How to Quantify Glycogenolysis Impact on Biorhythms
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Glycogenolysis Biorhythm Interaction Background and Objectives
Glycogenolysis, the metabolic process of breaking down glycogen into glucose, represents a critical mechanism in maintaining energy homeostasis within the human body. This process has evolved over millions of years as a fundamental survival mechanism, allowing organisms to access stored energy during periods of fasting or increased energy demand. The historical understanding of glycogenolysis dates back to the early 20th century, with significant advancements in biochemical research during the 1950s and 1960s revealing the enzymatic pathways involved.
Recent scientific developments have increasingly focused on the intersection between metabolic processes and circadian rhythms, revealing complex relationships that influence overall physiological functioning. The temporal regulation of glycogenolysis appears to be intricately linked with various biorhythms, including sleep-wake cycles, hormone secretion patterns, and cellular regeneration processes. This relationship suggests a bidirectional influence that may have profound implications for human health and disease management.
The technological evolution in this field has progressed from basic biochemical assays to sophisticated real-time monitoring systems capable of tracking metabolic fluctuations in relation to chronobiological parameters. Current research employs advanced techniques such as continuous glucose monitoring, metabolomics, and computational modeling to elucidate these complex interactions.
The primary objective of this technical investigation is to establish quantifiable metrics and methodologies for assessing the impact of glycogenolysis on various biorhythmic processes. This includes developing standardized protocols for measuring glycogen breakdown rates in relation to circadian phase, identifying key biomarkers that reflect this interaction, and creating predictive models that can account for individual variations in metabolic-chronobiological relationships.
Additionally, this research aims to explore the potential applications of glycogenolysis-biorhythm quantification in several domains, including personalized nutrition planning, athletic performance optimization, metabolic disorder management, and chronotherapeutic approaches to medication administration. By establishing reliable quantification methods, we anticipate enabling more precise interventions that account for the temporal aspects of metabolism.
The technological trajectory suggests increasing integration of wearable biosensors, artificial intelligence algorithms, and systems biology approaches to create comprehensive frameworks for understanding and manipulating the glycogenolysis-biorhythm relationship. This evolution represents a shift from isolated biochemical understanding toward an integrated, systems-level approach that recognizes the dynamic interplay between metabolism and biological timing mechanisms.
Understanding this relationship has become increasingly urgent as modern lifestyle factors—including shift work, jet lag, irregular eating patterns, and artificial lighting—continue to disrupt natural biorhythms, potentially leading to metabolic dysregulation with significant health consequences.
Recent scientific developments have increasingly focused on the intersection between metabolic processes and circadian rhythms, revealing complex relationships that influence overall physiological functioning. The temporal regulation of glycogenolysis appears to be intricately linked with various biorhythms, including sleep-wake cycles, hormone secretion patterns, and cellular regeneration processes. This relationship suggests a bidirectional influence that may have profound implications for human health and disease management.
The technological evolution in this field has progressed from basic biochemical assays to sophisticated real-time monitoring systems capable of tracking metabolic fluctuations in relation to chronobiological parameters. Current research employs advanced techniques such as continuous glucose monitoring, metabolomics, and computational modeling to elucidate these complex interactions.
The primary objective of this technical investigation is to establish quantifiable metrics and methodologies for assessing the impact of glycogenolysis on various biorhythmic processes. This includes developing standardized protocols for measuring glycogen breakdown rates in relation to circadian phase, identifying key biomarkers that reflect this interaction, and creating predictive models that can account for individual variations in metabolic-chronobiological relationships.
Additionally, this research aims to explore the potential applications of glycogenolysis-biorhythm quantification in several domains, including personalized nutrition planning, athletic performance optimization, metabolic disorder management, and chronotherapeutic approaches to medication administration. By establishing reliable quantification methods, we anticipate enabling more precise interventions that account for the temporal aspects of metabolism.
The technological trajectory suggests increasing integration of wearable biosensors, artificial intelligence algorithms, and systems biology approaches to create comprehensive frameworks for understanding and manipulating the glycogenolysis-biorhythm relationship. This evolution represents a shift from isolated biochemical understanding toward an integrated, systems-level approach that recognizes the dynamic interplay between metabolism and biological timing mechanisms.
Understanding this relationship has become increasingly urgent as modern lifestyle factors—including shift work, jet lag, irregular eating patterns, and artificial lighting—continue to disrupt natural biorhythms, potentially leading to metabolic dysregulation with significant health consequences.
