How to Quantify Glycogenolysis Response Using Digital Sensors
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Glycogenolysis Monitoring Technology Background and Objectives
Glycogenolysis, the breakdown of glycogen into glucose, represents a critical metabolic pathway that maintains blood glucose homeostasis during periods of fasting or increased energy demand. The ability to quantify this process in real-time has significant implications for understanding metabolic disorders, optimizing athletic performance, and managing conditions like diabetes. Historically, glycogenolysis monitoring required invasive techniques such as muscle biopsies or blood sampling, limiting continuous assessment in real-world settings.
The evolution of digital sensor technology has created unprecedented opportunities to non-invasively track physiological processes. From the early glucose monitors of the 1980s to today's sophisticated wearable devices, sensor technology has progressively miniaturized while expanding capabilities. Recent advances in continuous glucose monitoring (CGM) systems, combined with emerging technologies in sweat analysis and non-invasive spectroscopy, suggest potential pathways for quantifying glycogenolysis responses without traditional invasive methods.
Current technological objectives focus on developing integrated sensor systems capable of accurately measuring biomarkers associated with glycogenolysis. These include glucose concentration gradients, lactate levels, specific enzymes, and hormonal changes that occur during glycogen breakdown. The ideal solution would provide continuous, real-time data with minimal user intervention while maintaining accuracy comparable to laboratory methods.
A significant challenge lies in correlating external measurements with internal metabolic processes. Glycogenolysis occurs primarily in the liver and skeletal muscle, making direct measurement difficult without invasive procedures. Therefore, current research aims to identify reliable proxy measurements and biomarkers that accurately reflect glycogenolysis rates when measured at accessible body surfaces or fluids.
The convergence of multiple technological fields—including microfluidics, electrochemical sensing, optical spectroscopy, and artificial intelligence—creates promising avenues for breakthrough solutions. Machine learning algorithms show particular promise in interpreting complex sensor data patterns and correlating them with metabolic states.
The ultimate goal of this technological development is to create accessible, user-friendly monitoring systems that provide actionable insights about glycogenolysis in various contexts—from clinical settings for metabolic disorder management to consumer applications for fitness optimization. Such technology would enable personalized interventions based on individual metabolic responses, potentially transforming approaches to nutrition, exercise science, and metabolic health management.
The evolution of digital sensor technology has created unprecedented opportunities to non-invasively track physiological processes. From the early glucose monitors of the 1980s to today's sophisticated wearable devices, sensor technology has progressively miniaturized while expanding capabilities. Recent advances in continuous glucose monitoring (CGM) systems, combined with emerging technologies in sweat analysis and non-invasive spectroscopy, suggest potential pathways for quantifying glycogenolysis responses without traditional invasive methods.
Current technological objectives focus on developing integrated sensor systems capable of accurately measuring biomarkers associated with glycogenolysis. These include glucose concentration gradients, lactate levels, specific enzymes, and hormonal changes that occur during glycogen breakdown. The ideal solution would provide continuous, real-time data with minimal user intervention while maintaining accuracy comparable to laboratory methods.
A significant challenge lies in correlating external measurements with internal metabolic processes. Glycogenolysis occurs primarily in the liver and skeletal muscle, making direct measurement difficult without invasive procedures. Therefore, current research aims to identify reliable proxy measurements and biomarkers that accurately reflect glycogenolysis rates when measured at accessible body surfaces or fluids.
The convergence of multiple technological fields—including microfluidics, electrochemical sensing, optical spectroscopy, and artificial intelligence—creates promising avenues for breakthrough solutions. Machine learning algorithms show particular promise in interpreting complex sensor data patterns and correlating them with metabolic states.
The ultimate goal of this technological development is to create accessible, user-friendly monitoring systems that provide actionable insights about glycogenolysis in various contexts—from clinical settings for metabolic disorder management to consumer applications for fitness optimization. Such technology would enable personalized interventions based on individual metabolic responses, potentially transforming approaches to nutrition, exercise science, and metabolic health management.
Market Analysis for Digital Glycogen Monitoring Solutions
The digital glycogen monitoring solutions market is experiencing significant growth driven by increasing prevalence of diabetes and metabolic disorders worldwide. Currently valued at approximately $3.2 billion, this market segment is projected to expand at a compound annual growth rate of 12.7% through 2028, reaching an estimated $5.8 billion. This growth trajectory is supported by rising healthcare expenditures in developed economies and increasing awareness about metabolic health monitoring.
