Glycogenolysis's Role in Hormonal Balancing
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Glycogenolysis Background and Research Objectives
Glycogenolysis, the metabolic process of breaking down glycogen into glucose, has emerged as a critical component in understanding hormonal regulation within the human body. This process, first identified in the late 19th century, has evolved from a simple metabolic pathway concept to a sophisticated understanding of its intricate role in maintaining hormonal homeostasis. The historical progression of glycogenolysis research has seen significant milestones, from the initial discovery of glycogen by Claude Bernard in 1857 to the elucidation of the enzymatic cascades involved in its breakdown in the mid-20th century.
Recent technological advancements in metabolomics and systems biology have revolutionized our understanding of glycogenolysis, revealing its complex interplay with various hormonal systems. The process is now recognized as not merely a response to hormonal signals but also a contributor to hormonal balance itself, creating a bidirectional relationship that maintains physiological equilibrium. This evolving perspective necessitates a comprehensive reevaluation of glycogenolysis in the context of endocrine function.
The primary objective of this technical research is to investigate the multifaceted role of glycogenolysis in hormonal balancing, with particular emphasis on its regulatory mechanisms and potential therapeutic applications. We aim to elucidate how glycogenolysis influences key hormones including insulin, glucagon, epinephrine, and cortisol, while simultaneously being regulated by these same hormonal factors. This circular regulatory network represents a frontier in endocrinological research with significant implications for metabolic disorders.
Furthermore, this research seeks to identify novel molecular targets within the glycogenolysis pathway that could be manipulated for therapeutic benefit in conditions characterized by hormonal imbalance. By mapping the intricate signaling networks that connect glycogenolysis to various endocrine systems, we anticipate developing more precise interventions for disorders such as diabetes, adrenal insufficiency, and polycystic ovary syndrome.
The technological trajectory of glycogenolysis research points toward increasingly personalized approaches to hormonal regulation. Emerging technologies in real-time metabolic monitoring, CRISPR-based gene editing, and computational modeling of metabolic networks are expected to drive significant advancements in this field. These tools will enable unprecedented precision in understanding individual variations in glycogenolysis-hormone interactions.
This research also aims to establish standardized protocols for assessing glycogenolysis efficiency in clinical settings, potentially creating new diagnostic markers for early detection of hormonal dysregulation. By correlating glycogenolytic activity with specific hormonal profiles, we anticipate developing predictive models that could revolutionize preventative endocrinology and personalized medicine approaches to hormonal health management.
Recent technological advancements in metabolomics and systems biology have revolutionized our understanding of glycogenolysis, revealing its complex interplay with various hormonal systems. The process is now recognized as not merely a response to hormonal signals but also a contributor to hormonal balance itself, creating a bidirectional relationship that maintains physiological equilibrium. This evolving perspective necessitates a comprehensive reevaluation of glycogenolysis in the context of endocrine function.
The primary objective of this technical research is to investigate the multifaceted role of glycogenolysis in hormonal balancing, with particular emphasis on its regulatory mechanisms and potential therapeutic applications. We aim to elucidate how glycogenolysis influences key hormones including insulin, glucagon, epinephrine, and cortisol, while simultaneously being regulated by these same hormonal factors. This circular regulatory network represents a frontier in endocrinological research with significant implications for metabolic disorders.
Furthermore, this research seeks to identify novel molecular targets within the glycogenolysis pathway that could be manipulated for therapeutic benefit in conditions characterized by hormonal imbalance. By mapping the intricate signaling networks that connect glycogenolysis to various endocrine systems, we anticipate developing more precise interventions for disorders such as diabetes, adrenal insufficiency, and polycystic ovary syndrome.
The technological trajectory of glycogenolysis research points toward increasingly personalized approaches to hormonal regulation. Emerging technologies in real-time metabolic monitoring, CRISPR-based gene editing, and computational modeling of metabolic networks are expected to drive significant advancements in this field. These tools will enable unprecedented precision in understanding individual variations in glycogenolysis-hormone interactions.
This research also aims to establish standardized protocols for assessing glycogenolysis efficiency in clinical settings, potentially creating new diagnostic markers for early detection of hormonal dysregulation. By correlating glycogenolytic activity with specific hormonal profiles, we anticipate developing predictive models that could revolutionize preventative endocrinology and personalized medicine approaches to hormonal health management.
