Detect Glycogenolysis Pathways Using Mass Spectrometry
AUG 28, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Glycogenolysis Detection Background and Objectives
Glycogenolysis, the metabolic pathway that breaks down glycogen into glucose, represents a critical process in maintaining blood glucose homeostasis during periods of fasting or increased energy demand. The scientific exploration of glycogenolysis pathways dates back to the early 20th century, with significant advancements occurring in the 1950s through biochemical assays. However, these traditional methods often lacked sensitivity and specificity required for comprehensive pathway analysis.
The advent of mass spectrometry (MS) in biological research has revolutionized our ability to detect and quantify metabolic intermediates with unprecedented precision. Since the 1990s, MS techniques have evolved from simple analyte identification to sophisticated approaches capable of tracking complex metabolic networks in real-time. The integration of liquid chromatography with tandem mass spectrometry (LC-MS/MS) has particularly enhanced our capacity to monitor glycogenolysis pathways by enabling simultaneous detection of multiple metabolites.
Current technological trends indicate a shift toward higher resolution instruments, improved ionization techniques, and advanced data processing algorithms specifically optimized for metabolic pathway analysis. These developments align with the growing recognition of glycogenolysis dysregulation in various pathological conditions, including diabetes, glycogen storage diseases, and certain cancers.
The primary objective of this technical research is to develop and validate robust MS-based methodologies for comprehensive detection and quantification of glycogenolysis pathway components. Specifically, we aim to establish protocols capable of simultaneously monitoring key metabolic intermediates, regulatory enzymes, and post-translational modifications that govern glycogen breakdown.
Secondary objectives include determining the temporal dynamics of glycogenolysis under various physiological and pathological conditions, identifying novel regulatory mechanisms through untargeted metabolomic approaches, and developing standardized analytical workflows that can be implemented in both research and clinical settings.
Long-term goals encompass the integration of glycogenolysis pathway data with broader metabolic networks to create comprehensive models of energy metabolism. This would enable prediction of metabolic responses to therapeutic interventions and facilitate personalized medicine approaches for metabolic disorders. Additionally, we seek to miniaturize and automate these analytical procedures to enable point-of-care testing for glycogen metabolism disorders.
The successful development of MS-based glycogenolysis detection methods would significantly advance our understanding of energy metabolism regulation and potentially lead to novel diagnostic tools and therapeutic strategies for metabolic disorders. This research aligns with the broader scientific trend toward systems biology approaches that integrate multiple levels of biological information to understand complex physiological processes.
The advent of mass spectrometry (MS) in biological research has revolutionized our ability to detect and quantify metabolic intermediates with unprecedented precision. Since the 1990s, MS techniques have evolved from simple analyte identification to sophisticated approaches capable of tracking complex metabolic networks in real-time. The integration of liquid chromatography with tandem mass spectrometry (LC-MS/MS) has particularly enhanced our capacity to monitor glycogenolysis pathways by enabling simultaneous detection of multiple metabolites.
Current technological trends indicate a shift toward higher resolution instruments, improved ionization techniques, and advanced data processing algorithms specifically optimized for metabolic pathway analysis. These developments align with the growing recognition of glycogenolysis dysregulation in various pathological conditions, including diabetes, glycogen storage diseases, and certain cancers.
The primary objective of this technical research is to develop and validate robust MS-based methodologies for comprehensive detection and quantification of glycogenolysis pathway components. Specifically, we aim to establish protocols capable of simultaneously monitoring key metabolic intermediates, regulatory enzymes, and post-translational modifications that govern glycogen breakdown.
Secondary objectives include determining the temporal dynamics of glycogenolysis under various physiological and pathological conditions, identifying novel regulatory mechanisms through untargeted metabolomic approaches, and developing standardized analytical workflows that can be implemented in both research and clinical settings.
Long-term goals encompass the integration of glycogenolysis pathway data with broader metabolic networks to create comprehensive models of energy metabolism. This would enable prediction of metabolic responses to therapeutic interventions and facilitate personalized medicine approaches for metabolic disorders. Additionally, we seek to miniaturize and automate these analytical procedures to enable point-of-care testing for glycogen metabolism disorders.
