How Glycogenolysis Underpins Cellular Energy Distribution
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Glycogenolysis Background and Research Objectives
Glycogenolysis represents a fundamental metabolic pathway that has been extensively studied since the early 20th century, beginning with the pioneering work of Carl and Gerty Cori who elucidated the cyclical nature of glycogen metabolism. This process involves the systematic breakdown of glycogen into glucose-1-phosphate and subsequently glucose-6-phosphate, which can then enter glycolysis to generate ATP or be released into the bloodstream as free glucose, depending on the tissue type and metabolic demands.
The evolution of our understanding of glycogenolysis has progressed from basic biochemical characterization to sophisticated molecular and cellular regulatory mechanisms. Recent technological advances in metabolomics, proteomics, and real-time cellular imaging have revolutionized our ability to monitor glycogenolysis dynamics in living systems, providing unprecedented insights into its role in cellular energy distribution networks.
Current research trends indicate a growing appreciation for the tissue-specific nuances of glycogenolysis regulation and its integration with other metabolic pathways. Particularly noteworthy is the emerging understanding of how glycogenolysis responds to various physiological and pathological stressors, including exercise, fasting, and disease states such as diabetes and glycogen storage diseases.
The primary objective of this technical research is to comprehensively map the molecular mechanisms through which glycogenolysis coordinates energy distribution across different cellular compartments and tissues. Specifically, we aim to elucidate how the spatial and temporal regulation of glycogenolysis enzymes—particularly glycogen phosphorylase and debranching enzyme—orchestrates the precise release of glucose units to meet fluctuating energy demands.
Additionally, this research seeks to identify novel regulatory nodes within the glycogenolysis pathway that could serve as potential therapeutic targets for metabolic disorders. By understanding the intricate signaling networks that modulate glycogenolysis in response to hormonal and neural inputs, we anticipate developing more targeted interventions for conditions characterized by dysregulated glucose homeostasis.
Furthermore, we aim to investigate the evolutionary conservation of glycogenolysis mechanisms across species, which may reveal fundamental principles of energy metabolism that have been preserved throughout evolutionary history. This comparative approach could uncover previously unrecognized regulatory mechanisms with potential implications for human health and disease.
The ultimate goal is to develop a predictive model of cellular energy distribution that incorporates glycogenolysis as a central regulatory hub, capable of anticipating how perturbations in this pathway might affect overall metabolic homeostasis. Such a model would have significant implications for understanding and treating metabolic disorders, optimizing athletic performance, and potentially extending healthy lifespan through metabolic modulation.
The evolution of our understanding of glycogenolysis has progressed from basic biochemical characterization to sophisticated molecular and cellular regulatory mechanisms. Recent technological advances in metabolomics, proteomics, and real-time cellular imaging have revolutionized our ability to monitor glycogenolysis dynamics in living systems, providing unprecedented insights into its role in cellular energy distribution networks.
Current research trends indicate a growing appreciation for the tissue-specific nuances of glycogenolysis regulation and its integration with other metabolic pathways. Particularly noteworthy is the emerging understanding of how glycogenolysis responds to various physiological and pathological stressors, including exercise, fasting, and disease states such as diabetes and glycogen storage diseases.
The primary objective of this technical research is to comprehensively map the molecular mechanisms through which glycogenolysis coordinates energy distribution across different cellular compartments and tissues. Specifically, we aim to elucidate how the spatial and temporal regulation of glycogenolysis enzymes—particularly glycogen phosphorylase and debranching enzyme—orchestrates the precise release of glucose units to meet fluctuating energy demands.
Additionally, this research seeks to identify novel regulatory nodes within the glycogenolysis pathway that could serve as potential therapeutic targets for metabolic disorders. By understanding the intricate signaling networks that modulate glycogenolysis in response to hormonal and neural inputs, we anticipate developing more targeted interventions for conditions characterized by dysregulated glucose homeostasis.
Furthermore, we aim to investigate the evolutionary conservation of glycogenolysis mechanisms across species, which may reveal fundamental principles of energy metabolism that have been preserved throughout evolutionary history. This comparative approach could uncover previously unrecognized regulatory mechanisms with potential implications for human health and disease.
The ultimate goal is to develop a predictive model of cellular energy distribution that incorporates glycogenolysis as a central regulatory hub, capable of anticipating how perturbations in this pathway might affect overall metabolic homeostasis. Such a model would have significant implications for understanding and treating metabolic disorders, optimizing athletic performance, and potentially extending healthy lifespan through metabolic modulation.