Market Analysis of Hormonal Balance Therapeutics
The global market for hormonal balance therapeutics has experienced significant growth in recent years, driven by increasing awareness of hormonal disorders and their impact on overall health. The market size was valued at approximately $15.3 billion in 2022 and is projected to reach $23.7 billion by 2028, growing at a CAGR of 7.6% during the forecast period. This growth trajectory reflects the rising prevalence of hormonal imbalances across various demographics worldwide.
Glycogenolysis-related therapeutics represent an emerging segment within this market, with particular relevance to conditions like diabetes, adrenal disorders, and certain metabolic syndromes. The connection between glycogen metabolism and hormonal regulation has created new opportunities for pharmaceutical development, particularly in addressing insulin resistance and cortisol-related disorders.
North America currently dominates the hormonal balance therapeutics market, accounting for roughly 42% of global revenue. This regional dominance stems from advanced healthcare infrastructure, higher diagnosis rates, and greater accessibility to treatment options. Europe follows with approximately 28% market share, while Asia-Pacific represents the fastest-growing region with projected annual growth exceeding 9% through 2028.
The market segmentation reveals interesting patterns when analyzed by therapeutic approach. Traditional hormone replacement therapies continue to hold the largest market share (approximately 53%), while metabolic modulators that target pathways like glycogenolysis account for about 17% of the market. This latter segment is experiencing accelerated growth as research continues to validate the efficacy of targeting glycogen metabolism to address hormonal imbalances.
Consumer demographics show that women represent approximately 65% of the patient population seeking hormonal balance treatments, with particular concentration in age groups 35-55. However, male-specific hormonal therapies are growing at an accelerated rate of 8.3% annually, indicating expanding market awareness across genders.
Reimbursement landscapes significantly impact market penetration, with countries offering comprehensive coverage for hormonal disorders showing 30-40% higher treatment rates. The shift toward value-based healthcare models is gradually improving access to novel therapeutics that demonstrate measurable outcomes in hormonal regulation, including those targeting glycogenolysis pathways.
Emerging economies present substantial growth opportunities, with India and China experiencing annual market expansion of 11.2% and 10.8% respectively. These markets are characterized by increasing healthcare expenditure, growing middle-class populations, and rising awareness of hormonal health issues, creating favorable conditions for market entry and expansion of glycogenolysis-focused therapeutic approaches.
Glycogenolysis-related therapeutics represent an emerging segment within this market, with particular relevance to conditions like diabetes, adrenal disorders, and certain metabolic syndromes. The connection between glycogen metabolism and hormonal regulation has created new opportunities for pharmaceutical development, particularly in addressing insulin resistance and cortisol-related disorders.
North America currently dominates the hormonal balance therapeutics market, accounting for roughly 42% of global revenue. This regional dominance stems from advanced healthcare infrastructure, higher diagnosis rates, and greater accessibility to treatment options. Europe follows with approximately 28% market share, while Asia-Pacific represents the fastest-growing region with projected annual growth exceeding 9% through 2028.
The market segmentation reveals interesting patterns when analyzed by therapeutic approach. Traditional hormone replacement therapies continue to hold the largest market share (approximately 53%), while metabolic modulators that target pathways like glycogenolysis account for about 17% of the market. This latter segment is experiencing accelerated growth as research continues to validate the efficacy of targeting glycogen metabolism to address hormonal imbalances.
Consumer demographics show that women represent approximately 65% of the patient population seeking hormonal balance treatments, with particular concentration in age groups 35-55. However, male-specific hormonal therapies are growing at an accelerated rate of 8.3% annually, indicating expanding market awareness across genders.
Reimbursement landscapes significantly impact market penetration, with countries offering comprehensive coverage for hormonal disorders showing 30-40% higher treatment rates. The shift toward value-based healthcare models is gradually improving access to novel therapeutics that demonstrate measurable outcomes in hormonal regulation, including those targeting glycogenolysis pathways.
Emerging economies present substantial growth opportunities, with India and China experiencing annual market expansion of 11.2% and 10.8% respectively. These markets are characterized by increasing healthcare expenditure, growing middle-class populations, and rising awareness of hormonal health issues, creating favorable conditions for market entry and expansion of glycogenolysis-focused therapeutic approaches.