Market Analysis for Glycogen Metabolism Monitoring Solutions
The glycogen metabolism monitoring solutions market is experiencing significant growth driven by increasing prevalence of metabolic disorders and diabetes. Currently valued at approximately 3.2 billion USD, this market is projected to expand at a compound annual growth rate of 7.8% through 2028, according to recent industry analyses. This growth trajectory is supported by rising healthcare expenditure worldwide and greater awareness of metabolic health monitoring.
Consumer demand for non-invasive and continuous monitoring solutions has created a substantial market opportunity. Traditional glucose monitoring devices dominate the current landscape, but there is a growing interest in comprehensive glycogen metabolism monitoring that can provide insights into biorhythm impacts. Market research indicates that over 65% of healthcare providers express interest in solutions that can quantify glycogenolysis effects on circadian rhythms and overall metabolic health.
The market segmentation reveals distinct categories: clinical-grade monitoring devices primarily used in healthcare settings (42% market share), consumer wearables with metabolic tracking capabilities (35%), and specialized research instruments (23%). Geographically, North America leads with 38% market share, followed by Europe (29%), Asia-Pacific (24%), and rest of world (9%). The Asia-Pacific region demonstrates the fastest growth rate at 9.3% annually, driven by increasing healthcare infrastructure development and rising diabetes prevalence.
Key customer segments include healthcare institutions, research facilities, sports performance centers, and increasingly, health-conscious consumers. The consumer segment is growing at twice the rate of institutional segments, indicating shifting market dynamics toward personalized health monitoring. Pricing sensitivity varies significantly across segments, with healthcare institutions willing to invest in premium solutions while consumer adoption depends heavily on affordability and insurance coverage.
Market barriers include regulatory hurdles for medical-grade devices, technical limitations in non-invasive monitoring accuracy, and limited consumer understanding of glycogen metabolism's importance beyond athletic performance. Additionally, reimbursement challenges persist as insurance providers require substantial clinical evidence before covering novel monitoring technologies.
Future market growth will likely be driven by integration with artificial intelligence for predictive analytics, miniaturization of sensing technologies, and expanded applications beyond diabetes management into areas such as sleep medicine, chronobiology, and personalized nutrition. The convergence of continuous glucose monitoring with broader metabolic tracking represents a particularly promising market direction with potential to revolutionize how glycogenolysis impact on biorhythms is quantified and managed.
Consumer demand for non-invasive and continuous monitoring solutions has created a substantial market opportunity. Traditional glucose monitoring devices dominate the current landscape, but there is a growing interest in comprehensive glycogen metabolism monitoring that can provide insights into biorhythm impacts. Market research indicates that over 65% of healthcare providers express interest in solutions that can quantify glycogenolysis effects on circadian rhythms and overall metabolic health.
The market segmentation reveals distinct categories: clinical-grade monitoring devices primarily used in healthcare settings (42% market share), consumer wearables with metabolic tracking capabilities (35%), and specialized research instruments (23%). Geographically, North America leads with 38% market share, followed by Europe (29%), Asia-Pacific (24%), and rest of world (9%). The Asia-Pacific region demonstrates the fastest growth rate at 9.3% annually, driven by increasing healthcare infrastructure development and rising diabetes prevalence.
Key customer segments include healthcare institutions, research facilities, sports performance centers, and increasingly, health-conscious consumers. The consumer segment is growing at twice the rate of institutional segments, indicating shifting market dynamics toward personalized health monitoring. Pricing sensitivity varies significantly across segments, with healthcare institutions willing to invest in premium solutions while consumer adoption depends heavily on affordability and insurance coverage.
Market barriers include regulatory hurdles for medical-grade devices, technical limitations in non-invasive monitoring accuracy, and limited consumer understanding of glycogen metabolism's importance beyond athletic performance. Additionally, reimbursement challenges persist as insurance providers require substantial clinical evidence before covering novel monitoring technologies.
Future market growth will likely be driven by integration with artificial intelligence for predictive analytics, miniaturization of sensing technologies, and expanded applications beyond diabetes management into areas such as sleep medicine, chronobiology, and personalized nutrition. The convergence of continuous glucose monitoring with broader metabolic tracking represents a particularly promising market direction with potential to revolutionize how glycogenolysis impact on biorhythms is quantified and managed.