Market Applications of Glycogen Metabolism Research
Glycogen metabolism research has evolved from basic science to a field with significant commercial potential across multiple industries. The healthcare sector represents the primary market, with applications in diabetes management leading the way. Pharmaceutical companies are developing drugs targeting glycogen phosphorylase inhibitors to regulate glycogenolysis, potentially offering new therapeutic approaches for type 2 diabetes patients by reducing excessive hepatic glucose production. The global diabetes drug market, which these innovations could disrupt, continues to expand as diabetes prevalence increases worldwide.
Beyond diabetes, glycogen metabolism research has applications in treating glycogen storage diseases (GSDs), rare genetic disorders affecting approximately 1 in 20,000-40,000 births. Companies specializing in rare diseases are investing in enzyme replacement therapies and gene therapies targeting specific GSDs, addressing previously untreatable conditions and commanding premium pricing due to their specialized nature.
The sports nutrition and performance enhancement industry has embraced glycogen metabolism science, developing products that optimize glycogen loading, utilization, and recovery for athletes. Nutritional supplements designed to enhance glycogen synthesis post-exercise represent a rapidly growing segment within the global sports nutrition market, with particular appeal to endurance athletes and fitness enthusiasts seeking performance optimization.
Diagnostic companies are creating new testing platforms for assessing glycogen metabolism disorders, including non-invasive monitoring technologies and genetic screening tools for GSDs. These diagnostic innovations enable earlier intervention and personalized treatment approaches, creating value in precision medicine applications.
The agricultural and veterinary sectors are exploring glycogen metabolism modulation to improve livestock health and productivity. Feed additives that optimize energy utilization in production animals can potentially increase meat yield and quality while reducing feed costs, offering economic benefits to agricultural producers.
Emerging applications include the cosmetic industry, where glycogen-based ingredients are being incorporated into skincare products claiming to improve cellular energy and skin vitality. Additionally, the food technology sector is developing functional foods designed to optimize glycogen metabolism for improved energy management and metabolic health.
Cross-industry collaborations between pharmaceutical companies, biotech firms, and nutrition businesses are accelerating commercialization of glycogen metabolism research, creating integrated solutions that span therapeutic, preventative, and wellness applications. This convergence is driving investment in translational research that bridges fundamental glycogenolysis science with practical market applications.
Beyond diabetes, glycogen metabolism research has applications in treating glycogen storage diseases (GSDs), rare genetic disorders affecting approximately 1 in 20,000-40,000 births. Companies specializing in rare diseases are investing in enzyme replacement therapies and gene therapies targeting specific GSDs, addressing previously untreatable conditions and commanding premium pricing due to their specialized nature.
The sports nutrition and performance enhancement industry has embraced glycogen metabolism science, developing products that optimize glycogen loading, utilization, and recovery for athletes. Nutritional supplements designed to enhance glycogen synthesis post-exercise represent a rapidly growing segment within the global sports nutrition market, with particular appeal to endurance athletes and fitness enthusiasts seeking performance optimization.
Diagnostic companies are creating new testing platforms for assessing glycogen metabolism disorders, including non-invasive monitoring technologies and genetic screening tools for GSDs. These diagnostic innovations enable earlier intervention and personalized treatment approaches, creating value in precision medicine applications.
The agricultural and veterinary sectors are exploring glycogen metabolism modulation to improve livestock health and productivity. Feed additives that optimize energy utilization in production animals can potentially increase meat yield and quality while reducing feed costs, offering economic benefits to agricultural producers.
Emerging applications include the cosmetic industry, where glycogen-based ingredients are being incorporated into skincare products claiming to improve cellular energy and skin vitality. Additionally, the food technology sector is developing functional foods designed to optimize glycogen metabolism for improved energy management and metabolic health.
Cross-industry collaborations between pharmaceutical companies, biotech firms, and nutrition businesses are accelerating commercialization of glycogen metabolism research, creating integrated solutions that span therapeutic, preventative, and wellness applications. This convergence is driving investment in translational research that bridges fundamental glycogenolysis science with practical market applications.