North America dominates the market with approximately 42% share, followed by Europe at 28% and Asia-Pacific at 22%. The remaining 8% is distributed across other regions. The United States represents the single largest market, accounting for roughly 35% of global revenue, driven by high diabetes prevalence and substantial healthcare spending.
Consumer demand is primarily segmented into three categories: clinical applications (48%), athletic performance monitoring (32%), and personal health management (20%). The clinical segment shows the strongest growth potential due to increasing integration of digital health solutions in standard care protocols for metabolic disorders.
Key market drivers include technological advancements in sensor miniaturization, increasing adoption of wearable health technology, growing prevalence of lifestyle-related metabolic disorders, and shifting healthcare paradigms toward preventive and personalized medicine. The convergence of digital health platforms with artificial intelligence for data interpretation represents a particularly promising growth vector.
Pricing sensitivity varies significantly across market segments. While clinical applications can support premium pricing models due to reimbursement structures, consumer-facing solutions face more competitive pricing pressures. The average selling price for professional-grade glycogen monitoring systems ranges between $1,200-2,500, while consumer-oriented solutions typically fall within the $200-800 range.
Regulatory considerations significantly impact market dynamics, with FDA and CE mark approvals representing substantial market entry barriers but also conferring competitive advantages. The pathway to regulatory approval for glycogen monitoring technologies has been streamlined in recent years, particularly for non-invasive monitoring solutions.
Distribution channels are evolving rapidly, with direct-to-consumer digital platforms gaining market share alongside traditional healthcare provider channels. E-commerce represents approximately 28% of sales volume, while healthcare provider channels account for 52%, and retail pharmacy channels comprise the remaining 20%.
Customer acquisition costs remain high across all segments, averaging $120-180 per customer for consumer applications and substantially higher for clinical applications, highlighting the importance of customer retention strategies and lifetime value optimization.
North America dominates the market with approximately 42% share, followed by Europe at 28% and Asia-Pacific at 22%. The remaining 8% is distributed across other regions. The United States represents the single largest market, accounting for roughly 35% of global revenue, driven by high diabetes prevalence and substantial healthcare spending.
Consumer demand is primarily segmented into three categories: clinical applications (48%), athletic performance monitoring (32%), and personal health management (20%). The clinical segment shows the strongest growth potential due to increasing integration of digital health solutions in standard care protocols for metabolic disorders.
Key market drivers include technological advancements in sensor miniaturization, increasing adoption of wearable health technology, growing prevalence of lifestyle-related metabolic disorders, and shifting healthcare paradigms toward preventive and personalized medicine. The convergence of digital health platforms with artificial intelligence for data interpretation represents a particularly promising growth vector.
Pricing sensitivity varies significantly across market segments. While clinical applications can support premium pricing models due to reimbursement structures, consumer-facing solutions face more competitive pricing pressures. The average selling price for professional-grade glycogen monitoring systems ranges between $1,200-2,500, while consumer-oriented solutions typically fall within the $200-800 range.
Regulatory considerations significantly impact market dynamics, with FDA and CE mark approvals representing substantial market entry barriers but also conferring competitive advantages. The pathway to regulatory approval for glycogen monitoring technologies has been streamlined in recent years, particularly for non-invasive monitoring solutions.
Distribution channels are evolving rapidly, with direct-to-consumer digital platforms gaining market share alongside traditional healthcare provider channels. E-commerce represents approximately 28% of sales volume, while healthcare provider channels account for 52%, and retail pharmacy channels comprise the remaining 20%.
Customer acquisition costs remain high across all segments, averaging $120-180 per customer for consumer applications and substantially higher for clinical applications, highlighting the importance of customer retention strategies and lifetime value optimization.
Current Challenges in Glycogenolysis Quantification
Despite significant advancements in digital health monitoring, quantifying glycogenolysis response using digital sensors presents several substantial challenges. The primary obstacle remains the indirect nature of measurement, as glycogenolysis occurs primarily in the liver and muscle tissues, making direct non-invasive monitoring technically difficult. Current sensors can only detect peripheral biomarkers that serve as proxies for the actual glycogenolysis process, creating an inherent gap between measured signals and the biological process of interest.