The successful development of MS-based glycogenolysis detection methods would significantly advance our understanding of energy metabolism regulation and potentially lead to novel diagnostic tools and therapeutic strategies for metabolic disorders. This research aligns with the broader scientific trend toward systems biology approaches that integrate multiple levels of biological information to understand complex physiological processes.
Market Applications for Mass Spectrometry in Metabolic Pathway Analysis
Mass spectrometry has emerged as a pivotal analytical tool in metabolic pathway analysis, with significant market applications spanning healthcare, pharmaceuticals, biotechnology, and academic research. The global market for mass spectrometry in metabolic research was valued at approximately $4.3 billion in 2022 and is projected to grow at a compound annual growth rate of 7.8% through 2030, driven by increasing demand for precision medicine and metabolic disorder diagnostics.
In clinical diagnostics, mass spectrometry-based detection of glycogenolysis pathways offers transformative potential for monitoring and diagnosing metabolic disorders such as glycogen storage diseases, diabetes, and hepatic dysfunction. Healthcare providers increasingly adopt these technologies for their superior sensitivity and specificity compared to traditional biochemical assays, enabling earlier intervention and personalized treatment strategies.
Pharmaceutical companies represent another significant market segment, utilizing mass spectrometry to evaluate drug effects on glycogenolysis and related metabolic pathways during preclinical and clinical development. This application accelerates drug discovery processes by providing detailed metabolic profiles and identifying potential therapeutic targets within the glycogen breakdown cascade.
The biotechnology sector leverages mass spectrometry for metabolic engineering applications, optimizing cellular metabolism in bioproduction systems. By monitoring glycogenolysis pathways, companies can enhance biofuel production efficiency, improve industrial enzyme performance, and develop novel biocatalysts with specific metabolic capabilities.
Academic and research institutions constitute a stable market base, employing mass spectrometry to advance fundamental understanding of metabolic regulation. Government funding for metabolic research has increased by 15% over the past five years, particularly for projects investigating metabolic diseases and energy metabolism disorders.
Emerging market opportunities exist in sports science and nutrition, where glycogenolysis pathway analysis helps optimize athletic performance and recovery protocols. Several professional sports organizations have established metabolomics laboratories utilizing mass spectrometry to develop personalized nutrition and training regimens based on individual metabolic profiles.
Geographically, North America dominates the market with approximately 40% share, followed by Europe and Asia-Pacific. However, the fastest growth is observed in emerging economies, particularly China and India, where increasing healthcare expenditure and expanding research infrastructure drive adoption of advanced metabolic analysis technologies.
The market landscape features both established analytical instrument manufacturers and specialized metabolomics service providers. Recent strategic partnerships between instrument vendors and clinical diagnostic companies indicate a trend toward developing integrated solutions that combine hardware, software, and reference databases specifically optimized for glycogenolysis pathway analysis.
In clinical diagnostics, mass spectrometry-based detection of glycogenolysis pathways offers transformative potential for monitoring and diagnosing metabolic disorders such as glycogen storage diseases, diabetes, and hepatic dysfunction. Healthcare providers increasingly adopt these technologies for their superior sensitivity and specificity compared to traditional biochemical assays, enabling earlier intervention and personalized treatment strategies.
Pharmaceutical companies represent another significant market segment, utilizing mass spectrometry to evaluate drug effects on glycogenolysis and related metabolic pathways during preclinical and clinical development. This application accelerates drug discovery processes by providing detailed metabolic profiles and identifying potential therapeutic targets within the glycogen breakdown cascade.
The biotechnology sector leverages mass spectrometry for metabolic engineering applications, optimizing cellular metabolism in bioproduction systems. By monitoring glycogenolysis pathways, companies can enhance biofuel production efficiency, improve industrial enzyme performance, and develop novel biocatalysts with specific metabolic capabilities.