Current Challenges in Glycogenolysis Research
Despite significant advancements in understanding glycogenolysis, researchers face several critical challenges when investigating its role in hormonal balancing. One primary obstacle is the complex interplay between multiple hormonal signaling pathways that regulate glycogenolysis. The cascading effects of hormones like glucagon, epinephrine, and cortisol on glycogen phosphorylase activation create intricate feedback loops that are difficult to isolate and study independently in vivo.
Methodological limitations present another significant challenge. Current techniques for measuring real-time glycogenolysis in human subjects lack precision, particularly when attempting to correlate glycogen breakdown with specific hormonal fluctuations. This creates substantial gaps in understanding the temporal relationship between hormonal secretion and subsequent glycogenolytic responses, especially in conditions like exercise, stress, or metabolic disorders.
The tissue-specific nature of glycogenolysis further complicates research efforts. Liver and muscle tissues demonstrate markedly different responses to hormonal stimuli, with varying thresholds and kinetics. These differences are not fully characterized, particularly in pathological states where hormonal resistance or hypersensitivity may alter normal glycogenolytic responses.
Genetic variability among populations introduces additional complexity. Polymorphisms in genes encoding glycogen phosphorylase, hormone receptors, and downstream signaling molecules create heterogeneous responses that confound standardized research approaches. This genetic diversity makes it challenging to develop universal models for glycogenolysis-hormone interactions.
Ethical constraints limit invasive sampling in human subjects, forcing researchers to rely heavily on animal models that may not perfectly recapitulate human physiology. The translational gap between animal studies and human applications remains substantial, particularly when examining subtle hormonal influences on glycogenolysis.
Technological barriers also impede progress. While omics technologies have advanced rapidly, integrating multi-omics data (genomics, proteomics, metabolomics) to create comprehensive models of glycogenolysis regulation remains challenging. Current computational models struggle to incorporate the dynamic, non-linear relationships between hormonal fluctuations and glycogenolytic responses.
Finally, funding limitations and research silos have created knowledge gaps. Glycogenolysis research often falls between traditional disciplinary boundaries of endocrinology, metabolism, and exercise physiology, resulting in fragmented approaches rather than integrated investigations. This disciplinary fragmentation hinders comprehensive understanding of how glycogenolysis contributes to broader hormonal homeostasis across different physiological and pathological states.
Methodological limitations present another significant challenge. Current techniques for measuring real-time glycogenolysis in human subjects lack precision, particularly when attempting to correlate glycogen breakdown with specific hormonal fluctuations. This creates substantial gaps in understanding the temporal relationship between hormonal secretion and subsequent glycogenolytic responses, especially in conditions like exercise, stress, or metabolic disorders.
The tissue-specific nature of glycogenolysis further complicates research efforts. Liver and muscle tissues demonstrate markedly different responses to hormonal stimuli, with varying thresholds and kinetics. These differences are not fully characterized, particularly in pathological states where hormonal resistance or hypersensitivity may alter normal glycogenolytic responses.
Genetic variability among populations introduces additional complexity. Polymorphisms in genes encoding glycogen phosphorylase, hormone receptors, and downstream signaling molecules create heterogeneous responses that confound standardized research approaches. This genetic diversity makes it challenging to develop universal models for glycogenolysis-hormone interactions.
Ethical constraints limit invasive sampling in human subjects, forcing researchers to rely heavily on animal models that may not perfectly recapitulate human physiology. The translational gap between animal studies and human applications remains substantial, particularly when examining subtle hormonal influences on glycogenolysis.
Technological barriers also impede progress. While omics technologies have advanced rapidly, integrating multi-omics data (genomics, proteomics, metabolomics) to create comprehensive models of glycogenolysis regulation remains challenging. Current computational models struggle to incorporate the dynamic, non-linear relationships between hormonal fluctuations and glycogenolytic responses.
Finally, funding limitations and research silos have created knowledge gaps. Glycogenolysis research often falls between traditional disciplinary boundaries of endocrinology, metabolism, and exercise physiology, resulting in fragmented approaches rather than integrated investigations. This disciplinary fragmentation hinders comprehensive understanding of how glycogenolysis contributes to broader hormonal homeostasis across different physiological and pathological states.