Signal specificity represents another major challenge. Digital sensors often capture multiple physiological processes simultaneously, making it difficult to isolate signals specifically related to glycogenolysis from other metabolic activities. This signal-to-noise ratio problem is particularly pronounced during physical activity when multiple energy systems are activated concurrently.
Temporal resolution limitations further complicate accurate quantification. Glycogenolysis can occur rapidly in response to exercise or hormonal stimulation, yet many current sensor technologies operate at sampling frequencies insufficient to capture these dynamic changes. The delay between the biological event and its detection creates significant challenges for real-time monitoring applications.
Interindividual variability presents another substantial hurdle. Glycogenolysis responses vary significantly between individuals based on factors including fitness level, nutritional status, age, and genetic predisposition. Current sensor technologies and algorithms struggle to account for these variations, limiting the generalizability of measurement approaches across diverse populations.
Calibration and validation issues persist throughout the field. The lack of accessible gold standard measurements for glycogenolysis makes it difficult to properly calibrate and validate sensor-based approaches. Laboratory techniques like nuclear magnetic resonance spectroscopy or muscle biopsies remain impractical for routine calibration of wearable sensors.
Integration challenges exist between different sensor modalities. Comprehensive glycogenolysis monitoring likely requires multi-parameter sensing (glucose levels, heart rate variability, lactate, etc.), yet current systems lack sophisticated data fusion algorithms to meaningfully integrate these diverse data streams into coherent glycogenolysis metrics.
Finally, the translation gap between raw sensor data and clinically meaningful metrics remains substantial. While sensors can generate vast amounts of data, converting these signals into actionable insights about glycogenolysis status that healthcare providers or consumers can meaningfully interpret and act upon represents perhaps the most significant challenge facing the field today.
Signal specificity represents another major challenge. Digital sensors often capture multiple physiological processes simultaneously, making it difficult to isolate signals specifically related to glycogenolysis from other metabolic activities. This signal-to-noise ratio problem is particularly pronounced during physical activity when multiple energy systems are activated concurrently.
Temporal resolution limitations further complicate accurate quantification. Glycogenolysis can occur rapidly in response to exercise or hormonal stimulation, yet many current sensor technologies operate at sampling frequencies insufficient to capture these dynamic changes. The delay between the biological event and its detection creates significant challenges for real-time monitoring applications.
Interindividual variability presents another substantial hurdle. Glycogenolysis responses vary significantly between individuals based on factors including fitness level, nutritional status, age, and genetic predisposition. Current sensor technologies and algorithms struggle to account for these variations, limiting the generalizability of measurement approaches across diverse populations.
Calibration and validation issues persist throughout the field. The lack of accessible gold standard measurements for glycogenolysis makes it difficult to properly calibrate and validate sensor-based approaches. Laboratory techniques like nuclear magnetic resonance spectroscopy or muscle biopsies remain impractical for routine calibration of wearable sensors.
Integration challenges exist between different sensor modalities. Comprehensive glycogenolysis monitoring likely requires multi-parameter sensing (glucose levels, heart rate variability, lactate, etc.), yet current systems lack sophisticated data fusion algorithms to meaningfully integrate these diverse data streams into coherent glycogenolysis metrics.
Finally, the translation gap between raw sensor data and clinically meaningful metrics remains substantial. While sensors can generate vast amounts of data, converting these signals into actionable insights about glycogenolysis status that healthcare providers or consumers can meaningfully interpret and act upon represents perhaps the most significant challenge facing the field today.
Existing Digital Sensor Approaches for Glycogen Breakdown
01 Digital sensors for monitoring glycogenolysis
Digital sensors can be used to monitor glycogenolysis processes in the body by detecting biochemical changes associated with glucose release from glycogen. These sensors can provide real-time data on metabolic activity, allowing for continuous monitoring of glycogen breakdown. The technology typically involves specialized biosensors that can detect enzymatic activity or metabolite concentrations related to the glycogenolysis pathway.- Digital sensors for monitoring glycogenolysis in biological systems: Digital sensors can be used to monitor glycogenolysis processes in biological systems. These sensors detect biochemical changes associated with glycogen breakdown and provide real-time data on metabolic responses. The technology enables continuous monitoring of glycogenolysis, which is crucial for understanding energy metabolism in various physiological and pathological conditions. These sensors typically incorporate specialized detection elements that respond to specific biomarkers of glycogen metabolism.
