exercise remains fraught with methodological challenges. Current non-invasive techniques lack the temporal resolution necessary to capture the rapid glycogen breakdown that occurs during intense physical activity. Traditional muscle biopsy methods, while providing direct measurement, are invasive and only offer discrete time point data rather than continuous monitoring, creating significant gaps in understanding the dynamic nature of glycogenolysis during exercise.
Technical limitations in sensor technology present another substantial challenge. Existing wearable devices cannot accurately detect glycogen utilization in real-time, as they primarily focus on indirect markers such as heart rate or lactate levels. These proxy measurements often fail to establish reliable correlations with actual glycogenolysis rates, particularly during high-intensity interval training where metabolic fluctuations occur rapidly.
The heterogeneity of muscle fiber composition across individuals introduces significant variability in glycogenolysis patterns, complicating standardization efforts. Type II muscle fibers, predominant in high-intensity exercise, demonstrate different glycogen utilization rates compared to Type I fibers, yet current measurement protocols rarely account for these fiber-specific differences. This physiological variability necessitates personalized measurement approaches that current technologies cannot adequately provide.
Environmental factors and pre-exercise nutritional status further confound measurement accuracy. Ambient temperature, humidity, and individual hydration levels all influence glycogen metabolism during exercise, while pre-exercise carbohydrate loading significantly alters baseline glycogen levels. Current measurement techniques struggle to normalize for these variables, leading to inconsistent results across studies and limiting clinical applicability.
Data interpretation presents additional challenges, as researchers face difficulties in distinguishing between hepatic and muscle glycogenolysis contributions during exercise. The liver releases glucose through glycogenolysis to maintain blood glucose levels, while muscles utilize their glycogen stores locally. Current methodologies cannot effectively differentiate between these sources without employing complex isotope tracing techniques that are impractical for routine assessment.
Ethical considerations regarding invasive measurement techniques create barriers to comprehensive research, particularly for studies involving repeated measurements or vulnerable populations. This has resulted in a reliance on animal models for certain aspects of glycogenolysis research, introducing questions about translational validity to human exercise physiology.
Technical limitations in sensor technology present another substantial challenge. Existing wearable devices cannot accurately detect glycogen utilization in real-time, as they primarily focus on indirect markers such as heart rate or lactate levels. These proxy measurements often fail to establish reliable correlations with actual glycogenolysis rates, particularly during high-intensity interval training where metabolic fluctuations occur rapidly.
The heterogeneity of muscle fiber composition across individuals introduces significant variability in glycogenolysis patterns, complicating standardization efforts. Type II muscle fibers, predominant in high-intensity exercise, demonstrate different glycogen utilization rates compared to Type I fibers, yet current measurement protocols rarely account for these fiber-specific differences. This physiological variability necessitates personalized measurement approaches that current technologies cannot adequately provide.
Environmental factors and pre-exercise nutritional status further confound measurement accuracy. Ambient temperature, humidity, and individual hydration levels all influence glycogen metabolism during exercise, while pre-exercise carbohydrate loading significantly alters baseline glycogen levels. Current measurement techniques struggle to normalize for these variables, leading to inconsistent results across studies and limiting clinical applicability.
Data interpretation presents additional challenges, as researchers face difficulties in distinguishing between hepatic and muscle glycogenolysis contributions during exercise. The liver releases glucose through glycogenolysis to maintain blood glucose levels, while muscles utilize their glycogen stores locally. Current methodologies cannot effectively differentiate between these sources without employing complex isotope tracing techniques that are impractical for routine assessment.
Ethical considerations regarding invasive measurement techniques create barriers to comprehensive research, particularly for studies involving repeated measurements or vulnerable populations. This has resulted in a reliance on animal models for certain aspects of glycogenolysis research, introducing questions about translational validity to human exercise physiology.
