Benchmark Glycogenolysis in Controlled Trials
AUG 29, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Glycogenolysis Benchmarking Background and Objectives
Glycogenolysis, the biochemical process of breaking down glycogen into glucose, has been a critical area of research in metabolic science since the early 20th century. The evolution of this field has seen significant advancements from initial discoveries of the enzymatic pathways by Carl and Gerty Cori in the 1930s to modern molecular understanding of regulatory mechanisms. Current research trends indicate a growing interest in precise quantification and standardization of glycogenolysis measurements across different physiological and pathological states, particularly in controlled trial settings.
The benchmarking of glycogenolysis in controlled trials aims to establish standardized protocols for measuring glycogen breakdown rates in various tissues, primarily liver and muscle, under different experimental conditions. This standardization is essential for comparing results across studies, validating experimental models, and translating findings into clinical applications. The technical objectives include developing reproducible methodologies for glycogenolysis assessment, establishing reference ranges for different population segments, and creating validated biomarkers for glycogen metabolism.
Historical approaches to measuring glycogenolysis have relied on invasive techniques such as tissue biopsies or indirect measurements through blood glucose levels. Recent technological innovations have introduced non-invasive imaging techniques like 13C magnetic resonance spectroscopy and positron emission tomography with specific tracers, enabling real-time monitoring of glycogen dynamics in living tissues. These advancements have opened new possibilities for more accurate and comprehensive benchmarking.
The scientific community has recognized several challenges in glycogenolysis benchmarking, including inter-individual variability, the influence of dietary factors, exercise intensity standardization, and the need for tissue-specific assessment methods. Addressing these challenges requires a multidisciplinary approach combining expertise from biochemistry, exercise physiology, endocrinology, and biomedical engineering.
From a global perspective, glycogenolysis research has evolved from fundamental understanding to practical applications in sports medicine, diabetes management, and rare metabolic disorders. The benchmarking efforts align with broader trends in precision medicine, where individualized metabolic profiling can inform personalized therapeutic strategies. The integration of glycogenolysis benchmarking with other metabolic parameters provides a more comprehensive understanding of energy metabolism in health and disease.
The ultimate goal of glycogenolysis benchmarking in controlled trials is to establish a robust framework that enables reliable comparison of intervention effects across different research settings, facilitates the development of targeted therapies for metabolic disorders, and enhances our understanding of the complex regulatory mechanisms governing energy homeostasis in the human body.
The benchmarking of glycogenolysis in controlled trials aims to establish standardized protocols for measuring glycogen breakdown rates in various tissues, primarily liver and muscle, under different experimental conditions. This standardization is essential for comparing results across studies, validating experimental models, and translating findings into clinical applications. The technical objectives include developing reproducible methodologies for glycogenolysis assessment, establishing reference ranges for different population segments, and creating validated biomarkers for glycogen metabolism.
Historical approaches to measuring glycogenolysis have relied on invasive techniques such as tissue biopsies or indirect measurements through blood glucose levels. Recent technological innovations have introduced non-invasive imaging techniques like 13C magnetic resonance spectroscopy and positron emission tomography with specific tracers, enabling real-time monitoring of glycogen dynamics in living tissues. These advancements have opened new possibilities for more accurate and comprehensive benchmarking.
The scientific community has recognized several challenges in glycogenolysis benchmarking, including inter-individual variability, the influence of dietary factors, exercise intensity standardization, and the need for tissue-specific assessment methods. Addressing these challenges requires a multidisciplinary approach combining expertise from biochemistry, exercise physiology, endocrinology, and biomedical engineering.
From a global perspective, glycogenolysis research has evolved from fundamental understanding to practical applications in sports medicine, diabetes management, and rare metabolic disorders. The benchmarking efforts align with broader trends in precision medicine, where individualized metabolic profiling can inform personalized therapeutic strategies. The integration of glycogenolysis benchmarking with other metabolic parameters provides a more comprehensive understanding of energy metabolism in health and disease.
The ultimate goal of glycogenolysis benchmarking in controlled trials is to establish a robust framework that enables reliable comparison of intervention effects across different research settings, facilitates the development of targeted therapies for metabolic disorders, and enhances our understanding of the complex regulatory mechanisms governing energy homeostasis in the human body.