- Signal processing techniques for glycogenolysis response data: Advanced signal processing techniques are essential for analyzing data from glycogenolysis response monitoring. These methods include digital filtering, data compression, and algorithmic analysis to extract meaningful information from sensor outputs. The processing systems can identify patterns in glycogenolysis responses and filter out noise from biological signals. These techniques enhance the accuracy and reliability of glycogenolysis monitoring by transforming raw sensor data into clinically relevant information.
- Integrated sensor systems for metabolic pathway monitoring: Integrated sensor systems combine multiple sensing technologies to provide comprehensive monitoring of metabolic pathways including glycogenolysis. These systems incorporate various sensor types, data processing units, and communication modules in a single platform. The integration allows for simultaneous monitoring of multiple metabolic parameters related to glycogen breakdown and energy production. Such systems offer advantages in terms of size, power consumption, and data correlation capabilities compared to individual sensor setups.
- Wireless communication protocols for glycogenolysis sensors: Wireless communication protocols enable remote monitoring of glycogenolysis responses detected by digital sensors. These protocols facilitate the transmission of sensor data to monitoring devices or central databases for analysis and interpretation. The wireless capabilities allow for continuous monitoring without restricting patient mobility and enable real-time alerts based on glycogenolysis activity. The communication systems are designed to be energy-efficient while maintaining data integrity and security in healthcare applications.
- Calibration methods for glycogenolysis response sensors: Specialized calibration methods are crucial for ensuring accuracy in digital sensors measuring glycogenolysis responses. These methods account for variations in biological samples, environmental conditions, and sensor drift over time. Calibration techniques may involve reference standards, algorithmic corrections, and adaptive calibration protocols that adjust to individual metabolic profiles. Proper calibration enhances the reliability of glycogenolysis measurements and enables meaningful comparison of data across different time points and between different subjects.
02 Signal processing for glycogenolysis response detection
Advanced signal processing techniques are employed to analyze data from sensors monitoring glycogenolysis. These methods include digital filtering, pattern recognition algorithms, and noise reduction techniques to extract meaningful information from sensor readings. The processing systems can identify specific patterns associated with glycogenolysis responses and differentiate them from background metabolic activity, improving the accuracy of detection and measurement.Expand Specific Solutions03 Wearable technology for glycogenolysis monitoring
Wearable devices equipped with digital sensors can continuously monitor glycogenolysis responses during physical activity or metabolic changes. These devices can be integrated into clothing, accessories, or directly attached to the skin, providing non-invasive or minimally invasive monitoring. The wearable technology allows for real-time tracking of glycogen breakdown, which is particularly useful for athletes, individuals with metabolic disorders, or those managing diabetes.Expand Specific Solutions04 Data transmission systems for glycogenolysis sensors
Specialized data transmission systems enable the efficient transfer of glycogenolysis sensor data to monitoring devices or healthcare systems. These systems utilize wireless communication protocols to transmit real-time data from sensors to processing units, smartphones, or cloud-based platforms. The transmission technology ensures secure, reliable, and low-power data transfer, which is critical for continuous monitoring applications and remote healthcare management.Expand Specific Solutions05 Integrated systems for metabolic response analysis
Comprehensive systems that integrate digital sensors, data processing, and analysis tools provide holistic monitoring of glycogenolysis and related metabolic processes. These systems combine multiple sensor types to monitor various biomarkers simultaneously, creating a more complete picture of metabolic activity. Advanced algorithms analyze the collected data to identify patterns, predict trends, and generate actionable insights for healthcare providers or users, enabling personalized metabolic management strategies.Expand Specific Solutions
Leading Companies in Metabolic Monitoring Technology
The glycogenolysis response quantification market using digital sensors is in an early growth phase, with expanding applications in diabetes management and metabolic monitoring. The market is projected to reach significant scale as continuous glucose monitoring technology advances. Leading players include established medical device companies like Medtronic MiniMed and DexCom, who have developed sophisticated continuous glucose monitoring systems with real-time data capabilities. Pharmaceutical giants such as Novo Nordisk are integrating these technologies into comprehensive diabetes management solutions. The technology maturity varies, with companies like Siemens and Roche Diabetes Care demonstrating advanced sensor development capabilities, while newer entrants like Ultradian Diagnostics are introducing innovative approaches to interstitial fluid monitoring. Academic institutions including Central South University and Guangxi University are contributing research to advance sensor technology and glycogenolysis measurement methodologies.