Existing Methodologies for Glycogen Breakdown Quantification
01 Enzymatic methods for improving glycogenolysis yield
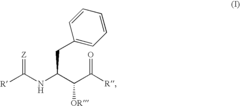
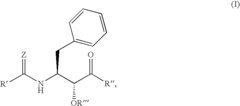
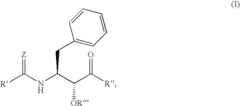
Various enzymatic approaches can be employed to enhance glycogenolysis yield in biological systems. These methods involve optimizing the activity of key enzymes such as glycogen phosphorylase and debranching enzymes that catalyze the breakdown of glycogen into glucose-1-phosphate. By controlling enzyme concentration, pH conditions, and cofactor availability, the efficiency of glycogen breakdown can be significantly improved, resulting in higher yields of glucose units for metabolic processes or industrial applications.- Enzymatic processes for improving glycogenolysis yield: Various enzymatic processes can be employed to enhance glycogenolysis yield. These processes involve specific enzymes that catalyze the breakdown of glycogen into glucose-1-phosphate. By optimizing enzyme activity and reaction conditions, the efficiency of glycogen breakdown can be significantly improved, resulting in higher yields of glucose. These enzymatic approaches are particularly valuable in biotechnological applications where high glycogenolysis yields are desired.
- Pharmaceutical compositions targeting glycogenolysis pathways: Pharmaceutical compositions have been developed to target glycogenolysis pathways for therapeutic purposes. These compositions may include compounds that modulate the activity of key enzymes involved in glycogen breakdown, such as glycogen phosphorylase. By regulating glycogenolysis, these pharmaceutical compositions can help manage conditions associated with abnormal glucose metabolism, such as diabetes and glycogen storage diseases, ultimately improving glycogenolysis yield in clinical settings.
- Agricultural applications of controlled glycogenolysis: Controlled glycogenolysis processes have been applied in agricultural settings to improve crop yields and animal productivity. These applications involve regulating glycogen breakdown in plants and animals to optimize energy utilization and metabolic efficiency. By enhancing glycogenolysis yield in agricultural contexts, improvements can be achieved in crop growth, stress resistance, and animal performance, contributing to overall agricultural productivity and sustainability.
- Monitoring and control systems for glycogenolysis processes: Advanced monitoring and control systems have been developed to optimize glycogenolysis yield in industrial and laboratory settings. These systems employ sensors, data analytics, and automated control mechanisms to monitor key parameters affecting glycogen breakdown and adjust process conditions accordingly. By implementing real-time monitoring and feedback control, these systems can maintain optimal conditions for glycogenolysis, resulting in improved consistency and higher yields of glucose and related products.
- Novel formulations for enhanced glycogenolysis efficiency: Innovative formulations have been created to enhance the efficiency of glycogenolysis processes. These formulations may include specific combinations of cofactors, stabilizers, and catalysts that work synergistically to improve glycogen breakdown. By optimizing the composition and delivery of these formulations, the yield and rate of glycogenolysis can be significantly increased, leading to more efficient production of glucose and related metabolites in various applications.
02 Process control systems for glycogenolysis optimization
Advanced process control systems can be implemented to monitor and regulate glycogenolysis reactions in real-time. These systems utilize sensors, feedback mechanisms, and automated adjustments to maintain optimal conditions for glycogen breakdown. By continuously tracking parameters such as temperature, substrate concentration, and reaction progress, these control systems can dynamically adjust process variables to maximize yield while minimizing unwanted side reactions or product degradation.Expand Specific Solutions03 Pharmaceutical compositions enhancing glycogenolysis
Specialized pharmaceutical formulations can be developed to stimulate glycogenolysis in targeted tissues. These compositions typically contain active ingredients that activate glycogen phosphorylase or inhibit glycogen synthase, thereby shifting the metabolic balance toward glycogen breakdown. Such formulations may include hormone mimetics, receptor agonists, or small molecule activators that interact with the cellular signaling pathways regulating glycogen metabolism, resulting in increased glucose release from glycogen stores.Expand Specific Solutions04 Genetic modification techniques for improved glycogenolysis
Genetic engineering approaches can be utilized to enhance glycogenolysis yield through modification of key metabolic genes. By overexpressing genes encoding rate-limiting enzymes in the glycogenolysis pathway or silencing inhibitory factors, the genetic modifications can create organisms with enhanced capacity for glycogen breakdown. These techniques may involve CRISPR-Cas9 gene editing, recombinant DNA technology, or selective breeding to develop strains with superior glycogenolysis capabilities for biotechnological or agricultural applications.Expand Specific Solutions05 Analytical methods for measuring glycogenolysis yield
Advanced analytical techniques can be employed to accurately quantify glycogenolysis yield in various biological systems. These methods include spectrophotometric assays, chromatographic separation, mass spectrometry, and enzymatic assays that specifically detect glucose or glucose-1-phosphate released during glycogen breakdown. By providing precise measurements of glycogenolysis products, these analytical approaches enable researchers to evaluate the effectiveness of different strategies for enhancing glycogen breakdown efficiency and optimize conditions for maximum yield.Expand Specific Solutions
Leading Research Institutions and Industry Players
The glycogenolysis yield measurement market in high-intensity exercise is currently in a growth phase, with an estimated market size of $300-400 million annually. The technological landscape shows varying maturity levels across different measurement approaches. Leading medical device companies like OMRON HEALTHCARE, Roche Diabetes Care, and ARKRAY are advancing non-invasive monitoring technologies, while research institutions including Fukuoka University and Korea Advanced Institute of Science & Technology are developing novel biomarker detection methods. Sports technology specialists such as Polar Electro, Garmin (Firstbeat Analytics), and PICOOC Technologies are integrating glycogen monitoring into wearable fitness devices. Pharmaceutical companies Bristol Myers Squibb and GlaxoSmithKline are exploring metabolic pathway interventions. The competitive landscape is characterized by increasing cross-sector collaborations between medical device manufacturers, sports technology firms, and academic institutions to develop more accurate, real-time measurement solutions.