Academic and research institutions constitute a stable market base, employing mass spectrometry to advance fundamental understanding of metabolic regulation. Government funding for metabolic research has increased by 15% over the past five years, particularly for projects investigating metabolic diseases and energy metabolism disorders.
Emerging market opportunities exist in sports science and nutrition, where glycogenolysis pathway analysis helps optimize athletic performance and recovery protocols. Several professional sports organizations have established metabolomics laboratories utilizing mass spectrometry to develop personalized nutrition and training regimens based on individual metabolic profiles.
Geographically, North America dominates the market with approximately 40% share, followed by Europe and Asia-Pacific. However, the fastest growth is observed in emerging economies, particularly China and India, where increasing healthcare expenditure and expanding research infrastructure drive adoption of advanced metabolic analysis technologies.
The market landscape features both established analytical instrument manufacturers and specialized metabolomics service providers. Recent strategic partnerships between instrument vendors and clinical diagnostic companies indicate a trend toward developing integrated solutions that combine hardware, software, and reference databases specifically optimized for glycogenolysis pathway analysis.
Current Challenges in Glycogenolysis Pathway Detection
Despite significant advancements in mass spectrometry (MS) technologies, detecting and analyzing glycogenolysis pathways presents numerous technical challenges that impede comprehensive metabolic research. The complexity of glycogen metabolism intermediates creates substantial analytical hurdles, as these compounds exhibit similar molecular structures and chemical properties, making their separation and identification exceptionally difficult using conventional MS approaches.
Signal-to-noise ratio remains a persistent obstacle, particularly when attempting to detect low-abundance metabolites involved in glycogenolysis. Many key intermediates exist at concentrations near or below current detection limits, especially in clinical samples where sample volumes are often restricted. This limitation significantly hampers the ability to track pathway dynamics in real-time or in minimally invasive diagnostic applications.
Sample preparation introduces another layer of complexity, as glycogen pathway intermediates are highly susceptible to degradation and modification during extraction procedures. The instability of phosphorylated intermediates, particularly glucose-1-phosphate and glucose-6-phosphate, requires specialized handling protocols that are not standardized across laboratories, leading to inconsistent results and poor reproducibility in multi-center studies.
Quantification accuracy presents further complications due to matrix effects and ion suppression phenomena that disproportionately affect carbohydrate detection in complex biological samples. Current internal standardization methods fail to adequately compensate for these effects across the diverse range of intermediates in the glycogenolysis pathway, resulting in unreliable quantitative measurements.
Data processing and interpretation challenges are equally significant. The absence of comprehensive spectral libraries specific to glycogenolysis intermediates forces researchers to rely on manual annotation, introducing subjectivity and increasing analysis time. Existing bioinformatics tools struggle with pathway-specific analysis, particularly when distinguishing between glycogenolysis and other interconnected metabolic pathways like glycolysis or pentose phosphate pathway.
Temporal resolution limitations prevent effective monitoring of the rapid enzymatic cascade characteristic of glycogenolysis. Current MS workflows typically provide static snapshots rather than dynamic pathway information, obscuring the sequential nature of the process and regulatory mechanisms that control the rate and extent of glycogen breakdown.
Integration challenges with other omics technologies further complicate comprehensive pathway analysis. While proteomics can identify the enzymes involved in glycogenolysis, and genomics can reveal genetic variants affecting pathway function, the technical barriers to integrating these data streams with metabolomics results create significant gaps in pathway understanding and interpretation.
Signal-to-noise ratio remains a persistent obstacle, particularly when attempting to detect low-abundance metabolites involved in glycogenolysis. Many key intermediates exist at concentrations near or below current detection limits, especially in clinical samples where sample volumes are often restricted. This limitation significantly hampers the ability to track pathway dynamics in real-time or in minimally invasive diagnostic applications.
Sample preparation introduces another layer of complexity, as glycogen pathway intermediates are highly susceptible to degradation and modification during extraction procedures. The instability of phosphorylated intermediates, particularly glucose-1-phosphate and glucose-6-phosphate, requires specialized handling protocols that are not standardized across laboratories, leading to inconsistent results and poor reproducibility in multi-center studies.