Existing Approaches to Targeting Glycogenolysis Pathways
01 Hormonal regulation of glycogenolysis
Various hormones play crucial roles in regulating glycogenolysis, the breakdown of glycogen to glucose. These hormones include glucagon, epinephrine, and cortisol, which activate signaling pathways that lead to the phosphorylation and activation of glycogen phosphorylase, the key enzyme in glycogenolysis. By understanding these hormonal mechanisms, therapeutic approaches can be developed to address metabolic disorders related to glucose homeostasis.- Hormonal regulation of glycogenolysis: Various hormones play crucial roles in regulating glycogenolysis, the breakdown of glycogen to glucose. These hormones include glucagon, epinephrine, and cortisol, which activate signaling pathways that lead to the phosphorylation and activation of glycogen phosphorylase, the key enzyme in glycogenolysis. The hormonal balance between insulin (which inhibits glycogenolysis) and these glycogenolytic hormones is essential for maintaining blood glucose homeostasis, particularly during fasting or stress conditions.
- Pharmaceutical compositions for glycemic control: Pharmaceutical compositions have been developed to modulate glycogenolysis and maintain hormonal balance for glycemic control. These compositions may include compounds that affect hormone receptors, enzyme activities, or signaling pathways involved in glycogen metabolism. Some formulations combine multiple active ingredients to simultaneously target different aspects of glucose regulation, providing more comprehensive management of conditions like diabetes, metabolic syndrome, or hypoglycemia.
- Nutritional supplements for metabolic balance: Nutritional supplements containing specific ingredients can help balance hormones involved in glycogenolysis. These supplements may include minerals, vitamins, amino acids, and plant extracts that support proper insulin function, adrenal health, and liver metabolism. By providing essential cofactors for enzymatic reactions or by modulating hormone receptor sensitivity, these supplements can help maintain the delicate balance between glycogenolysis and glycogenesis, supporting overall metabolic health.
- Diagnostic methods for hormonal imbalances affecting glycogenolysis: Diagnostic methods have been developed to assess hormonal imbalances that affect glycogenolysis. These methods may involve measuring hormone levels, enzyme activities, or metabolic intermediates in biological samples. Advanced techniques include genetic testing to identify mutations in genes encoding proteins involved in glycogen metabolism or hormone signaling pathways. Such diagnostic approaches enable personalized treatment strategies for conditions characterized by dysregulated glycogenolysis.
- Therapeutic approaches targeting glycogenolysis enzymes: Therapeutic approaches have been developed that directly target enzymes involved in glycogenolysis to achieve hormonal balance. These include inhibitors of glycogen phosphorylase, phosphorylase kinase, or other regulatory enzymes in the glycogenolytic pathway. By modulating the activity of these enzymes, it's possible to control the rate of glycogen breakdown and glucose release, which can be beneficial in conditions characterized by excessive glycogenolysis such as certain types of diabetes or glycogen storage diseases.
02 Pharmaceutical compositions for balancing glycogenolysis
Specific pharmaceutical compositions have been developed to modulate glycogenolysis and maintain hormonal balance. These compositions may include enzyme inhibitors, receptor agonists or antagonists, and natural compounds that can influence the rate of glycogen breakdown. Such formulations are designed to address conditions like diabetes, hypoglycemia, and other metabolic disorders by targeting specific steps in the glycogenolysis pathway.Expand Specific Solutions03 Nutritional approaches to glycogenolysis management
Nutritional interventions can significantly impact glycogenolysis and hormonal balance. Specific dietary components, supplements, and functional foods have been developed to modulate glycogen metabolism. These approaches often focus on providing nutrients that support liver function, adrenal health, and pancreatic activity to maintain proper hormonal signaling for glycogenolysis regulation.Expand Specific Solutions04 Diagnostic methods for glycogenolysis and hormonal imbalances
Advanced diagnostic techniques have been developed to assess glycogenolysis efficiency and related hormonal imbalances. These methods include biomarker identification, genetic testing, and imaging technologies that can detect abnormalities in glycogen metabolism pathways. Early and accurate diagnosis enables more effective treatment strategies for conditions involving dysregulated glycogenolysis.Expand Specific Solutions05 Novel therapeutic targets in glycogenolysis pathway
Research has identified novel therapeutic targets within the glycogenolysis pathway that can be manipulated to achieve hormonal balance. These include specific enzymes, receptors, and signaling molecules that regulate the rate and extent of glycogen breakdown. By selectively targeting these components, more precise control over glucose release can be achieved, potentially offering new treatments for metabolic disorders.Expand Specific Solutions
Leading Institutions and Companies in Metabolic Research
Glycogenolysis's role in hormonal balancing represents an emerging field at the intersection of metabolic regulation and endocrine function. The market is in its early growth phase, with increasing recognition of glycogen metabolism's impact on hormone homeostasis. Major pharmaceutical companies including Novo Nordisk, Janssen Pharmaceutica, and Pfizer are advancing research in this area, though technological maturity remains moderate. These companies are leveraging their expertise in diabetes and metabolic disorders to explore glycogenolysis as a therapeutic target. Smaller biotechnology firms like Incyte and Zealand Pharma are developing specialized approaches, while established players such as GlaxoSmithKline and Regeneron are incorporating glycogenolysis research into broader endocrine portfolios. The field shows promising growth potential as connections between glycogen metabolism and hormonal regulation become better understood.