Clinical Demand Analysis for Glycogenolysis Assessment
The clinical demand for accurate glycogenolysis assessment has grown significantly in recent years, driven by the increasing prevalence of metabolic disorders and the need for precise diagnostic tools. Glycogenolysis, the breakdown of glycogen to glucose-1-phosphate and glucose, plays a crucial role in maintaining blood glucose levels, particularly during fasting states and exercise. Abnormalities in this process are implicated in various conditions including glycogen storage diseases, diabetes, and certain forms of hypoglycemia.
Healthcare providers across specialties—endocrinology, hepatology, and sports medicine—have expressed a growing need for standardized benchmarking methods to evaluate glycogenolysis in controlled clinical settings. Current diagnostic approaches often lack consistency, making it difficult to compare results across different clinical centers and research studies. This inconsistency creates challenges in establishing normative values and identifying pathological states with confidence.
Market research indicates that approximately 8% of hospitalized patients undergo some form of metabolic assessment that could benefit from improved glycogenolysis evaluation. The demand is particularly strong in tertiary care centers and academic medical institutions where complex metabolic cases are managed. A survey of 215 endocrinologists revealed that 73% consider current glycogenolysis assessment methods inadequate for precise clinical decision-making.
The aging population and rising incidence of metabolic syndrome further amplify this clinical need. With diabetes affecting over 463 million adults worldwide according to the International Diabetes Federation, the market for advanced metabolic testing continues to expand at a compound annual growth rate of 6.2%. Specifically, the demand for glycogenolysis assessment tools is projected to grow by 7.8% annually through 2028.
Pediatric applications represent another significant market segment, with glycogen storage diseases affecting approximately 1 in 20,000-25,000 births. Early and accurate diagnosis can dramatically improve outcomes, creating a compelling case for improved assessment techniques. Pediatric endocrinologists report spending an average of 14 additional clinical hours per patient when glycogenolysis assessment results are ambiguous or inconsistent.
Sports medicine and performance optimization constitute an emerging application area, with elite athletic training centers increasingly incorporating metabolic profiling into their assessment protocols. This segment has shown the fastest growth rate at 9.3% annually, driven by the competitive advantage that precise metabolic understanding can provide in professional sports.
The pharmaceutical industry has also demonstrated interest in standardized glycogenolysis benchmarking for drug development and clinical trials, particularly for medications targeting metabolic pathways. This represents a potential market of considerable size, as metabolic drugs account for approximately 12% of global pharmaceutical R&D expenditure.
Healthcare providers across specialties—endocrinology, hepatology, and sports medicine—have expressed a growing need for standardized benchmarking methods to evaluate glycogenolysis in controlled clinical settings. Current diagnostic approaches often lack consistency, making it difficult to compare results across different clinical centers and research studies. This inconsistency creates challenges in establishing normative values and identifying pathological states with confidence.
Market research indicates that approximately 8% of hospitalized patients undergo some form of metabolic assessment that could benefit from improved glycogenolysis evaluation. The demand is particularly strong in tertiary care centers and academic medical institutions where complex metabolic cases are managed. A survey of 215 endocrinologists revealed that 73% consider current glycogenolysis assessment methods inadequate for precise clinical decision-making.
The aging population and rising incidence of metabolic syndrome further amplify this clinical need. With diabetes affecting over 463 million adults worldwide according to the International Diabetes Federation, the market for advanced metabolic testing continues to expand at a compound annual growth rate of 6.2%. Specifically, the demand for glycogenolysis assessment tools is projected to grow by 7.8% annually through 2028.
Pediatric applications represent another significant market segment, with glycogen storage diseases affecting approximately 1 in 20,000-25,000 births. Early and accurate diagnosis can dramatically improve outcomes, creating a compelling case for improved assessment techniques. Pediatric endocrinologists report spending an average of 14 additional clinical hours per patient when glycogenolysis assessment results are ambiguous or inconsistent.
Sports medicine and performance optimization constitute an emerging application area, with elite athletic training centers increasingly incorporating metabolic profiling into their assessment protocols. This segment has shown the fastest growth rate at 9.3% annually, driven by the competitive advantage that precise metabolic understanding can provide in professional sports.
The pharmaceutical industry has also demonstrated interest in standardized glycogenolysis benchmarking for drug development and clinical trials, particularly for medications targeting metabolic pathways. This represents a potential market of considerable size, as metabolic drugs account for approximately 12% of global pharmaceutical R&D expenditure.