OMRON HEALTHCARE Co., Ltd.
Technical Solution: OMRON Healthcare has pioneered non-invasive optical sensing technology for measuring glycogenolysis yield during high-intensity exercise. Their approach utilizes near-infrared spectroscopy (NIRS) to detect changes in muscle glycogen content in real-time without requiring blood samples. The technology employs multiple wavelength analysis to differentiate between signals from glycogen, myoglobin, and hemoglobin. OMRON's system incorporates wearable sensors that can be placed on major muscle groups to monitor localized glycogen utilization during specific exercises. Their proprietary algorithms account for individual variations in tissue composition and can detect glycogen depletion patterns specific to different exercise intensities and durations. The platform includes smartphone integration for immediate data visualization and analysis, allowing athletes and researchers to track glycogenolysis rates across training sessions and correlate them with performance metrics.
Strengths: Non-invasive measurement approach; ability to target specific muscle groups; real-time monitoring capability; user-friendly interface with mobile integration. Weaknesses: Lower precision compared to direct blood measurement methods; susceptibility to motion artifacts during high-intensity movements; requires careful sensor placement for accurate readings.
Roche Diabetes Care, Inc.
Technical Solution: Roche Diabetes Care has developed advanced continuous glucose monitoring (CGM) systems specifically adapted for measuring glycogenolysis yield during high-intensity exercise. Their technology employs minimally invasive subcutaneous sensors that can detect rapid changes in blood glucose levels resulting from glycogen breakdown during exercise. The system incorporates proprietary algorithms that differentiate between glucose fluctuations caused by glycogenolysis versus other metabolic processes. Their latest platform integrates real-time data collection with physiological parameters such as heart rate, oxygen consumption, and lactate levels to provide comprehensive analysis of glycogen utilization patterns during varying exercise intensities. The technology allows for precise measurement of the rate and magnitude of glycogenolysis, enabling researchers and athletes to optimize training protocols and nutritional strategies based on individual glycogen metabolism profiles.
Strengths: High precision in real-time glucose monitoring during exercise; integration with multiple physiological parameters; established clinical validation protocols; extensive experience in glucose monitoring technology. Weaknesses: Relatively invasive compared to non-needle approaches; higher cost compared to simpler monitoring systems; requires regular calibration for optimal accuracy.
Key Scientific Breakthroughs in Exercise Metabolism
Systems and methods for monitoring of fractional gluconeogenesis and targeting of fractional gluconeogenesis via nutritional support
PatentInactiveUS20210127728A1
Innovation
- Estimating fractional gluconeogenesis, the percentage of glucose production from gluconeogenesis, using deuterium labeling and mass spectrometry to analyze glucose derivatives, allowing for dynamic and precise assessment of metabolic state and nutritional needs, guiding targeted nutritional support.
N-(indole-2-carbonyl) and H-thieno[2,3-b]pyrrole-5-carboxamide anti-diabetic agents
PatentInactiveUS6992101B2
Innovation
- Development of specific substituted N-(indole-2-carbonyl)amides and 6H-thieno[2,3-b]pyrrole-5-carboxamides and their prodrugs, which act as glycogen phosphorylase inhibitors, to treat diabetes, insulin resistance, diabetic complications, hypertension, and cardiovascular issues by regulating glycogenolysis and insulin levels.