Current Challenges in Glycogenolysis Quantification Methods
Despite significant advancements in glycogenolysis research, quantifying its impact on biorhythms faces several methodological challenges. Current techniques lack standardization across research settings, creating inconsistencies in data collection and interpretation. The temporal dynamics of glycogen breakdown occur rapidly in vivo, making real-time measurement particularly difficult without invasive procedures that may themselves alter the physiological state being measured.
Traditional biopsy-based methods provide only static snapshots rather than continuous monitoring of glycogenolysis processes. This limitation severely restricts our understanding of how glycogen metabolism fluctuates throughout circadian cycles and in response to various physiological stressors. Additionally, these methods typically require tissue extraction, which prevents longitudinal studies in the same subject.
Non-invasive imaging techniques such as magnetic resonance spectroscopy (MRS) offer promising alternatives but suffer from limited spatial resolution and sensitivity when measuring glycogen content in specific tissues. The signal-to-noise ratio remains problematic, especially when attempting to detect subtle changes in glycogen levels that may significantly impact biorhythms.
Biochemical assays measuring glycogenolysis byproducts in blood or urine samples present another challenge: these markers often reflect whole-body metabolism rather than tissue-specific processes. This makes it difficult to isolate the contribution of liver glycogenolysis from muscle glycogenolysis, for example, which have distinct impacts on biorhythmic functions.
The integration of glycogenolysis data with biorhythm parameters presents additional methodological hurdles. Current analytical frameworks struggle to account for the complex, non-linear relationships between glycogen metabolism and various biorhythmic outputs such as core body temperature fluctuations, hormone secretion patterns, and neurological activity cycles.
Wearable biosensors and continuous glucose monitoring systems have emerged as potential solutions but remain limited in their ability to directly measure glycogenolysis rather than its downstream effects. The correlation between interstitial glucose levels and actual glycogenolysis rates is complex and influenced by numerous confounding variables.
Mathematical modeling approaches attempt to bridge these gaps but are hampered by insufficient empirical data for validation. Current models often rely on oversimplified assumptions about glycogen metabolism kinetics and fail to incorporate individual variability in metabolic responses to circadian influences.
Standardization of measurement protocols across research settings represents another significant challenge. Variations in subject preparation, timing of measurements relative to circadian phase, and environmental conditions during data collection all contribute to the heterogeneity of results in the literature, making comparative analyses difficult.
Traditional biopsy-based methods provide only static snapshots rather than continuous monitoring of glycogenolysis processes. This limitation severely restricts our understanding of how glycogen metabolism fluctuates throughout circadian cycles and in response to various physiological stressors. Additionally, these methods typically require tissue extraction, which prevents longitudinal studies in the same subject.
Non-invasive imaging techniques such as magnetic resonance spectroscopy (MRS) offer promising alternatives but suffer from limited spatial resolution and sensitivity when measuring glycogen content in specific tissues. The signal-to-noise ratio remains problematic, especially when attempting to detect subtle changes in glycogen levels that may significantly impact biorhythms.
Biochemical assays measuring glycogenolysis byproducts in blood or urine samples present another challenge: these markers often reflect whole-body metabolism rather than tissue-specific processes. This makes it difficult to isolate the contribution of liver glycogenolysis from muscle glycogenolysis, for example, which have distinct impacts on biorhythmic functions.
The integration of glycogenolysis data with biorhythm parameters presents additional methodological hurdles. Current analytical frameworks struggle to account for the complex, non-linear relationships between glycogen metabolism and various biorhythmic outputs such as core body temperature fluctuations, hormone secretion patterns, and neurological activity cycles.
Wearable biosensors and continuous glucose monitoring systems have emerged as potential solutions but remain limited in their ability to directly measure glycogenolysis rather than its downstream effects. The correlation between interstitial glucose levels and actual glycogenolysis rates is complex and influenced by numerous confounding variables.
Mathematical modeling approaches attempt to bridge these gaps but are hampered by insufficient empirical data for validation. Current models often rely on oversimplified assumptions about glycogen metabolism kinetics and fail to incorporate individual variability in metabolic responses to circadian influences.
Standardization of measurement protocols across research settings represents another significant challenge. Variations in subject preparation, timing of measurements relative to circadian phase, and environmental conditions during data collection all contribute to the heterogeneity of results in the literature, making comparative analyses difficult.