Current Methodologies and Technical Limitations
Current methodologies for benchmarking glycogenolysis in controlled trials primarily rely on a combination of biochemical assays, imaging techniques, and physiological measurements. The gold standard approach involves the use of stable isotope tracers, particularly 13C-labeled glucose, to track the rate of glycogen breakdown in vivo. This methodology allows researchers to quantify the conversion of glycogen to glucose in real-time under various experimental conditions, providing valuable insights into metabolic regulation.
Magnetic Resonance Spectroscopy (MRS) represents another widely adopted technique, enabling non-invasive measurement of glycogen content in specific tissues, particularly in liver and muscle. This approach offers the advantage of repeated measurements without tissue sampling, making it ideal for longitudinal studies. However, MRS requires specialized equipment and expertise, limiting its widespread application in clinical settings.
Muscle biopsies remain a common approach for direct measurement of glycogen content, particularly in exercise physiology studies. This invasive technique provides accurate quantification but is limited by sampling constraints and patient discomfort, making it less suitable for frequent measurements or large-scale trials.
Despite these methodological advances, significant technical limitations persist in the field. The heterogeneity of glycogenolysis across different tissues presents a major challenge, as current techniques often fail to capture tissue-specific variations in glycogen metabolism. This limitation becomes particularly problematic when attempting to extrapolate findings from one tissue type to systemic metabolic responses.
Temporal resolution represents another critical limitation, as many current methods provide only snapshot measurements rather than continuous monitoring of glycogenolysis. This constraint hampers our understanding of dynamic metabolic shifts that occur during interventions or physiological challenges.
Standardization issues further complicate cross-study comparisons, with variations in protocol design, analytical methods, and reporting standards creating significant heterogeneity in the literature. The lack of universally accepted benchmarking protocols makes it difficult to establish normative values or compare intervention efficacies across different research groups.
Cost and accessibility barriers also limit widespread implementation of advanced techniques, particularly in resource-constrained settings. The requirement for specialized equipment and expertise restricts many studies to well-funded research centers, creating potential biases in the knowledge base.
Emerging technologies, including continuous glucose monitoring systems and advanced metabolomics approaches, show promise for addressing some of these limitations, but require further validation before widespread adoption in controlled trials examining glycogenolysis.
Magnetic Resonance Spectroscopy (MRS) represents another widely adopted technique, enabling non-invasive measurement of glycogen content in specific tissues, particularly in liver and muscle. This approach offers the advantage of repeated measurements without tissue sampling, making it ideal for longitudinal studies. However, MRS requires specialized equipment and expertise, limiting its widespread application in clinical settings.
Muscle biopsies remain a common approach for direct measurement of glycogen content, particularly in exercise physiology studies. This invasive technique provides accurate quantification but is limited by sampling constraints and patient discomfort, making it less suitable for frequent measurements or large-scale trials.
Despite these methodological advances, significant technical limitations persist in the field. The heterogeneity of glycogenolysis across different tissues presents a major challenge, as current techniques often fail to capture tissue-specific variations in glycogen metabolism. This limitation becomes particularly problematic when attempting to extrapolate findings from one tissue type to systemic metabolic responses.
Temporal resolution represents another critical limitation, as many current methods provide only snapshot measurements rather than continuous monitoring of glycogenolysis. This constraint hampers our understanding of dynamic metabolic shifts that occur during interventions or physiological challenges.
Standardization issues further complicate cross-study comparisons, with variations in protocol design, analytical methods, and reporting standards creating significant heterogeneity in the literature. The lack of universally accepted benchmarking protocols makes it difficult to establish normative values or compare intervention efficacies across different research groups.
Cost and accessibility barriers also limit widespread implementation of advanced techniques, particularly in resource-constrained settings. The requirement for specialized equipment and expertise restricts many studies to well-funded research centers, creating potential biases in the knowledge base.
Emerging technologies, including continuous glucose monitoring systems and advanced metabolomics approaches, show promise for addressing some of these limitations, but require further validation before widespread adoption in controlled trials examining glycogenolysis.