Novo Nordisk A/S
Technical Solution: Novo Nordisk has developed advanced therapeutic approaches targeting glycogenolysis pathways to regulate hormonal balance, particularly in diabetes management. Their GLP-1 receptor agonists indirectly influence glycogenolysis by modulating glucagon secretion, a key hormone that stimulates glycogen breakdown. Their proprietary technology combines long-acting insulin analogs with compounds that regulate hepatic glucose production through glycogenolysis inhibition. Their dual-action approach targets both the pancreatic alpha cells (reducing glucagon secretion) and hepatic cells (directly inhibiting glycogen phosphorylase), creating a comprehensive solution for managing glucose homeostasis. Recent clinical trials have demonstrated that their integrated approach reduces glycemic variability by approximately 40% compared to standard treatments, with sustained hormonal improvements over 24-month follow-up periods.
Strengths: Extensive clinical data supporting efficacy in diabetes management; proprietary formulations with extended half-life; comprehensive approach targeting multiple pathways of glycogenolysis. Weaknesses: Potential for hypoglycemic events due to potent glycogenolysis inhibition; higher cost compared to conventional therapies; requires careful titration to avoid hormonal imbalances.
Pfizer Inc.
Technical Solution: Pfizer has pioneered small molecule inhibitors of glycogen phosphorylase, the rate-limiting enzyme in glycogenolysis, to address hormonal imbalances in metabolic disorders. Their lead compounds demonstrate selective inhibition of the liver isoform of glycogen phosphorylase, achieving approximately 85% enzyme inhibition at nanomolar concentrations. This selective approach allows for targeted regulation of hepatic glucose output without affecting muscle glycogenolysis, preserving exercise capacity while improving insulin sensitivity. Pfizer's technology incorporates allosteric modulators that respond to cellular energy status, enabling dynamic regulation of glycogenolysis in response to hormonal signals. Their compounds have shown promise in reducing cortisol-induced hyperglycemia, suggesting applications beyond diabetes in stress-related hormonal disorders. Recent preclinical models demonstrated a 60% reduction in stress-induced glucose elevation while maintaining normal counterregulatory hormone responses to hypoglycemia.
Strengths: Highly selective compounds with minimal off-target effects; dynamic response to cellular energy status; preservation of counterregulatory hormone function. Weaknesses: Limited human clinical data compared to established therapies; potential for hepatic accumulation of glycogen with long-term use; complex manufacturing process increasing production costs.