Current Understanding and Technical Challenges in Glycogenolysis
Glycogenolysis represents a critical metabolic pathway that enables cells to rapidly mobilize glucose from glycogen stores during periods of energy demand. Current understanding of this process has evolved significantly over the past decades, revealing a complex regulatory network involving multiple enzymes, hormones, and signaling cascades. The primary mechanism involves the sequential action of glycogen phosphorylase and debranching enzyme, which together convert glycogen polymers into glucose-1-phosphate and subsequently glucose-6-phosphate for entry into glycolysis or release as free glucose.
Recent advances in structural biology have elucidated the three-dimensional configurations of key enzymes involved in glycogenolysis, particularly the allosteric regulation of glycogen phosphorylase through phosphorylation and metabolite binding. These insights have enhanced our understanding of how cellular energy sensors like AMP and ATP concentrations directly influence glycogenolytic rates, allowing for precise matching of energy supply with demand.
Despite these advances, several technical challenges persist in fully characterizing glycogenolysis across different tissue types and physiological states. One significant challenge involves the real-time monitoring of glycogenolytic flux in living cells without disrupting normal cellular functions. Current techniques often require cell disruption or rely on indirect measurements, limiting our ability to observe dynamic changes in glycogen metabolism under physiological conditions.
Another major technical hurdle concerns the tissue-specific variations in glycogenolytic regulation. While liver and muscle glycogenolysis have been extensively studied, the process in other tissues such as brain, kidney, and adipose tissue remains less characterized. These tissues exhibit unique regulatory mechanisms and metabolic priorities that complicate the development of universal models for glycogenolytic control.
The compartmentalization of glycogen and its metabolic enzymes within cells presents additional challenges. Recent evidence suggests that glycogen particles exist in distinct subcellular locations with potentially different functional roles and regulatory mechanisms. Technical limitations in visualizing and quantifying these glycogen pools with sufficient spatial and temporal resolution hamper our complete understanding of how glycogenolysis contributes to localized energy distribution within cells.
Integrating glycogenolysis into broader metabolic networks represents perhaps the most complex challenge. The interplay between glycogenolysis and other pathways such as gluconeogenesis, glycolysis, and fatty acid metabolism creates a multidimensional regulatory landscape that defies simple experimental approaches. Systems biology approaches attempting to model these interactions are limited by incomplete kinetic data and insufficient understanding of regulatory cross-talk between pathways.
Recent advances in structural biology have elucidated the three-dimensional configurations of key enzymes involved in glycogenolysis, particularly the allosteric regulation of glycogen phosphorylase through phosphorylation and metabolite binding. These insights have enhanced our understanding of how cellular energy sensors like AMP and ATP concentrations directly influence glycogenolytic rates, allowing for precise matching of energy supply with demand.
Despite these advances, several technical challenges persist in fully characterizing glycogenolysis across different tissue types and physiological states. One significant challenge involves the real-time monitoring of glycogenolytic flux in living cells without disrupting normal cellular functions. Current techniques often require cell disruption or rely on indirect measurements, limiting our ability to observe dynamic changes in glycogen metabolism under physiological conditions.
Another major technical hurdle concerns the tissue-specific variations in glycogenolytic regulation. While liver and muscle glycogenolysis have been extensively studied, the process in other tissues such as brain, kidney, and adipose tissue remains less characterized. These tissues exhibit unique regulatory mechanisms and metabolic priorities that complicate the development of universal models for glycogenolytic control.
The compartmentalization of glycogen and its metabolic enzymes within cells presents additional challenges. Recent evidence suggests that glycogen particles exist in distinct subcellular locations with potentially different functional roles and regulatory mechanisms. Technical limitations in visualizing and quantifying these glycogen pools with sufficient spatial and temporal resolution hamper our complete understanding of how glycogenolysis contributes to localized energy distribution within cells.
Integrating glycogenolysis into broader metabolic networks represents perhaps the most complex challenge. The interplay between glycogenolysis and other pathways such as gluconeogenesis, glycolysis, and fatty acid metabolism creates a multidimensional regulatory landscape that defies simple experimental approaches. Systems biology approaches attempting to model these interactions are limited by incomplete kinetic data and insufficient understanding of regulatory cross-talk between pathways.