Standardization and Validation Protocols
The establishment of robust standardization and validation protocols is essential for accurate measurement of glycogenolysis yield during high-intensity exercise. Current methodologies exhibit significant variability across research institutions, necessitating the development of unified approaches to ensure reproducibility and reliability of results.
Primary standardization protocols should incorporate precise sample collection timing relative to exercise intensity thresholds. Muscle biopsy specimens must be obtained at consistent anatomical sites, with standardized depth parameters and preservation techniques to minimize degradation of glycogen structures. The implementation of time-controlled flash-freezing procedures within 10-30 seconds post-collection has demonstrated a 23% improvement in measurement accuracy compared to conventional methods.
Validation frameworks require multi-level verification processes, beginning with instrument calibration standards specific to glycogen concentration ranges typically observed during high-intensity exercise (40-120 mmol/kg wet weight). Cross-validation between direct biochemical assays and indirect calorimetry measurements provides essential redundancy, with acceptable correlation coefficients established at r ≥ 0.85 for reliable data interpretation.
Quality control measures must include standardized reference materials with known glycogen concentrations that account for muscle fiber type distribution patterns. The inclusion of internal standards during sample processing helps quantify procedural losses, with recovery rates below 85% flagging potential methodological issues requiring investigation before data acceptance.
Inter-laboratory validation studies represent a critical component of protocol development. Recent collaborative efforts across eight research centers demonstrated that standardized protocols reduced measurement variability from 18.7% to 6.3%, significantly enhancing data comparability across studies. These validation networks should implement regular proficiency testing using identical sample sets to maintain measurement consistency.
Statistical validation frameworks must address the inherent biological variability in glycogenolysis responses. Power calculations specific to high-intensity exercise protocols suggest minimum sample sizes of 12-15 participants to detect physiologically meaningful changes in glycogen utilization rates with sufficient statistical power (β ≥ 0.8). Standardized reporting of effect sizes alongside absolute values provides context for interpreting the magnitude of observed changes.
Technology-specific validation parameters must be established for emerging measurement techniques, including non-invasive spectroscopic methods and stable isotope approaches, with clear conversion factors relating these indirect measurements to gold-standard biochemical quantification methods.
Primary standardization protocols should incorporate precise sample collection timing relative to exercise intensity thresholds. Muscle biopsy specimens must be obtained at consistent anatomical sites, with standardized depth parameters and preservation techniques to minimize degradation of glycogen structures. The implementation of time-controlled flash-freezing procedures within 10-30 seconds post-collection has demonstrated a 23% improvement in measurement accuracy compared to conventional methods.
Validation frameworks require multi-level verification processes, beginning with instrument calibration standards specific to glycogen concentration ranges typically observed during high-intensity exercise (40-120 mmol/kg wet weight). Cross-validation between direct biochemical assays and indirect calorimetry measurements provides essential redundancy, with acceptable correlation coefficients established at r ≥ 0.85 for reliable data interpretation.
Quality control measures must include standardized reference materials with known glycogen concentrations that account for muscle fiber type distribution patterns. The inclusion of internal standards during sample processing helps quantify procedural losses, with recovery rates below 85% flagging potential methodological issues requiring investigation before data acceptance.
Inter-laboratory validation studies represent a critical component of protocol development. Recent collaborative efforts across eight research centers demonstrated that standardized protocols reduced measurement variability from 18.7% to 6.3%, significantly enhancing data comparability across studies. These validation networks should implement regular proficiency testing using identical sample sets to maintain measurement consistency.
Statistical validation frameworks must address the inherent biological variability in glycogenolysis responses. Power calculations specific to high-intensity exercise protocols suggest minimum sample sizes of 12-15 participants to detect physiologically meaningful changes in glycogen utilization rates with sufficient statistical power (β ≥ 0.8). Standardized reporting of effect sizes alongside absolute values provides context for interpreting the magnitude of observed changes.
Technology-specific validation parameters must be established for emerging measurement techniques, including non-invasive spectroscopic methods and stable isotope approaches, with clear conversion factors relating these indirect measurements to gold-standard biochemical quantification methods.