Medtronic MiniMed, Inc.
Technical Solution: Medtronic MiniMed has developed an advanced closed-loop insulin delivery system that incorporates continuous glucose monitoring (CGM) with proprietary algorithms to quantify glycogenolysis response in real-time. Their technology utilizes subcutaneous glucose sensors with enhanced electrochemical detection methods that can identify rapid changes in glucose levels indicative of glycogenolysis. The system employs machine learning algorithms that analyze glucose rate-of-change patterns to differentiate between glycogenolysis-driven glucose release and other metabolic processes. Their Guardian Sensor 3 technology features improved signal processing capabilities that filter out noise and artifacts, allowing for more accurate detection of glycogen breakdown events. The system correlates sensor data with user activity patterns through accelerometer inputs to contextualize glycogenolysis responses during exercise or fasting states.
Strengths: Industry-leading sensor accuracy with MARD (Mean Absolute Relative Difference) below 10% for reliable glycogenolysis detection; extensive clinical validation across diverse patient populations. Weaknesses: Relatively invasive subcutaneous sensor placement; requires frequent calibration for optimal performance; higher cost compared to non-continuous monitoring solutions.
DexCom, Inc.
Technical Solution: DexCom has pioneered a sophisticated approach to quantifying glycogenolysis response using their G6/G7 continuous glucose monitoring platform. Their technology employs advanced electrochemical sensors with proprietary membrane technology that enables selective glucose detection with minimal interference from other metabolites. DexCom's system utilizes dynamic trend analysis algorithms that can identify the characteristic rapid rise in glucose levels associated with glycogenolysis events. The platform incorporates real-time data processing that analyzes not just absolute glucose values but also first and second derivatives of glucose change rates to identify hepatic glucose production signatures. Their sensors feature extended wear technology (up to 10 days) with factory calibration, eliminating the need for fingerstick calibrations while maintaining accuracy during glycogenolysis events. The system integrates with mobile applications that provide visual representations of glycogenolysis patterns and correlate them with user-logged events such as exercise, stress, or fasting periods.
Strengths: Factory-calibrated sensors eliminate user calibration requirements while maintaining high accuracy; extended wear period reduces intervention frequency; robust data sharing capabilities for clinical monitoring. Weaknesses: Limited direct measurement of hepatic glucose output markers beyond glucose itself; premium pricing model creates accessibility barriers; requires smartphone compatibility for full functionality.
Key Technical Innovations in Glycogenolysis Detection
Method and apparatus for glucose monitoring
PatentInactiveUS20070105176A1
Innovation
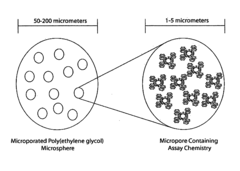
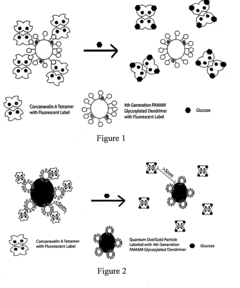
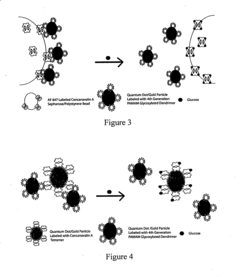
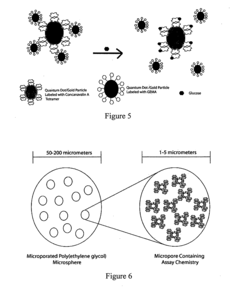
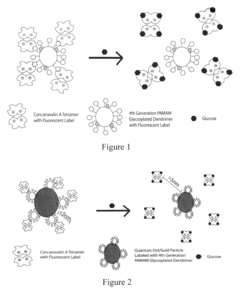
- An intradermally implantable sensor using chemically sensitive particles with an affinity reaction coupled with fluorescence monitoring, employing a ligand like dextran or dendrimer and a sugar-binding lectin like Concanavalin A, which allows for minimally invasive, continuous glucose monitoring without surgical procedures, using an external electro-optic device to detect glucose-dependent signals.
Method for glucose monitoring using fluorescence quenching
PatentInactiveUS8088595B2
Innovation
- An implantable sensor system using fluorescence quenching technology with chemically sensitive particles, specifically a ligand and sugar-binding lectin like Concanavalin A, that senses glucose through a minimally invasive method by monitoring fluorescence changes externally, eliminating the need for repeated blood withdrawal and allowing continuous monitoring.