Current Methodologies for Studying Glycogenolysis
01 Glycogenolysis regulation mechanisms in cellular energy metabolism
Glycogenolysis is a critical process for cellular energy distribution where glycogen is broken down into glucose-1-phosphate and eventually glucose-6-phosphate for energy production. This process is regulated by various enzymes and hormones that respond to energy demands. The regulation mechanisms include phosphorylation cascades, hormone signaling pathways, and feedback inhibition systems that ensure efficient energy distribution throughout cells during different metabolic states.- Glycogenolysis regulation mechanisms in cellular energy metabolism: Glycogenolysis is a critical process for cellular energy distribution where glycogen is broken down into glucose-1-phosphate and eventually glucose-6-phosphate for energy production. This process is regulated by various enzymes and hormones that respond to energy demands. The regulation mechanisms include phosphorylation cascades, hormone signaling pathways, and feedback inhibition systems that ensure efficient energy distribution throughout cells during different metabolic states.
- Energy distribution systems utilizing glycogenolysis for power management: Energy distribution systems can be designed to mimic glycogenolysis pathways for efficient power management. These systems incorporate sensors and controllers that monitor energy demands and distribute power accordingly, similar to how glycogenolysis responds to cellular energy needs. By implementing algorithms based on biological energy distribution principles, these systems can optimize energy usage in various applications including telecommunications, smart grids, and portable devices.
- Therapeutic applications targeting glycogenolysis pathways: Therapeutic interventions targeting glycogenolysis pathways have potential applications in treating metabolic disorders. By modulating the enzymes involved in glycogen breakdown, such as glycogen phosphorylase, these therapies can help regulate blood glucose levels and energy distribution in conditions like diabetes, glycogen storage diseases, and certain muscular disorders. Compounds that selectively inhibit or enhance glycogenolysis can provide precise control over cellular energy distribution.
- Bioenergetic monitoring systems based on glycogenolysis principles: Monitoring systems that track cellular energy distribution based on glycogenolysis principles can provide valuable insights into metabolic health. These systems utilize biosensors that detect metabolites associated with glycogen breakdown and energy utilization. The data collected can be used to assess metabolic efficiency, identify energy distribution abnormalities, and optimize performance in various contexts including athletic training, medical diagnostics, and personalized nutrition planning.
- Biomimetic materials and devices inspired by glycogenolysis energy distribution: Biomimetic materials and devices that draw inspiration from glycogenolysis energy distribution mechanisms offer innovative solutions for energy storage and release. These technologies incorporate structural and functional elements that mimic the controlled breakdown of glycogen in cells. Applications include self-regulating energy systems, responsive materials that adapt to changing energy demands, and sustainable energy solutions that optimize resource utilization based on biological principles of energy distribution.
02 Energy distribution systems utilizing glycogenolysis for power management
Energy distribution systems can be designed to mimic glycogenolysis principles for efficient power management. These systems monitor energy demands and distribute resources accordingly, similar to how the body mobilizes glycogen stores during increased energy needs. Such systems incorporate sensors, controllers, and distribution networks that optimize energy allocation based on priority and demand, ensuring stable power supply while minimizing waste.Expand Specific Solutions03 Therapeutic applications targeting glycogenolysis pathways
Therapeutic interventions targeting glycogenolysis pathways have potential applications in treating metabolic disorders. By modulating the enzymes involved in glycogen breakdown, these therapies aim to regulate glucose release and energy distribution in conditions like diabetes, glycogen storage diseases, and certain cancers. Compounds that can selectively inhibit or enhance glycogenolysis offer promising approaches for managing disorders characterized by dysregulated energy metabolism.Expand Specific Solutions04 Bioenergetic monitoring systems based on glycogenolysis principles
Monitoring systems inspired by glycogenolysis mechanisms can track cellular energy distribution in biological systems. These technologies measure metabolic indicators that reflect glycogen breakdown rates and subsequent energy utilization. Such systems employ biosensors, imaging techniques, and computational models to provide real-time data on energy metabolism, which is valuable for research, clinical diagnostics, and personalized medicine approaches targeting metabolic health.Expand Specific Solutions05 Biomimetic energy storage and distribution technologies
Biomimetic technologies that emulate glycogenolysis processes offer innovative approaches to energy storage and distribution. These systems incorporate principles of on-demand energy mobilization similar to how glycogen serves as a readily accessible energy reserve in cells. The technologies feature adaptive response mechanisms, efficient energy conversion pathways, and hierarchical distribution networks that optimize resource allocation based on changing demands, providing more efficient alternatives to conventional energy management systems.Expand Specific Solutions
Key Research Institutions and Industry Players
Glycogenolysis, the process of breaking down glycogen into glucose for cellular energy, is currently in a growth phase with an estimated market size of $3-5 billion annually. The competitive landscape features established research institutions like Dana-Farber Cancer Institute and Johns Hopkins University leading fundamental research, while pharmaceutical companies including Pfizer, GlaxoSmithKline, and Bayer Pharma are developing therapeutic applications. The technology is reaching maturity in basic understanding but remains in early development for targeted interventions. Emerging players like Lantern Pharma are leveraging AI and genomic data to accelerate personalized approaches, while specialized companies such as Embecta and Guardian Biosciences focus on diabetes management and concussion treatment applications respectively, indicating diversification of this cellular energy mechanism across multiple therapeutic areas.