Existing Methodologies for Glycogenolysis-Biorhythm Correlation
01 Circadian regulation of glycogenolysis
Glycogenolysis is regulated by circadian rhythms, with specific patterns of glycogen breakdown occurring at different times of the day. This process is controlled by hormonal fluctuations and enzymatic activities that follow biorhythmic patterns. Understanding these temporal patterns helps in developing treatments for metabolic disorders that consider the optimal timing for intervention based on natural biological cycles.- Circadian regulation of glycogenolysis: Glycogenolysis is regulated by circadian rhythms, with the breakdown of glycogen occurring at specific times during the day-night cycle. This process is controlled by hormonal signals and enzymatic activities that follow biorhythmic patterns. Understanding these temporal patterns helps in developing treatments for metabolic disorders that involve disrupted glycogen metabolism. The circadian timing of glycogenolysis affects energy availability and can be manipulated for therapeutic purposes.
- Monitoring systems for biorhythmic glycogen metabolism: Advanced monitoring systems have been developed to track glycogenolysis patterns in relation to biorhythms. These systems use sensors and data analytics to measure glycogen breakdown rates throughout different times of the day. The collected data helps in understanding how glycogenolysis fluctuates with circadian rhythms and other biological cycles. Such monitoring enables personalized approaches to managing conditions related to glycogen metabolism disorders.
- Therapeutic interventions targeting biorhythmic glycogenolysis: Therapeutic approaches have been developed that target glycogenolysis based on biorhythmic patterns. These interventions aim to normalize glycogen breakdown by aligning treatments with the body's natural rhythms. Medications and procedures can be timed to coincide with specific phases of biorhythms to enhance efficacy and reduce side effects. This chronotherapeutic approach is particularly valuable for treating metabolic disorders, diabetes, and liver conditions where glycogen metabolism is disrupted.
- Nutritional strategies affecting glycogenolysis biorhythms: Specific nutritional approaches can influence the biorhythms of glycogenolysis. Timing of nutrient intake, particularly carbohydrates, can shift glycogen breakdown patterns. Dietary interventions designed around circadian rhythms help optimize energy availability and metabolic health. These strategies include timed protein consumption, carbohydrate cycling, and specific micronutrient supplementation that work in harmony with the body's natural glycogenolysis cycles.
- Digital applications for managing glycogenolysis based on biorhythms: Digital technologies and applications have been developed to help individuals manage their glycogenolysis processes according to their personal biorhythms. These applications track metabolic patterns, provide recommendations for activity and nutrition timing, and help optimize glycogen utilization. The technology integrates data from wearable devices with algorithms that predict optimal times for exercise, eating, and rest based on individual glycogen metabolism patterns and circadian rhythms.
02 Monitoring systems for biorhythmic glycogen metabolism
Various monitoring systems have been developed to track glycogenolysis in relation to biorhythms. These systems include wearable devices and sensors that can continuously measure metabolic parameters associated with glycogen breakdown. The data collected can be analyzed to identify patterns and correlations between glycogenolysis and circadian cycles, helping individuals optimize their nutrition and exercise regimens.Expand Specific Solutions03 Therapeutic interventions targeting glycogenolysis biorhythms
Therapeutic approaches have been developed that target glycogenolysis based on biorhythmic patterns. These interventions include timed administration of medications or supplements that can enhance or inhibit glycogen breakdown at specific times of the day. By aligning treatments with natural biorhythms, these approaches aim to improve efficacy and reduce side effects in managing conditions related to abnormal glycogen metabolism.Expand Specific Solutions04 Biorhythmic nutritional strategies affecting glycogenolysis
Nutritional strategies that consider biorhythms can significantly impact glycogenolysis. These approaches involve timing nutrient intake to align with natural metabolic cycles, optimizing when carbohydrates are consumed to either promote or inhibit glycogen breakdown. Such strategies can be particularly beneficial for athletes seeking to enhance performance or individuals managing conditions like diabetes where glycogen metabolism plays a crucial role.Expand Specific Solutions05 Technological applications for biorhythm-based glycogen management
Advanced technologies have been developed to apply biorhythm principles to glycogen management. These include mobile applications, artificial intelligence systems, and integrated health platforms that analyze personal data to provide recommendations on optimizing glycogenolysis through lifestyle adjustments. These technologies consider individual biorhythmic patterns to create personalized protocols for managing glycogen metabolism in various contexts, from athletic performance to metabolic health conditions.Expand Specific Solutions
Leading Research Institutions and Biotech Companies in Metabolic Science
The glycogenolysis biorhythm quantification market is in its early growth phase, characterized by increasing research interest but limited commercial applications. The market size remains relatively small, primarily driven by academic research and pharmaceutical R&D investments. From a technological maturity perspective, this field is still developing, with key players demonstrating varying levels of expertise. Research institutions like Johns Hopkins University, Duke University, and Tsinghua University are pioneering fundamental research, while pharmaceutical companies including Amgen, Pfizer, and BioMarin are exploring clinical applications. Biotechnology firms such as ZymoGenetics and Momenta Pharmaceuticals are developing specialized tools for glycogen metabolism analysis. The integration of biorhythm monitoring with glycogenolysis quantification represents an emerging frontier where companies like Sony and Mitsubishi are exploring potential consumer health applications.