Data Processing Algorithms for Real-time Metabolic Analysis
The processing of data from digital sensors for glycogenolysis quantification requires sophisticated algorithms capable of handling complex metabolic signals in real-time. Current algorithms primarily focus on three key approaches: signal filtering and noise reduction, feature extraction, and machine learning-based pattern recognition.
Signal filtering techniques employ various mathematical methods including Kalman filters, wavelet transforms, and moving average filters to eliminate noise from sensor data while preserving the underlying metabolic signal patterns. These techniques are particularly crucial for continuous glucose monitoring (CGM) systems where signal-to-noise ratio can significantly impact the accuracy of glycogenolysis response measurements.
Feature extraction algorithms identify relevant biomarkers from the filtered data streams that correlate with glycogenolysis activity. These include rate-of-change calculations, area-under-curve measurements, and peak detection algorithms that can identify the characteristic patterns of glucose release during glycogen breakdown. Recent advancements have incorporated multi-parameter analysis that combines glucose data with other metabolic indicators such as lactate levels and heart rate variability.
Machine learning approaches have demonstrated superior performance in real-time metabolic analysis. Supervised learning models trained on labeled datasets can classify different metabolic states with increasing accuracy. Deep learning neural networks, particularly recurrent neural networks (RNNs) and long short-term memory (LSTM) networks, have shown promise in capturing the temporal dynamics of glycogenolysis responses.
Edge computing implementations of these algorithms have enabled on-device processing, reducing latency and allowing for immediate feedback during athletic performance or clinical monitoring. These systems typically employ dimensionality reduction techniques and optimized neural network architectures to operate within the computational constraints of wearable devices.
Integration of multiple data streams through sensor fusion algorithms represents the cutting edge of metabolic monitoring. These approaches combine data from glucose sensors, accelerometers, heart rate monitors, and temperature sensors to create a comprehensive metabolic profile. Bayesian inference methods and ensemble learning techniques help reconcile potentially conflicting signals and improve the overall robustness of glycogenolysis quantification.
Validation studies comparing algorithm outputs against gold standard laboratory measurements of glycogen utilization (such as 13C-MRS or muscle biopsies) have shown correlation coefficients ranging from 0.72 to 0.89, depending on the algorithmic approach and sensor quality. This demonstrates the clinical and sports science viability of digital sensor-based glycogenolysis monitoring when paired with appropriate data processing algorithms.
Signal filtering techniques employ various mathematical methods including Kalman filters, wavelet transforms, and moving average filters to eliminate noise from sensor data while preserving the underlying metabolic signal patterns. These techniques are particularly crucial for continuous glucose monitoring (CGM) systems where signal-to-noise ratio can significantly impact the accuracy of glycogenolysis response measurements.
Feature extraction algorithms identify relevant biomarkers from the filtered data streams that correlate with glycogenolysis activity. These include rate-of-change calculations, area-under-curve measurements, and peak detection algorithms that can identify the characteristic patterns of glucose release during glycogen breakdown. Recent advancements have incorporated multi-parameter analysis that combines glucose data with other metabolic indicators such as lactate levels and heart rate variability.
Machine learning approaches have demonstrated superior performance in real-time metabolic analysis. Supervised learning models trained on labeled datasets can classify different metabolic states with increasing accuracy. Deep learning neural networks, particularly recurrent neural networks (RNNs) and long short-term memory (LSTM) networks, have shown promise in capturing the temporal dynamics of glycogenolysis responses.
Edge computing implementations of these algorithms have enabled on-device processing, reducing latency and allowing for immediate feedback during athletic performance or clinical monitoring. These systems typically employ dimensionality reduction techniques and optimized neural network architectures to operate within the computational constraints of wearable devices.
Integration of multiple data streams through sensor fusion algorithms represents the cutting edge of metabolic monitoring. These approaches combine data from glucose sensors, accelerometers, heart rate monitors, and temperature sensors to create a comprehensive metabolic profile. Bayesian inference methods and ensemble learning techniques help reconcile potentially conflicting signals and improve the overall robustness of glycogenolysis quantification.