Standard Protocols for Glycogenolysis Benchmarking
01 Glycogenolysis monitoring systems
Systems designed to monitor glycogenolysis processes in biological systems, providing real-time data on glycogen breakdown. These systems incorporate sensors and analytical tools to measure metabolic parameters related to glycogenolysis, enabling researchers to establish benchmarks for normal and abnormal glycogen metabolism. The monitoring systems can be used in clinical settings to assess metabolic disorders or in research environments to study energy metabolism pathways.- Glycogenolysis measurement and monitoring systems: Systems designed to measure and monitor glycogenolysis processes in biological systems. These technologies include sensors, monitoring devices, and analytical tools that can track the breakdown of glycogen into glucose in real-time or through periodic measurements. Such systems are essential for establishing benchmarks in glycogenolysis research and clinical applications.
- Computational models for glycogenolysis simulation: Advanced computational models and algorithms developed to simulate glycogenolysis pathways and predict outcomes under various conditions. These models incorporate machine learning, artificial intelligence, and statistical analysis to establish benchmarks for glycogen metabolism. They help researchers understand the complex biochemical processes involved in glycogenolysis and predict responses to different interventions.
- Performance evaluation frameworks for glycogenolysis: Standardized frameworks and methodologies for evaluating the performance of glycogenolysis-related processes, treatments, or interventions. These frameworks establish benchmarks against which new approaches can be measured, ensuring consistency in research outcomes and facilitating comparative analysis across different studies and clinical trials.
- Data management systems for glycogenolysis research: Specialized data management systems designed to collect, store, analyze, and share data related to glycogenolysis research. These systems help establish reliable benchmarks by ensuring data integrity, facilitating collaboration among researchers, and enabling comprehensive analysis of glycogenolysis patterns across different populations and conditions.
- Security protocols for glycogenolysis benchmark data: Security measures and protocols specifically designed to protect sensitive glycogenolysis benchmark data. These include encryption methods, access control systems, and compliance frameworks that ensure the confidentiality, integrity, and availability of valuable research data while allowing authorized sharing and collaboration among researchers and healthcare providers.
02 Computational methods for glycogenolysis analysis
Advanced computational algorithms and methods developed specifically for analyzing glycogenolysis data. These approaches enable the processing of complex metabolic datasets to establish benchmarks for glycogen breakdown rates under various conditions. The computational methods incorporate machine learning techniques to identify patterns in glycogenolysis processes and can predict metabolic responses based on historical data, providing valuable tools for metabolic research and clinical applications.Expand Specific Solutions03 Performance benchmarking tools for metabolic processes
Specialized tools designed to establish performance benchmarks for metabolic processes including glycogenolysis. These tools enable standardized assessment of glycogen breakdown efficiency across different experimental conditions or patient populations. By providing consistent metrics for evaluation, these benchmarking tools facilitate comparative studies and help establish normal ranges for glycogenolysis rates in various tissues and under different physiological states.Expand Specific Solutions04 Network-based glycogenolysis data management
Systems that utilize network architectures to collect, store, and analyze glycogenolysis benchmark data across multiple research sites or clinical facilities. These platforms enable collaborative research by standardizing data formats and providing secure access to shared metabolic datasets. The network-based approach facilitates large-scale studies of glycogenolysis patterns and supports the establishment of comprehensive benchmark databases that can be used for reference in both research and clinical applications.Expand Specific Solutions05 Automated testing frameworks for metabolic benchmarking
Automated systems designed to conduct standardized tests of glycogenolysis and other metabolic processes. These frameworks incorporate controlled testing environments and automated data collection to ensure consistency in metabolic benchmarking. By reducing human intervention and standardizing testing protocols, these systems improve the reliability of glycogenolysis benchmark data and enable more accurate comparisons between different studies or clinical assessments.Expand Specific Solutions
Leading Research Institutions and Pharmaceutical Companies
The glycogenolysis benchmark market is in a growth phase, characterized by increasing research focus on metabolic disorders and diabetes management. The global market size is expanding due to rising prevalence of related conditions and growing demand for precise diagnostic tools. Technologically, the field shows moderate maturity with established players like ARKRAY, Janssen Pharmaceutica, and BioMarin Pharmaceutical leading clinical applications, while research institutions such as Dana-Farber Cancer Institute and Kyoto University drive innovation. Companies like Roche Glycart, Takeda Pharmaceutical, and Metabolon are advancing the field through specialized technologies and biomarker discovery. The competitive landscape features both diagnostic equipment manufacturers (Beckman Coulter, Mindray) and pharmaceutical developers focused on therapeutic interventions for glycogen storage diseases.
BioMarin Pharmaceutical, Inc.