Quantification accuracy presents further complications due to matrix effects and ion suppression phenomena that disproportionately affect carbohydrate detection in complex biological samples. Current internal standardization methods fail to adequately compensate for these effects across the diverse range of intermediates in the glycogenolysis pathway, resulting in unreliable quantitative measurements.
Data processing and interpretation challenges are equally significant. The absence of comprehensive spectral libraries specific to glycogenolysis intermediates forces researchers to rely on manual annotation, introducing subjectivity and increasing analysis time. Existing bioinformatics tools struggle with pathway-specific analysis, particularly when distinguishing between glycogenolysis and other interconnected metabolic pathways like glycolysis or pentose phosphate pathway.
Temporal resolution limitations prevent effective monitoring of the rapid enzymatic cascade characteristic of glycogenolysis. Current MS workflows typically provide static snapshots rather than dynamic pathway information, obscuring the sequential nature of the process and regulatory mechanisms that control the rate and extent of glycogen breakdown.
Integration challenges with other omics technologies further complicate comprehensive pathway analysis. While proteomics can identify the enzymes involved in glycogenolysis, and genomics can reveal genetic variants affecting pathway function, the technical barriers to integrating these data streams with metabolomics results create significant gaps in pathway understanding and interpretation.
Current Mass Spectrometry Methods for Glycogen Breakdown Analysis
01 Mass spectrometry instrumentation and components
Various innovations in mass spectrometry hardware components that improve detection capabilities. These include specialized ion sources, detectors, analyzers, and vacuum systems that enhance sensitivity, resolution, and accuracy. Advancements in instrument design allow for better ionization efficiency, improved ion transmission, and reduced signal-to-noise ratios, resulting in more precise mass measurements and compound identification.- Mass spectrometry instrumentation and components: Various innovations in mass spectrometry instrumentation focus on improving detection capabilities through enhanced component design. These include specialized ion sources, detectors, analyzers, and vacuum systems that work together to improve sensitivity, resolution, and accuracy. Advanced components enable better ionization efficiency, ion transmission, and detection of target analytes even at low concentrations.
- Ion mobility and separation techniques: Ion mobility spectrometry combined with mass spectrometry enhances detection capabilities by adding an additional dimension of separation. These techniques separate ions based on their mobility in a carrier gas or electric field before mass analysis, allowing for improved discrimination between compounds with similar mass-to-charge ratios but different structures. This approach is particularly valuable for complex sample analysis and isomer differentiation.
- Data processing and analysis methods: Advanced computational methods for processing mass spectrometry data significantly improve detection capabilities. These include machine learning algorithms, statistical analysis tools, and specialized software that can identify patterns, reduce noise, and extract meaningful information from complex spectra. Such methods enable more accurate compound identification, quantification, and characterization even in challenging samples.
- Sample preparation and introduction systems: Innovative sample preparation and introduction techniques enhance mass spectrometry detection by improving the efficiency of analyte extraction, concentration, and delivery to the instrument. These methods include automated sample handling, specialized ionization interfaces, and chromatographic separation techniques that can be coupled with mass spectrometry to reduce matrix effects and increase sensitivity for target compounds.
- Application-specific detection methods: Specialized mass spectrometry detection methods have been developed for specific applications such as biomarker discovery, environmental monitoring, pharmaceutical analysis, and forensic investigations. These methods involve optimized parameters, targeted analysis approaches, and custom hardware configurations designed to address the unique challenges of detecting particular compounds or analytes in specific sample types or matrices.