Validation studies comparing algorithm outputs against gold standard laboratory measurements of glycogen utilization (such as 13C-MRS or muscle biopsies) have shown correlation coefficients ranging from 0.72 to 0.89, depending on the algorithmic approach and sensor quality. This demonstrates the clinical and sports science viability of digital sensor-based glycogenolysis monitoring when paired with appropriate data processing algorithms.
Clinical Validation Requirements for Metabolic Monitoring Devices
Clinical validation of metabolic monitoring devices for glycogenolysis response quantification requires rigorous testing protocols to ensure accuracy, reliability, and clinical utility. These devices must undergo comprehensive validation processes that adhere to regulatory standards established by organizations such as the FDA, EMA, and other international regulatory bodies.
The validation process should begin with laboratory-based analytical validation to establish the fundamental performance characteristics of the sensors. This includes determining precision, accuracy, linearity, detection limits, and analytical specificity when measuring biomarkers associated with glycogenolysis, such as glucose fluctuations, lactate levels, and potentially ketone bodies. Interference testing must be conducted to identify potential confounding factors that might affect measurement accuracy.
Clinical validation studies must follow, involving diverse patient populations that represent the intended user base. These studies should include subjects with various metabolic conditions, age groups, and fitness levels to ensure the device performs consistently across different physiological states. Particular attention should be paid to validating performance during exercise, fasting, and other conditions that trigger glycogenolysis.
Comparison studies against gold standard methods are essential. For glycogenolysis monitoring, this typically involves correlation with laboratory measurements of glucose, lactate, and potentially liver glycogen content through techniques such as magnetic resonance spectroscopy or biopsy in research settings. The validation protocol should establish acceptable limits of agreement between the digital sensor and reference methods.
Real-world validation is equally important, as controlled clinical environments may not fully represent daily use conditions. This includes testing the device's performance during various physical activities, in different environmental conditions, and over extended periods to assess drift and stability of measurements.
User factors must also be evaluated, including the impact of sensor placement, skin characteristics, hydration status, and user technique on measurement accuracy. This is particularly relevant for wearable sensors that may be affected by motion artifacts during exercise when glycogenolysis is most active.
Longitudinal validation studies are necessary to establish the device's ability to track changes in glycogenolysis response over time, which is crucial for monitoring metabolic adaptations to interventions or disease progression. These studies should demonstrate that the device can reliably detect clinically significant changes in metabolic parameters.
Finally, validation protocols must include comprehensive documentation of all testing methodologies, statistical analyses, and results interpretation guidelines to support regulatory submissions and clinical adoption of the technology.
The validation process should begin with laboratory-based analytical validation to establish the fundamental performance characteristics of the sensors. This includes determining precision, accuracy, linearity, detection limits, and analytical specificity when measuring biomarkers associated with glycogenolysis, such as glucose fluctuations, lactate levels, and potentially ketone bodies. Interference testing must be conducted to identify potential confounding factors that might affect measurement accuracy.
Clinical validation studies must follow, involving diverse patient populations that represent the intended user base. These studies should include subjects with various metabolic conditions, age groups, and fitness levels to ensure the device performs consistently across different physiological states. Particular attention should be paid to validating performance during exercise, fasting, and other conditions that trigger glycogenolysis.
Comparison studies against gold standard methods are essential. For glycogenolysis monitoring, this typically involves correlation with laboratory measurements of glucose, lactate, and potentially liver glycogen content through techniques such as magnetic resonance spectroscopy or biopsy in research settings. The validation protocol should establish acceptable limits of agreement between the digital sensor and reference methods.
Real-world validation is equally important, as controlled clinical environments may not fully represent daily use conditions. This includes testing the device's performance during various physical activities, in different environmental conditions, and over extended periods to assess drift and stability of measurements.
User factors must also be evaluated, including the impact of sensor placement, skin characteristics, hydration status, and user technique on measurement accuracy. This is particularly relevant for wearable sensors that may be affected by motion artifacts during exercise when glycogenolysis is most active.
Longitudinal validation studies are necessary to establish the device's ability to track changes in glycogenolysis response over time, which is crucial for monitoring metabolic adaptations to interventions or disease progression. These studies should demonstrate that the device can reliably detect clinically significant changes in metabolic parameters.
Finally, validation protocols must include comprehensive documentation of all testing methodologies, statistical analyses, and results interpretation guidelines to support regulatory submissions and clinical adoption of the technology.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!