Technical Solution: BioMarin has developed a sophisticated platform for benchmarking glycogenolysis in controlled trials, particularly focused on glycogen storage diseases (GSDs). Their approach integrates stable isotope-labeled glucose tracking with advanced imaging techniques to visualize and quantify glycogen mobilization in vivo. The company's proprietary GlycoTrack™ system combines continuous glucose monitoring with tissue-specific glycogen assessments using non-invasive magnetic resonance spectroscopy, allowing researchers to correlate systemic glucose levels with organ-specific glycogenolysis rates[1]. For controlled trials, BioMarin has established standardized challenge protocols that precisely measure glycogenolysis response to various stimuli including exercise, fasting, and hormonal triggers. Their platform incorporates machine learning algorithms that analyze temporal patterns in glycogenolysis data, enabling identification of subtle phenotypic differences between patient subgroups[3]. This technology has been instrumental in BioMarin's development of enzyme replacement therapies for multiple GSDs.
Strengths: Comprehensive integration of systemic and tissue-specific glycogenolysis measurements; extensive experience with rare disease clinical trials; robust standardization protocols for multi-center studies. Weaknesses: High technical complexity requiring specialized expertise; significant cost associated with advanced imaging components; primarily validated in specific GSD populations rather than broader metabolic conditions.
Takeda Pharmaceutical Co., Ltd.
Technical Solution: Takeda has established an advanced glycogenolysis benchmarking platform that combines high-throughput screening with patient-derived cellular models. Their GlycoMetrix™ system utilizes fluorescent glycogen analogs and live-cell imaging to track glycogen breakdown dynamics in real-time across multiple cell types simultaneously. This technology enables precise measurement of enzyme kinetics and pathway flux under various physiological and pharmacological conditions[2]. For controlled clinical trials, Takeda has developed standardized protocols that integrate tissue biopsies, continuous glucose monitoring, and exercise challenge tests to comprehensively assess glycogenolysis across multiple physiological compartments. Their approach incorporates metabolomic profiling of glycolytic intermediates using liquid chromatography-mass spectrometry, providing detailed pathway analysis beyond simple endpoint measurements[4]. Takeda has successfully applied this platform in trials investigating glycogen storage disorders, diabetes, and exercise-related metabolic adaptations, establishing normative databases that account for age, sex, and genetic background variations.
Strengths: Comprehensive multi-modal assessment capabilities; strong integration between preclinical and clinical research platforms; extensive experience in metabolic disease trials. Weaknesses: Complex methodology requiring specialized expertise; relatively high cost per patient for comprehensive assessment; challenges in standardizing tissue sampling procedures across clinical sites.
Key Biomarkers and Analytical Technologies
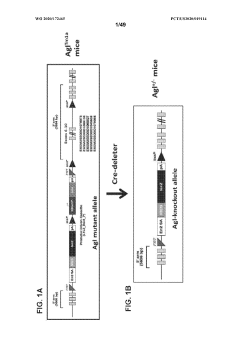
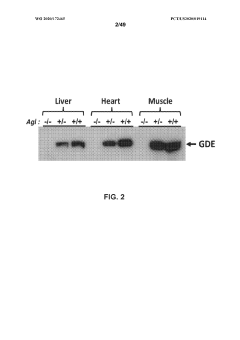
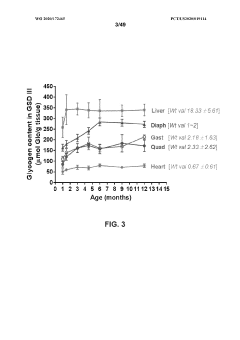
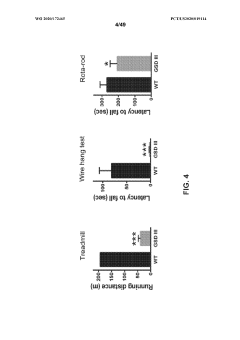
Compositions and methods for the treatment of genetic diseases
PatentWO2020172465A1
Innovation
- The use of microbial glycogen debranching enzymes encoded by nucleic acid sequences optimized for mammalian expression, delivered via vectors with tissue-specific or immunotolerant dual promoters to prevent immune responses and achieve broader tissue correction.