02 Tandem mass spectrometry techniques
Methods involving multiple stages of mass analysis for enhanced compound identification and structural elucidation. These techniques typically involve fragmenting selected ions and analyzing the resulting fragment ions to provide detailed structural information. Applications include proteomics, metabolomics, and pharmaceutical analysis where complex mixtures require advanced separation and identification capabilities.Expand Specific Solutions03 Sample preparation and ionization methods
Innovative approaches for preparing and introducing samples into mass spectrometers, including various ionization techniques. These methods address challenges in analyzing different sample types and improve ionization efficiency for diverse compounds. Developments include ambient ionization techniques, specialized sample introduction systems, and methods for reducing matrix effects that can interfere with accurate detection.Expand Specific Solutions04 Data processing and analysis algorithms
Software solutions and computational methods for processing and interpreting mass spectrometry data. These include algorithms for peak detection, spectral deconvolution, compound identification, and quantification. Advanced data processing techniques help manage large datasets, improve signal processing, reduce noise, and enhance the accuracy of compound identification and quantification in complex samples.Expand Specific Solutions05 Specialized applications and integrated systems
Mass spectrometry detection systems designed for specific applications or integrated with other analytical techniques. These include hyphenated techniques like LC-MS and GC-MS, portable mass spectrometry systems, and specialized configurations for particular industries or research areas. Integrated approaches combine mass spectrometry with other analytical methods to provide comprehensive analysis solutions for complex analytical challenges.Expand Specific Solutions
Leading Research Institutions and Instrument Manufacturers
The glycogenolysis pathways detection market using mass spectrometry is currently in a growth phase, with increasing adoption across research and clinical applications. The market size is expanding as metabolic disorder diagnostics gain prominence, estimated to reach significant value in the coming years. Technologically, companies like Shimadzu Corp. and Waters Corporation (through Micromass UK) lead with advanced mass spectrometry platforms, while bioMérieux and Quest Diagnostics focus on clinical applications. Academic institutions including MIT, Northwestern University, and Harvard College drive fundamental research innovations. Japanese firms (ARKRAY, Sumitomo Bakelite) are making notable contributions in specialized glycan analysis tools. The competitive landscape shows a mix of established analytical instrument manufacturers and specialized biotech companies, with increasing collaboration between academic and commercial entities to advance glycogenolysis pathway detection technologies.
Shimadzu Corp.
Technical Solution: Shimadzu has developed advanced LC-MS/MS (Liquid Chromatography-Tandem Mass Spectrometry) systems specifically optimized for glycogenolysis pathway analysis. Their LCMS-8060NX triple quadrupole mass spectrometer achieves ultra-fast scanning speeds of 30,000 u/sec and polarity switching time of 5 msec, enabling comprehensive detection of glycolytic intermediates in a single run. The system incorporates their proprietary ion focusing technology that significantly enhances sensitivity for phosphorylated metabolites common in glycogenolysis pathways. Shimadzu's approach combines high-resolution chromatographic separation with multiple reaction monitoring (MRM) to quantify key metabolites like glucose-1-phosphate, glucose-6-phosphate, and glycogen phosphorylase activity products simultaneously. Their integrated software platform provides automated pathway mapping and flux analysis capabilities, allowing researchers to visualize dynamic changes in glycogen metabolism under various physiological conditions.
Strengths: Industry-leading sensitivity and scanning speed optimized for low-abundance metabolites; comprehensive software for pathway analysis; robust quantification capabilities for phosphorylated compounds. Weaknesses: Higher cost compared to competitors; requires significant technical expertise to fully utilize advanced features; sample preparation protocols can be complex for certain tissue types.
Massachusetts Institute of Technology
Technical Solution: MIT has developed an innovative isotope tracing mass spectrometry approach for dynamic analysis of glycogenolysis pathways. Their technology combines stable isotope labeling with high-resolution mass spectrometry to track the flux through glycogen breakdown pathways in real-time. The approach utilizes 13C-labeled glucose to pre-label glycogen stores, followed by stimulation of glycogenolysis and time-course sampling to monitor the appearance of labeled intermediates. MIT's platform incorporates a custom-built microfluidic sampling interface that enables automated sampling at 10-second intervals, providing unprecedented temporal resolution of glycogenolysis dynamics. Their analytical workflow employs a hybrid Orbitrap-based mass spectrometer with sub-ppm mass accuracy, allowing for confident identification of labeled metabolites without chromatographic separation in some applications. The MIT team has developed specialized computational algorithms for isotopologue distribution analysis that can determine the relative contributions of glycogenolysis versus gluconeogenesis to glucose production under various physiological stresses. This approach has been successfully applied to study glycogenolysis in primary hepatocytes, muscle tissue samples, and in vivo using microdialysis sampling coupled to their mass spectrometry platform.