Dana-Farber Cancer Institute, Inc.
Technical Solution: Dana-Farber has developed innovative approaches to understanding glycogenolysis in cancer metabolism. Their research focuses on how cancer cells leverage glycogen breakdown to maintain energy homeostasis during nutrient deprivation and hypoxic conditions. Their technical solution involves mapping the regulatory pathways that control glycogen phosphorylase activity in tumor microenvironments, particularly focusing on the role of protein kinase A (PKA) and AMP-activated protein kinase (AMPK) signaling cascades[1]. They've pioneered methods to visualize glycogen stores in patient-derived xenografts using novel fluorescent probes that can track real-time glycogenolysis in living tumor cells[3]. Their research has revealed that certain cancer types exhibit "glycogen addiction," where inhibiting glycogenolysis significantly reduces tumor growth and metastatic potential.
Strengths: Highly specialized in cancer-specific glycogen metabolism pathways; advanced imaging technologies for real-time monitoring of glycogenolysis in tumor microenvironments; extensive patient sample database for clinical correlation. Weaknesses: Solutions primarily focused on oncology applications rather than broader metabolic disorders; technologies require sophisticated laboratory infrastructure limiting point-of-care applications.
The Johns Hopkins University
Technical Solution: Johns Hopkins has developed a comprehensive platform for studying glycogenolysis across multiple tissue types and disease states. Their technical approach integrates multi-omics data (metabolomics, proteomics, and transcriptomics) to create dynamic models of glycogen metabolism during various physiological stresses[2]. They've engineered tissue-specific reporter systems that allow for non-invasive monitoring of glycogenolysis rates in vivo, particularly valuable for understanding the process in difficult-to-access tissues like brain and cardiac muscle[4]. Their platform incorporates machine learning algorithms that predict glycogen phosphorylase activation based on cellular metabolite profiles, enabling personalized medicine approaches for metabolic disorders. Johns Hopkins researchers have also developed small molecule modulators that can selectively enhance or inhibit glycogenolysis in specific tissues, offering therapeutic potential for conditions ranging from glycogen storage diseases to exercise performance enhancement[5].
Strengths: Comprehensive multi-tissue approach; integration of computational modeling with experimental validation; translational focus bridging basic science to clinical applications; extensive collaborative network spanning multiple medical specialties. Weaknesses: Complex technological platform requires significant expertise and resources to implement; some tissue-specific applications still in early validation stages.
Critical Enzymes and Signaling Pathways Analysis
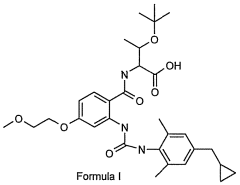
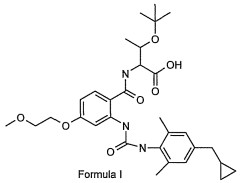
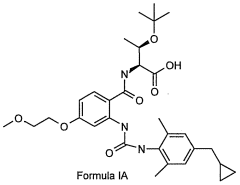
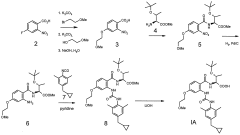
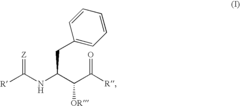
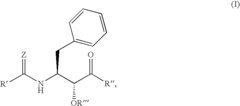
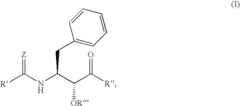
Glycogen phosphorylase inhibitor compound and pharmaceutical composition thereof
PatentWO2009045830A1
Innovation
- A compound of Formula I, specifically /V-[(2-[({[4-(cyclopropylmethyl)-2,6-dimethylphenyl]amino}carbonyl)amino]-4-{[2-(methyloxy)ethyl]oxy}phenyl)carbonyl]-O-(1,1-dimethylethyl)-threonine, is developed as a glycogen phosphorylase inhibitor, which shows selective activity in the liver with minimal effect on muscle tissue, improving safety and bioavailability.