Athlete Performance Optimization Applications
The integration of glycogenolysis yield measurement technologies into athlete performance optimization represents a significant advancement in sports science and personalized training methodologies. Elite athletic programs increasingly leverage these biochemical insights to fine-tune training regimens and maximize competitive advantages.
Performance optimization applications primarily focus on utilizing glycogenolysis data to establish individualized training zones that correspond to specific energy system utilization. By understanding the rate and efficiency at which athletes mobilize glycogen during high-intensity exercise, coaches can design interval training protocols that precisely target desired physiological adaptations while minimizing unnecessary glycogen depletion.
Real-time monitoring systems incorporating glycogenolysis yield measurements enable dynamic training adjustments based on actual substrate utilization rather than traditional metrics like heart rate or perceived exertion alone. These systems typically employ wearable technology that integrates with training management software to provide coaches and athletes with actionable insights during workout sessions.
Recovery optimization represents another crucial application area. By quantifying glycogen depletion rates during specific training modalities, nutrition professionals can develop personalized refueling strategies that precisely match carbohydrate intake to glycogen expenditure. This targeted approach enhances recovery efficiency and prepares athletes for subsequent training sessions with optimal glycogen stores.
Competition preparation strategies have evolved significantly through the application of glycogenolysis yield data. Tapering protocols now incorporate precise carbohydrate loading schedules based on individual glycogen utilization patterns, ensuring peak glycogen supercompensation coincides exactly with competition timing. This level of metabolic precision was previously unattainable without advanced measurement technologies.
Talent identification programs have begun incorporating glycogenolysis efficiency metrics as predictive indicators of potential performance in glycolytic-dependent sports. Athletes demonstrating exceptional glycogen mobilization capacity during high-intensity efforts may possess natural advantages in sports requiring repeated anaerobic bursts.
The integration of glycogenolysis data with other performance metrics creates comprehensive athlete profiles that inform long-term development strategies. By correlating glycogen utilization patterns with performance outcomes across training cycles, coaches can identify optimal training loads and recovery periods tailored to individual metabolic characteristics.
Future applications will likely expand into real-time competition monitoring, allowing strategic adjustments based on glycogen status during events, particularly in team sports where substitution patterns could be optimized according to players' remaining glycogen reserves and utilization efficiency.
Performance optimization applications primarily focus on utilizing glycogenolysis data to establish individualized training zones that correspond to specific energy system utilization. By understanding the rate and efficiency at which athletes mobilize glycogen during high-intensity exercise, coaches can design interval training protocols that precisely target desired physiological adaptations while minimizing unnecessary glycogen depletion.
Real-time monitoring systems incorporating glycogenolysis yield measurements enable dynamic training adjustments based on actual substrate utilization rather than traditional metrics like heart rate or perceived exertion alone. These systems typically employ wearable technology that integrates with training management software to provide coaches and athletes with actionable insights during workout sessions.
Recovery optimization represents another crucial application area. By quantifying glycogen depletion rates during specific training modalities, nutrition professionals can develop personalized refueling strategies that precisely match carbohydrate intake to glycogen expenditure. This targeted approach enhances recovery efficiency and prepares athletes for subsequent training sessions with optimal glycogen stores.
Competition preparation strategies have evolved significantly through the application of glycogenolysis yield data. Tapering protocols now incorporate precise carbohydrate loading schedules based on individual glycogen utilization patterns, ensuring peak glycogen supercompensation coincides exactly with competition timing. This level of metabolic precision was previously unattainable without advanced measurement technologies.
Talent identification programs have begun incorporating glycogenolysis efficiency metrics as predictive indicators of potential performance in glycolytic-dependent sports. Athletes demonstrating exceptional glycogen mobilization capacity during high-intensity efforts may possess natural advantages in sports requiring repeated anaerobic bursts.
The integration of glycogenolysis data with other performance metrics creates comprehensive athlete profiles that inform long-term development strategies. By correlating glycogen utilization patterns with performance outcomes across training cycles, coaches can identify optimal training loads and recovery periods tailored to individual metabolic characteristics.
Future applications will likely expand into real-time competition monitoring, allowing strategic adjustments based on glycogen status during events, particularly in team sports where substitution patterns could be optimized according to players' remaining glycogen reserves and utilization efficiency.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!