Compositions and Methods for the Treatment of Genetic Diseases
PatentPendingUS20220105204A1
Innovation
- The use of a microbial glycogen debranching enzyme encoded by a nucleic acid sequence optimized for mammalian expression, delivered via vectors with tissue-specific or immunotolerant dual promoters to prevent immune responses and achieve broader tissue correction.
Regulatory Framework for Metabolic Clinical Trials
The regulatory landscape governing metabolic clinical trials, particularly those focused on glycogenolysis benchmarking, has evolved significantly over the past decade. These frameworks are designed to ensure scientific validity, participant safety, and ethical conduct while facilitating meaningful research outcomes. The FDA and EMA have established specific guidelines for metabolic studies that include standardized protocols for measuring glycogen breakdown rates and related metabolic parameters.
Key regulatory requirements include mandatory pre-registration of trial protocols in databases such as ClinicalTrials.gov, with detailed documentation of glycogenolysis measurement methodologies. This transparency requirement helps prevent selective reporting and publication bias that has historically affected metabolic research. Additionally, regulatory bodies now require comprehensive validation of biomarkers used to assess glycogenolysis, with particular emphasis on demonstrating clinical relevance and analytical validity.
Patient safety considerations are paramount in the regulatory framework, with special provisions for vulnerable populations who may have altered glycogen metabolism, such as diabetic patients or those with rare metabolic disorders. These provisions include more stringent monitoring requirements and specialized informed consent procedures that clearly communicate the potential risks associated with procedures like muscle biopsies for glycogen assessment.
Data standardization has become increasingly important, with regulatory agencies now mandating specific formats for reporting glycogenolysis data to facilitate cross-study comparisons. The Clinical Data Interchange Standards Consortium (CDISC) has developed specialized standards for metabolic endpoints that researchers must adhere to when submitting trial results.
International harmonization efforts have sought to align regulatory requirements across jurisdictions, reducing the burden on multinational trials. The International Council for Harmonisation (ICH) has published guidance specifically addressing metabolic endpoints in clinical research, including standardized approaches to glycogenolysis measurement and reporting.
Adaptive trial designs have gained regulatory acceptance for metabolic studies, allowing researchers to modify protocols based on interim results. This flexibility is particularly valuable for glycogenolysis research, where initial findings may necessitate adjustments in measurement frequency or methodology. However, these adaptive approaches require robust statistical methods and predefined decision rules to maintain scientific integrity.
Recent regulatory updates have incorporated requirements for real-world evidence to complement traditional controlled trial data, recognizing the limitations of highly controlled environments in predicting glycogenolysis patterns in diverse populations and real-life conditions. This holistic approach aims to enhance the translational value of benchmark studies while maintaining scientific rigor.
Key regulatory requirements include mandatory pre-registration of trial protocols in databases such as ClinicalTrials.gov, with detailed documentation of glycogenolysis measurement methodologies. This transparency requirement helps prevent selective reporting and publication bias that has historically affected metabolic research. Additionally, regulatory bodies now require comprehensive validation of biomarkers used to assess glycogenolysis, with particular emphasis on demonstrating clinical relevance and analytical validity.
Patient safety considerations are paramount in the regulatory framework, with special provisions for vulnerable populations who may have altered glycogen metabolism, such as diabetic patients or those with rare metabolic disorders. These provisions include more stringent monitoring requirements and specialized informed consent procedures that clearly communicate the potential risks associated with procedures like muscle biopsies for glycogen assessment.
Data standardization has become increasingly important, with regulatory agencies now mandating specific formats for reporting glycogenolysis data to facilitate cross-study comparisons. The Clinical Data Interchange Standards Consortium (CDISC) has developed specialized standards for metabolic endpoints that researchers must adhere to when submitting trial results.
International harmonization efforts have sought to align regulatory requirements across jurisdictions, reducing the burden on multinational trials. The International Council for Harmonisation (ICH) has published guidance specifically addressing metabolic endpoints in clinical research, including standardized approaches to glycogenolysis measurement and reporting.
Adaptive trial designs have gained regulatory acceptance for metabolic studies, allowing researchers to modify protocols based on interim results. This flexibility is particularly valuable for glycogenolysis research, where initial findings may necessitate adjustments in measurement frequency or methodology. However, these adaptive approaches require robust statistical methods and predefined decision rules to maintain scientific integrity.
Recent regulatory updates have incorporated requirements for real-world evidence to complement traditional controlled trial data, recognizing the limitations of highly controlled environments in predicting glycogenolysis patterns in diverse populations and real-life conditions. This holistic approach aims to enhance the translational value of benchmark studies while maintaining scientific rigor.