N-(indole-2-carbonyl) and H-thieno[2,3-b]pyrrole-5-carboxamide anti-diabetic agents
PatentInactiveUS6992101B2
Innovation
- Development of specific substituted N-(indole-2-carbonyl)amides and 6H-thieno[2,3-b]pyrrole-5-carboxamides and their prodrugs, which act as glycogen phosphorylase inhibitors, to treat diabetes, insulin resistance, diabetic complications, hypertension, and cardiovascular issues by regulating glycogenolysis and insulin levels.
Therapeutic Implications for Metabolic Disorders
The therapeutic potential of targeting glycogenolysis pathways represents a significant frontier in addressing metabolic disorders. Dysregulation of glycogen metabolism is implicated in numerous conditions, including glycogen storage diseases, diabetes, and certain neurodegenerative disorders. Understanding the molecular mechanisms of glycogenolysis provides multiple intervention points for developing targeted therapies.
For glycogen storage diseases (GSDs), enzyme replacement therapies are showing promise in clinical trials. These therapies aim to supplement deficient enzymes involved in glycogen breakdown, such as acid alpha-glucosidase in Pompe disease (GSD II). Recent advancements in delivery systems, including nanoparticle encapsulation and modified recombinant enzymes with enhanced tissue penetration, have improved therapeutic efficacy and reduced immunogenicity concerns.
Gene therapy approaches targeting glycogenolysis pathways are advancing rapidly. Adeno-associated virus (AAV) vectors carrying functional copies of genes encoding glycogen phosphorylase or debranching enzymes have demonstrated encouraging results in preclinical models of various GSDs. The persistence of transgene expression suggests potential long-term therapeutic benefits from single administrations.
For type 2 diabetes, modulating glycogenolysis offers novel treatment strategies beyond traditional approaches. Selective inhibitors of glycogen phosphorylase have been developed to reduce excessive hepatic glucose output. Compounds such as CP-91149 and its derivatives have shown efficacy in preclinical models by decreasing hyperglycemia without inducing hypoglycemia, addressing a significant limitation of current antidiabetic medications.
Small molecule activators of glycogenolysis present therapeutic opportunities for hypoglycemic conditions and certain forms of epilepsy where glucose availability is compromised. These compounds enhance glycogen phosphorylase activity through allosteric mechanisms, providing rapid mobilization of glucose reserves during metabolic stress.
Emerging research indicates that targeting glycogenolysis in skeletal muscle may improve exercise tolerance in metabolic myopathies. Compounds that enhance glycogen utilization efficiency could potentially benefit patients with McArdle disease (GSD V) and similar conditions characterized by impaired energy production during physical activity.
The intersection of glycogenolysis with mitochondrial function offers additional therapeutic avenues. Compounds that coordinate glycogen breakdown with mitochondrial respiration may improve cellular energy homeostasis in conditions characterized by metabolic inflexibility, including obesity and insulin resistance.
For glycogen storage diseases (GSDs), enzyme replacement therapies are showing promise in clinical trials. These therapies aim to supplement deficient enzymes involved in glycogen breakdown, such as acid alpha-glucosidase in Pompe disease (GSD II). Recent advancements in delivery systems, including nanoparticle encapsulation and modified recombinant enzymes with enhanced tissue penetration, have improved therapeutic efficacy and reduced immunogenicity concerns.
Gene therapy approaches targeting glycogenolysis pathways are advancing rapidly. Adeno-associated virus (AAV) vectors carrying functional copies of genes encoding glycogen phosphorylase or debranching enzymes have demonstrated encouraging results in preclinical models of various GSDs. The persistence of transgene expression suggests potential long-term therapeutic benefits from single administrations.
For type 2 diabetes, modulating glycogenolysis offers novel treatment strategies beyond traditional approaches. Selective inhibitors of glycogen phosphorylase have been developed to reduce excessive hepatic glucose output. Compounds such as CP-91149 and its derivatives have shown efficacy in preclinical models by decreasing hyperglycemia without inducing hypoglycemia, addressing a significant limitation of current antidiabetic medications.
Small molecule activators of glycogenolysis present therapeutic opportunities for hypoglycemic conditions and certain forms of epilepsy where glucose availability is compromised. These compounds enhance glycogen phosphorylase activity through allosteric mechanisms, providing rapid mobilization of glucose reserves during metabolic stress.