Strengths: Unparalleled temporal resolution for dynamic pathway analysis; ability to distinguish between different sources of glucose production; innovative microfluidic sampling enhances reproducibility. Weaknesses: Requires specialized isotope labeling expertise; higher complexity in data interpretation; currently limited to research applications rather than clinical diagnostics.
Key Technical Innovations in Metabolite Detection
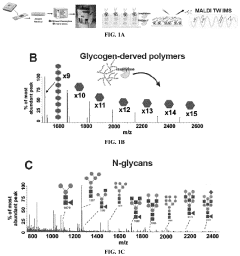
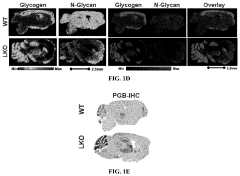
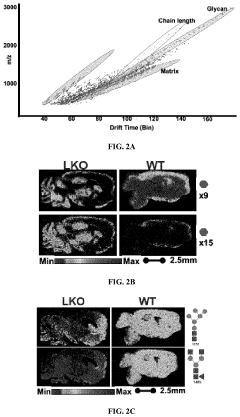
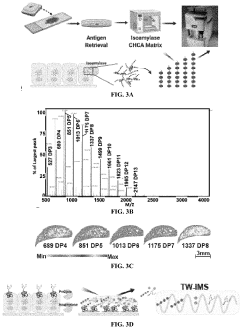
Methods of detecting glycogen and polyglucan
PatentPendingUS20220229026A1
Innovation
- A method involving gas-chromatography coupled with mass spectrometry for detecting sugar monomers and phosphates, and the use of isoamylase with matrix-assisted laser desorption ionization (MALDI) mass spectrometry for analyzing glycogen, including the cleavage of glucose chains and release of N-linked glycans, to achieve high sensitivity and spatial resolution.
Method for cleavage of sugar chain from glycoprotein, mass spectrometry for sugar chain, and mass spectrometry for glycoprotein
PatentWO2006109858A1
Innovation
- An on-membrane enzymatic reaction method using a sugar chain-releasing enzyme in a volatile reaction buffer solution, such as (NH4)HCO3, CH3C2NH4, or (NH4)2CO3, allows for direct MALDI-TOF MS analysis of glycoproteins by cleaving sugar chains without inhibiting enzymatic reactions or interfering with the MS process.
Data Processing Algorithms for Metabolic Pathway Identification
The field of metabolic pathway identification has witnessed significant advancements in data processing algorithms, particularly for glycogenolysis pathway detection using mass spectrometry. These algorithms serve as the computational backbone for transforming complex spectral data into meaningful biological insights about metabolic processes.
Current state-of-the-art algorithms employ multi-stage processing pipelines that begin with raw mass spectrometry data preprocessing. This includes noise reduction techniques such as Savitzky-Golay filtering and baseline correction algorithms that effectively separate metabolite signals from instrumental noise. Advanced peak detection algorithms utilizing continuous wavelet transforms have demonstrated superior performance in identifying metabolite peaks with high sensitivity and specificity.
Feature extraction represents another critical component, where algorithms extract quantifiable characteristics from spectral data. Machine learning approaches, particularly deep learning models like convolutional neural networks (CNNs), have revolutionized this process by automatically identifying relevant spectral features without explicit programming. These models can detect subtle patterns in mass spectrometry data that traditional algorithms might miss.