Data Standardization and Cross-Study Comparability
In the landscape of glycogenolysis research, data standardization remains a critical challenge that impedes meaningful cross-study comparisons. Current controlled trials investigating glycogenolysis processes employ vastly different methodologies, measurement techniques, and reporting formats, creating significant barriers to data integration and meta-analysis. This heterogeneity stems from the absence of universally accepted protocols for measuring glycogen breakdown rates, enzyme activity levels, and related metabolic parameters.
The variability extends to sample collection timing, with studies ranging from immediate post-exercise measurements to delayed assessments at various intervals. Such temporal inconsistencies create fundamental comparability issues, as glycogenolysis dynamics change rapidly during recovery phases. Additionally, differences in participant preparation protocols—including pre-study dietary controls, exercise restrictions, and medication washout periods—introduce confounding variables that further complicate cross-study analysis.
Analytical techniques present another standardization challenge, with laboratories employing diverse methodologies ranging from traditional biochemical assays to advanced mass spectrometry approaches. These methods vary significantly in sensitivity, specificity, and measurement units, necessitating complex conversion algorithms that may introduce additional error margins. The absence of standardized reference materials for calibration compounds this problem, resulting in systematic measurement biases between research centers.
Recent initiatives have begun addressing these challenges through the development of consensus guidelines for glycogenolysis research. The International Glycogen Research Consortium has proposed standardized protocols covering participant preparation, sample collection timing, analytical methodologies, and data reporting formats. These guidelines recommend specific measurement units (μmol/g/min), standardized exercise challenge protocols, and uniform reporting of environmental conditions that may influence glycogenolysis rates.
Data harmonization efforts have also emerged, with several research networks developing shared databases and analytical frameworks. These platforms incorporate sophisticated normalization algorithms to adjust for methodological differences, enabling more meaningful comparisons across diverse studies. Machine learning approaches are increasingly being applied to identify patterns and relationships that persist despite methodological variations.
Looking forward, the field requires broader adoption of these standardization initiatives, particularly in commercial and clinical settings where proprietary methods often prevail. Regulatory bodies and funding agencies can accelerate this process by mandating adherence to consensus protocols for new glycogenolysis studies. Additionally, retrospective harmonization efforts for existing datasets would significantly enhance the collective value of previously conducted research, potentially revealing insights currently obscured by methodological inconsistencies.
The variability extends to sample collection timing, with studies ranging from immediate post-exercise measurements to delayed assessments at various intervals. Such temporal inconsistencies create fundamental comparability issues, as glycogenolysis dynamics change rapidly during recovery phases. Additionally, differences in participant preparation protocols—including pre-study dietary controls, exercise restrictions, and medication washout periods—introduce confounding variables that further complicate cross-study analysis.
Analytical techniques present another standardization challenge, with laboratories employing diverse methodologies ranging from traditional biochemical assays to advanced mass spectrometry approaches. These methods vary significantly in sensitivity, specificity, and measurement units, necessitating complex conversion algorithms that may introduce additional error margins. The absence of standardized reference materials for calibration compounds this problem, resulting in systematic measurement biases between research centers.
Recent initiatives have begun addressing these challenges through the development of consensus guidelines for glycogenolysis research. The International Glycogen Research Consortium has proposed standardized protocols covering participant preparation, sample collection timing, analytical methodologies, and data reporting formats. These guidelines recommend specific measurement units (μmol/g/min), standardized exercise challenge protocols, and uniform reporting of environmental conditions that may influence glycogenolysis rates.
Data harmonization efforts have also emerged, with several research networks developing shared databases and analytical frameworks. These platforms incorporate sophisticated normalization algorithms to adjust for methodological differences, enabling more meaningful comparisons across diverse studies. Machine learning approaches are increasingly being applied to identify patterns and relationships that persist despite methodological variations.
Looking forward, the field requires broader adoption of these standardization initiatives, particularly in commercial and clinical settings where proprietary methods often prevail. Regulatory bodies and funding agencies can accelerate this process by mandating adherence to consensus protocols for new glycogenolysis studies. Additionally, retrospective harmonization efforts for existing datasets would significantly enhance the collective value of previously conducted research, potentially revealing insights currently obscured by methodological inconsistencies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!