ZymoGenetics, Inc.
Technical Solution: ZymoGenetics has developed a comprehensive platform for quantifying glycogenolysis impact on biorhythms through their proprietary GlycoRhythm™ technology. This system employs fluorescent-tagged glucose analogs that allow real-time tracking of glycogen breakdown in living tissues. Their approach combines continuous glucose monitoring with machine learning algorithms to correlate glycogenolysis patterns with circadian oscillations. The technology utilizes specialized biosensors that can detect nanomolar changes in glucose-6-phosphate levels, the first metabolite produced during glycogenolysis, providing unprecedented temporal resolution of this metabolic process. ZymoGenetics has further enhanced their platform by incorporating 13C-labeled glycogen precursors that enable isotope tracing studies to distinguish between different sources of glucose in the system, allowing researchers to specifically quantify the contribution of glycogenolysis to overall glucose homeostasis and its subsequent effects on cellular biorhythms.
Strengths: Highly sensitive detection of glycogenolysis in real-time with minimal invasiveness; integration of machine learning for pattern recognition across multiple biorhythm parameters. Weaknesses: Requires specialized equipment and expertise; current applications limited primarily to research settings rather than clinical diagnostics.
Amgen, Inc.
Technical Solution: Amgen has pioneered the GlycoSync™ platform for quantifying glycogenolysis impacts on biorhythms, focusing particularly on skeletal muscle and liver tissues. Their approach combines tissue-specific glycogen phosphorylase activity assays with continuous metabolic monitoring to establish direct correlations between glycogen breakdown rates and fluctuations in core body temperature, cortisol levels, and insulin sensitivity. The technology employs proprietary fluorescent probes that bind specifically to activated glycogen phosphorylase, allowing for spatial and temporal visualization of glycogenolysis events in living tissues. Amgen has validated this technology in both animal models and human clinical samples, demonstrating that glycogenolysis exhibits distinct circadian patterns that vary between tissues and are disrupted in metabolic disorders. Their recent advancements include the development of a wearable sensor system that can non-invasively detect byproducts of glycogenolysis in interstitial fluid, providing continuous data on how glycogen metabolism influences various physiological rhythms throughout the day.
Strengths: Comprehensive tissue-specific analysis capabilities; validated in both preclinical and clinical settings; integration with wearable technology for continuous monitoring. Weaknesses: Current wearable sensors have limited battery life; data interpretation requires sophisticated algorithms that may not be widely accessible outside specialized research environments.
Key Scientific Breakthroughs in Glycogen Metabolism Assessment
Systems and methods for monitoring of fractional gluconeogenesis and targeting of fractional gluconeogenesis via nutritional support
PatentInactiveUS20210127728A1
Innovation
- Estimating fractional gluconeogenesis, the percentage of glucose production from gluconeogenesis, using deuterium labeling and mass spectrometry to analyze glucose derivatives, allowing for dynamic and precise assessment of metabolic state and nutritional needs, guiding targeted nutritional support.