Pathway mapping algorithms constitute the final analytical stage, connecting identified metabolites to known biochemical pathways. Graph theory-based approaches have proven particularly effective for glycogenolysis pathway analysis, representing metabolites as nodes and biochemical reactions as edges. Algorithms such as PathFinder and MetaboNet employ topological analysis to identify pathway disruptions and metabolic bottlenecks with high accuracy.
Recent innovations include the integration of time-series analysis algorithms that capture dynamic changes in glycogenolysis pathways. These temporal algorithms can track metabolic flux through pathways, providing insights into the kinetics of glycogen breakdown under various physiological conditions. Bayesian statistical frameworks have enhanced the reliability of these analyses by incorporating uncertainty quantification.
Parallel computing architectures have addressed the computational intensity of these algorithms, with GPU-accelerated implementations reducing processing time by orders of magnitude. Cloud-based platforms now enable real-time processing of mass spectrometry data, facilitating rapid clinical decision-making in metabolic disorder diagnostics.
The integration of multi-omics data represents the frontier of algorithm development, where mass spectrometry data is analyzed alongside genomic, transcriptomic, and proteomic information. Ensemble algorithms that synthesize these diverse data types provide comprehensive views of glycogenolysis regulation, offering unprecedented insights into metabolic pathway dynamics and potential therapeutic targets.
Current state-of-the-art algorithms employ multi-stage processing pipelines that begin with raw mass spectrometry data preprocessing. This includes noise reduction techniques such as Savitzky-Golay filtering and baseline correction algorithms that effectively separate metabolite signals from instrumental noise. Advanced peak detection algorithms utilizing continuous wavelet transforms have demonstrated superior performance in identifying metabolite peaks with high sensitivity and specificity.
Feature extraction represents another critical component, where algorithms extract quantifiable characteristics from spectral data. Machine learning approaches, particularly deep learning models like convolutional neural networks (CNNs), have revolutionized this process by automatically identifying relevant spectral features without explicit programming. These models can detect subtle patterns in mass spectrometry data that traditional algorithms might miss.
Pathway mapping algorithms constitute the final analytical stage, connecting identified metabolites to known biochemical pathways. Graph theory-based approaches have proven particularly effective for glycogenolysis pathway analysis, representing metabolites as nodes and biochemical reactions as edges. Algorithms such as PathFinder and MetaboNet employ topological analysis to identify pathway disruptions and metabolic bottlenecks with high accuracy.
Recent innovations include the integration of time-series analysis algorithms that capture dynamic changes in glycogenolysis pathways. These temporal algorithms can track metabolic flux through pathways, providing insights into the kinetics of glycogen breakdown under various physiological conditions. Bayesian statistical frameworks have enhanced the reliability of these analyses by incorporating uncertainty quantification.
Parallel computing architectures have addressed the computational intensity of these algorithms, with GPU-accelerated implementations reducing processing time by orders of magnitude. Cloud-based platforms now enable real-time processing of mass spectrometry data, facilitating rapid clinical decision-making in metabolic disorder diagnostics.
The integration of multi-omics data represents the frontier of algorithm development, where mass spectrometry data is analyzed alongside genomic, transcriptomic, and proteomic information. Ensemble algorithms that synthesize these diverse data types provide comprehensive views of glycogenolysis regulation, offering unprecedented insights into metabolic pathway dynamics and potential therapeutic targets.
Clinical and Diagnostic Applications of Glycogenolysis Detection
The detection of glycogenolysis pathways using mass spectrometry has emerged as a powerful diagnostic tool in clinical settings. This technology enables healthcare professionals to identify abnormalities in glycogen metabolism, which are associated with various metabolic disorders including glycogen storage diseases, diabetes, and certain liver conditions.
Mass spectrometry-based detection of glycogenolysis offers significant advantages in clinical diagnostics, particularly in its ability to provide early detection of metabolic disorders before clinical symptoms manifest. This early intervention capability has proven crucial for conditions like von Gierke disease (Glycogen Storage Disease Type I) and McArdle disease (Glycogen Storage Disease Type V), where timely diagnosis can substantially improve patient outcomes through appropriate management strategies.