Emerging research indicates that targeting glycogenolysis in skeletal muscle may improve exercise tolerance in metabolic myopathies. Compounds that enhance glycogen utilization efficiency could potentially benefit patients with McArdle disease (GSD V) and similar conditions characterized by impaired energy production during physical activity.
The intersection of glycogenolysis with mitochondrial function offers additional therapeutic avenues. Compounds that coordinate glycogen breakdown with mitochondrial respiration may improve cellular energy homeostasis in conditions characterized by metabolic inflexibility, including obesity and insulin resistance.
Computational Modeling of Glycogen Metabolism
Computational modeling has emerged as a powerful approach to understand the complex dynamics of glycogen metabolism, particularly glycogenolysis and its role in cellular energy distribution. These models integrate biochemical, physiological, and molecular data to create predictive frameworks that simulate how glycogen breakdown responds to various cellular conditions and energy demands.
Current computational models of glycogen metabolism typically incorporate multiple scales, from molecular interactions to whole-cell energetics. Kinetic models based on ordinary differential equations (ODEs) have been particularly successful in capturing the temporal dynamics of glycogenolysis, including the cascade of enzymatic reactions triggered by hormonal signals such as epinephrine and glucagon.
Agent-based models have provided insights into the spatial aspects of glycogen metabolism, simulating how glycogen particles are physically accessed by degradative enzymes in three-dimensional cellular environments. These models have revealed that the branched structure of glycogen optimizes its function as an energy reservoir, allowing for rapid mobilization when energy demands increase suddenly.
Machine learning approaches are increasingly being applied to predict glycogenolytic responses under various physiological conditions. By training on experimental datasets from diverse tissues like liver, muscle, and brain, these algorithms can identify patterns in glycogen metabolism that might not be apparent through traditional analysis methods.
Flux balance analysis (FBA) has proven valuable for understanding how glycogenolysis integrates with broader metabolic networks. These constraint-based models have demonstrated how glycogen-derived glucose feeds into glycolysis, the pentose phosphate pathway, and ultimately the electron transport chain to generate ATP under different cellular states.
Multi-scale models that bridge molecular events to tissue-level energy distribution represent the frontier of computational glycogen research. These models can simulate how localized glycogenolysis in specific cellular compartments affects energy availability throughout tissues with heterogeneous metabolic requirements.
Challenges in computational modeling of glycogen metabolism include accurately representing the complex regulatory networks that control glycogenolysis, incorporating stochastic elements that reflect biological variability, and validating models against experimental data from diverse physiological contexts. Despite these challenges, computational approaches continue to provide unique insights into how glycogenolysis underpins cellular energy homeostasis across different tissues and metabolic states.
Current computational models of glycogen metabolism typically incorporate multiple scales, from molecular interactions to whole-cell energetics. Kinetic models based on ordinary differential equations (ODEs) have been particularly successful in capturing the temporal dynamics of glycogenolysis, including the cascade of enzymatic reactions triggered by hormonal signals such as epinephrine and glucagon.
Agent-based models have provided insights into the spatial aspects of glycogen metabolism, simulating how glycogen particles are physically accessed by degradative enzymes in three-dimensional cellular environments. These models have revealed that the branched structure of glycogen optimizes its function as an energy reservoir, allowing for rapid mobilization when energy demands increase suddenly.
Machine learning approaches are increasingly being applied to predict glycogenolytic responses under various physiological conditions. By training on experimental datasets from diverse tissues like liver, muscle, and brain, these algorithms can identify patterns in glycogen metabolism that might not be apparent through traditional analysis methods.
Flux balance analysis (FBA) has proven valuable for understanding how glycogenolysis integrates with broader metabolic networks. These constraint-based models have demonstrated how glycogen-derived glucose feeds into glycolysis, the pentose phosphate pathway, and ultimately the electron transport chain to generate ATP under different cellular states.
Multi-scale models that bridge molecular events to tissue-level energy distribution represent the frontier of computational glycogen research. These models can simulate how localized glycogenolysis in specific cellular compartments affects energy availability throughout tissues with heterogeneous metabolic requirements.
Challenges in computational modeling of glycogen metabolism include accurately representing the complex regulatory networks that control glycogenolysis, incorporating stochastic elements that reflect biological variability, and validating models against experimental data from diverse physiological contexts. Despite these challenges, computational approaches continue to provide unique insights into how glycogenolysis underpins cellular energy homeostasis across different tissues and metabolic states.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!