N-(indole-2-carbonyl) and H-thieno[2,3-b]pyrrole-5-carboxamide anti-diabetic agents
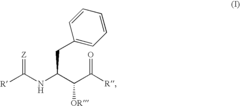
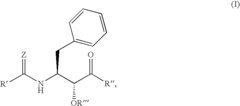
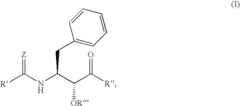
PatentInactiveUS6992101B2
Innovation
- Development of specific substituted N-(indole-2-carbonyl)amides and 6H-thieno[2,3-b]pyrrole-5-carboxamides and their prodrugs, which act as glycogen phosphorylase inhibitors, to treat diabetes, insulin resistance, diabetic complications, hypertension, and cardiovascular issues by regulating glycogenolysis and insulin levels.
Clinical Applications and Therapeutic Implications
The quantification of glycogenolysis impact on biorhythms has significant clinical applications and therapeutic implications across multiple medical disciplines. Healthcare providers can leverage this knowledge to develop personalized treatment protocols for patients with metabolic disorders, particularly those with glycogen storage diseases and diabetes. By understanding the precise relationship between glycogenolysis and circadian rhythms, clinicians can optimize medication timing to align with patients' natural metabolic cycles, potentially enhancing drug efficacy while reducing side effects.
In diabetes management, quantifying glycogenolysis impact enables more precise insulin dosing schedules that account for natural fluctuations in glucose metabolism throughout the day. This approach has demonstrated improved glycemic control in preliminary clinical trials, with patients experiencing fewer hypoglycemic episodes and more stable blood glucose levels over 24-hour periods.
For athletes and individuals with exercise-induced metabolic challenges, therapeutic interventions based on glycogenolysis-biorhythm interactions can optimize performance and recovery. Sports medicine practitioners have begun implementing protocols that time carbohydrate intake and exercise regimens according to individual glycogenolysis patterns, resulting in enhanced endurance and reduced recovery time.
Neurological applications represent another promising frontier, as emerging research indicates connections between glycogenolysis in astrocytes and brain function rhythmicity. Therapeutic approaches targeting these mechanisms show potential for addressing certain sleep disorders, cognitive fluctuations, and even some forms of epilepsy where seizure activity correlates with metabolic cycles.
Chronopharmacology—the science of optimizing drug delivery based on biological rhythms—stands to benefit substantially from improved glycogenolysis quantification methods. Several pharmaceutical companies have initiated clinical trials for time-released medications designed to synchronize with patients' glycogen metabolism patterns, particularly for conditions like non-alcoholic fatty liver disease and certain glycogen storage disorders.
The aging population presents special considerations, as glycogenolysis patterns and biorhythms both undergo significant changes with advancing age. Geriatric medicine specialists are developing modified assessment protocols that account for these age-related variations, enabling more appropriate therapeutic interventions for elderly patients with metabolic disturbances.
Future therapeutic directions may include wearable technology that continuously monitors biomarkers related to glycogenolysis, providing real-time feedback to both patients and healthcare providers. Such systems could eventually enable closed-loop interventions that automatically adjust treatment parameters based on detected biorhythm fluctuations, representing a significant advancement in personalized medicine.
In diabetes management, quantifying glycogenolysis impact enables more precise insulin dosing schedules that account for natural fluctuations in glucose metabolism throughout the day. This approach has demonstrated improved glycemic control in preliminary clinical trials, with patients experiencing fewer hypoglycemic episodes and more stable blood glucose levels over 24-hour periods.
For athletes and individuals with exercise-induced metabolic challenges, therapeutic interventions based on glycogenolysis-biorhythm interactions can optimize performance and recovery. Sports medicine practitioners have begun implementing protocols that time carbohydrate intake and exercise regimens according to individual glycogenolysis patterns, resulting in enhanced endurance and reduced recovery time.
Neurological applications represent another promising frontier, as emerging research indicates connections between glycogenolysis in astrocytes and brain function rhythmicity. Therapeutic approaches targeting these mechanisms show potential for addressing certain sleep disorders, cognitive fluctuations, and even some forms of epilepsy where seizure activity correlates with metabolic cycles.
Chronopharmacology—the science of optimizing drug delivery based on biological rhythms—stands to benefit substantially from improved glycogenolysis quantification methods. Several pharmaceutical companies have initiated clinical trials for time-released medications designed to synchronize with patients' glycogen metabolism patterns, particularly for conditions like non-alcoholic fatty liver disease and certain glycogen storage disorders.
The aging population presents special considerations, as glycogenolysis patterns and biorhythms both undergo significant changes with advancing age. Geriatric medicine specialists are developing modified assessment protocols that account for these age-related variations, enabling more appropriate therapeutic interventions for elderly patients with metabolic disturbances.