In pediatric medicine, glycogenolysis pathway detection has revolutionized the diagnosis of inherited metabolic disorders. The technique allows for precise identification of enzyme deficiencies in the glycogen breakdown pathway, enabling targeted therapeutic approaches. Recent clinical studies have demonstrated that mass spectrometry can detect abnormal metabolites with sensitivity exceeding 95% for several glycogen storage diseases, significantly outperforming traditional enzymatic assays.
The application extends to monitoring treatment efficacy in patients with established diagnoses. Clinicians can track changes in glycogen metabolism biomarkers over time, allowing for personalized adjustment of therapeutic interventions. This has been particularly valuable in managing patients with Pompe disease undergoing enzyme replacement therapy, where mass spectrometry provides objective measures of biochemical improvement.
In hepatology, glycogenolysis pathway detection serves as a valuable tool for assessing liver function and diagnosing conditions such as glycogen storage hepatopathy. The technique can distinguish between different forms of liver disease by identifying specific patterns of metabolite alterations, aiding in differential diagnosis when clinical presentations are similar.
Emergency medicine has also benefited from rapid glycogenolysis pathway analysis, particularly in cases of hypoglycemia of unknown origin. Mass spectrometry can quickly identify abnormalities in glycogen mobilization, guiding immediate treatment decisions in critical care settings. Several medical centers have implemented point-of-care mass spectrometry systems that can deliver results within 30 minutes, enabling time-sensitive clinical decision-making.
The integration of glycogenolysis pathway detection into routine clinical practice has been facilitated by advances in data interpretation software, which can automatically flag abnormal metabolite patterns and suggest potential diagnoses. This has expanded the accessibility of this technology beyond specialized metabolic centers to general hospitals and clinics, democratizing access to sophisticated metabolic diagnostics.
Mass spectrometry-based detection of glycogenolysis offers significant advantages in clinical diagnostics, particularly in its ability to provide early detection of metabolic disorders before clinical symptoms manifest. This early intervention capability has proven crucial for conditions like von Gierke disease (Glycogen Storage Disease Type I) and McArdle disease (Glycogen Storage Disease Type V), where timely diagnosis can substantially improve patient outcomes through appropriate management strategies.
In pediatric medicine, glycogenolysis pathway detection has revolutionized the diagnosis of inherited metabolic disorders. The technique allows for precise identification of enzyme deficiencies in the glycogen breakdown pathway, enabling targeted therapeutic approaches. Recent clinical studies have demonstrated that mass spectrometry can detect abnormal metabolites with sensitivity exceeding 95% for several glycogen storage diseases, significantly outperforming traditional enzymatic assays.
The application extends to monitoring treatment efficacy in patients with established diagnoses. Clinicians can track changes in glycogen metabolism biomarkers over time, allowing for personalized adjustment of therapeutic interventions. This has been particularly valuable in managing patients with Pompe disease undergoing enzyme replacement therapy, where mass spectrometry provides objective measures of biochemical improvement.
In hepatology, glycogenolysis pathway detection serves as a valuable tool for assessing liver function and diagnosing conditions such as glycogen storage hepatopathy. The technique can distinguish between different forms of liver disease by identifying specific patterns of metabolite alterations, aiding in differential diagnosis when clinical presentations are similar.
Emergency medicine has also benefited from rapid glycogenolysis pathway analysis, particularly in cases of hypoglycemia of unknown origin. Mass spectrometry can quickly identify abnormalities in glycogen mobilization, guiding immediate treatment decisions in critical care settings. Several medical centers have implemented point-of-care mass spectrometry systems that can deliver results within 30 minutes, enabling time-sensitive clinical decision-making.
The integration of glycogenolysis pathway detection into routine clinical practice has been facilitated by advances in data interpretation software, which can automatically flag abnormal metabolite patterns and suggest potential diagnoses. This has expanded the accessibility of this technology beyond specialized metabolic centers to general hospitals and clinics, democratizing access to sophisticated metabolic diagnostics.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!