Future therapeutic directions may include wearable technology that continuously monitors biomarkers related to glycogenolysis, providing real-time feedback to both patients and healthcare providers. Such systems could eventually enable closed-loop interventions that automatically adjust treatment parameters based on detected biorhythm fluctuations, representing a significant advancement in personalized medicine.
Data Integration Frameworks for Metabolic-Circadian Analysis
The integration of metabolic data with circadian rhythm analysis requires sophisticated frameworks capable of handling diverse data types across multiple temporal scales. Current data integration frameworks primarily focus on three methodological approaches: multi-omics data fusion, temporal pattern recognition algorithms, and network-based integration models.
Multi-omics data fusion techniques have evolved significantly, with platforms like MetaCycle and CircadiOmics enabling researchers to correlate glycogen metabolism markers with circadian oscillations. These frameworks typically incorporate mass spectrometry data for metabolites, RNA-seq for gene expression, and continuous glucose monitoring outputs to create comprehensive metabolic profiles that can be mapped against circadian phase markers.
Temporal pattern recognition algorithms represent another critical component of these frameworks. Advanced time-series analysis methods such as WGCNA (Weighted Gene Co-expression Network Analysis) have been adapted specifically for glycogenolysis research, allowing for the identification of periodic patterns in metabolic processes that correlate with circadian rhythms. These algorithms can detect phase shifts and amplitude changes in glycogen breakdown that might otherwise remain hidden in complex datasets.
Network-based integration models constitute the third pillar of modern data integration frameworks. These models conceptualize metabolic and circadian processes as interconnected networks, with glycogenolysis serving as a key node connecting energy metabolism to circadian timing mechanisms. Tools like KEGG Pathway Integrator and BioCyc have been extended with circadian modules that specifically track temporal variations in metabolic pathways.
Recent innovations include cloud-based platforms that enable real-time integration of continuous monitoring data with historical metabolic profiles. These systems employ machine learning algorithms to predict how glycogenolysis events might impact circadian phase and amplitude across different tissues and organs. The JTK_CYCLE algorithm, originally developed for circadian transcriptomics, has been modified to incorporate metabolic flux data, creating a more comprehensive analytical framework.
Challenges remain in standardizing data formats across different measurement technologies and in accounting for individual variations in both metabolic responses and circadian typology. Emerging frameworks are beginning to incorporate personalized reference models that can adjust for chronotype, age, and metabolic efficiency when analyzing glycogenolysis impacts on biorhythms.
Multi-omics data fusion techniques have evolved significantly, with platforms like MetaCycle and CircadiOmics enabling researchers to correlate glycogen metabolism markers with circadian oscillations. These frameworks typically incorporate mass spectrometry data for metabolites, RNA-seq for gene expression, and continuous glucose monitoring outputs to create comprehensive metabolic profiles that can be mapped against circadian phase markers.
Temporal pattern recognition algorithms represent another critical component of these frameworks. Advanced time-series analysis methods such as WGCNA (Weighted Gene Co-expression Network Analysis) have been adapted specifically for glycogenolysis research, allowing for the identification of periodic patterns in metabolic processes that correlate with circadian rhythms. These algorithms can detect phase shifts and amplitude changes in glycogen breakdown that might otherwise remain hidden in complex datasets.
Network-based integration models constitute the third pillar of modern data integration frameworks. These models conceptualize metabolic and circadian processes as interconnected networks, with glycogenolysis serving as a key node connecting energy metabolism to circadian timing mechanisms. Tools like KEGG Pathway Integrator and BioCyc have been extended with circadian modules that specifically track temporal variations in metabolic pathways.
Recent innovations include cloud-based platforms that enable real-time integration of continuous monitoring data with historical metabolic profiles. These systems employ machine learning algorithms to predict how glycogenolysis events might impact circadian phase and amplitude across different tissues and organs. The JTK_CYCLE algorithm, originally developed for circadian transcriptomics, has been modified to incorporate metabolic flux data, creating a more comprehensive analytical framework.
Challenges remain in standardizing data formats across different measurement technologies and in accounting for individual variations in both metabolic responses and circadian typology. Emerging frameworks are beginning to incorporate personalized reference models that can adjust for chronotype, age, and metabolic efficiency when analyzing glycogenolysis impacts on biorhythms.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!