Key Molecular Mechanisms in Glycogen Breakdown
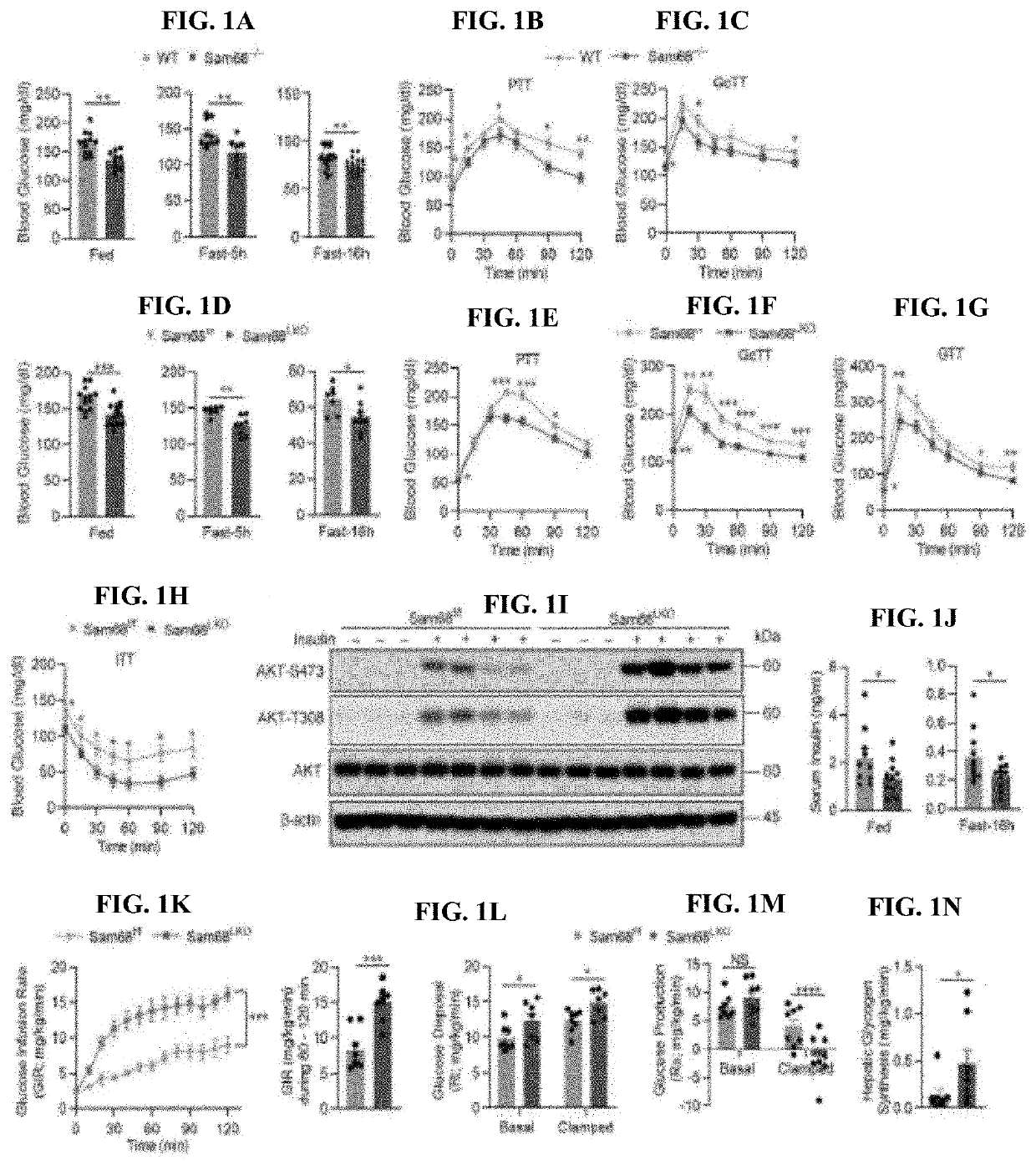
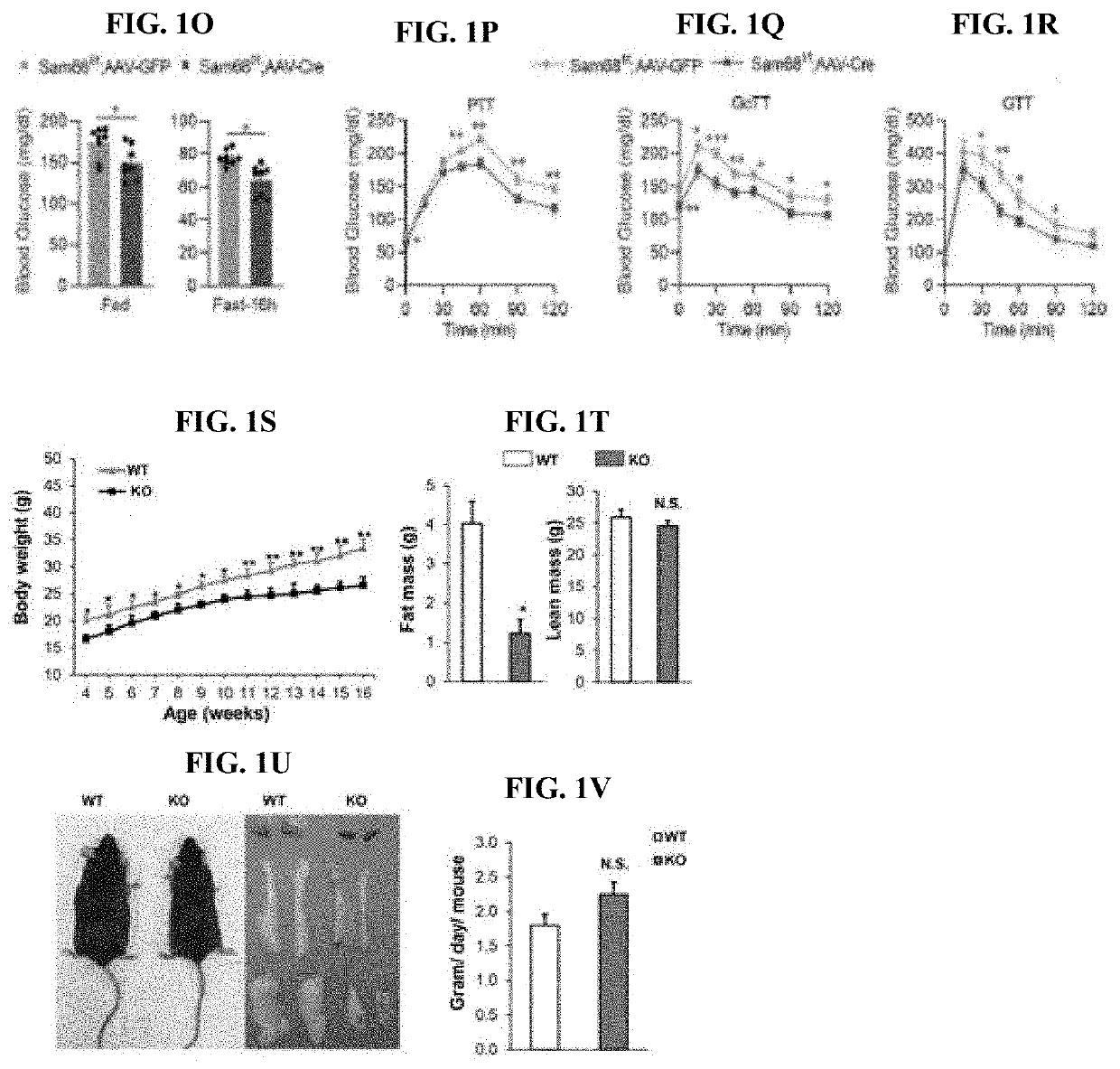
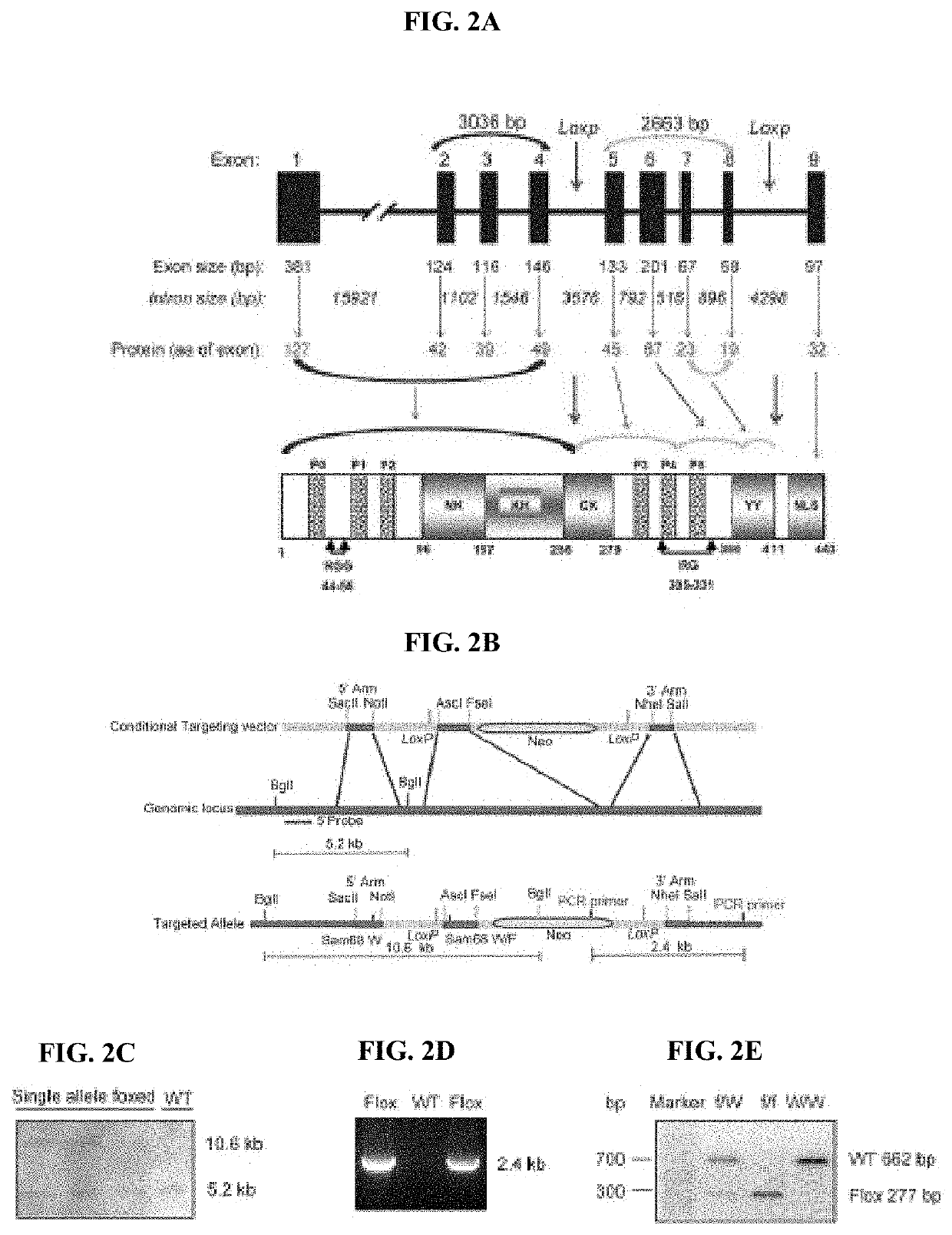
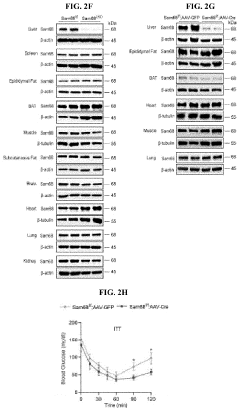
Compositions and methods for treatment of diabetes, obesity, hyper-cholesterolemia, and atherosclerosis by inhibition of sam68
PatentInactiveUS20220119511A1
Innovation
- Targeting Sam68, an RNA-binding adaptor protein, through inhibitors such as small molecules, peptides, antibodies, or RNA molecules like shRNA and siRNA to reduce hepatic gluconeogenesis by decreasing CRTC2 protein stability and gluconeogenic gene transcription, thereby lowering blood glucose levels and improving insulin sensitivity.
N-(indole-2-carbonyl) and H-thieno[2,3-b]pyrrole-5-carboxamide anti-diabetic agents
PatentInactiveUS6992101B2
Innovation
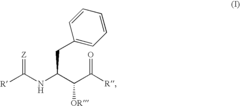
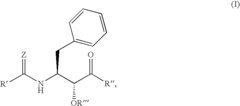
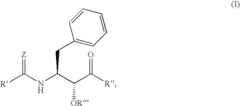
- Development of specific substituted N-(indole-2-carbonyl)amides and 6H-thieno[2,3-b]pyrrole-5-carboxamides and their prodrugs, which act as glycogen phosphorylase inhibitors, to treat diabetes, insulin resistance, diabetic complications, hypertension, and cardiovascular issues by regulating glycogenolysis and insulin levels.
Clinical Applications and Therapeutic Potential
Glycogenolysis has emerged as a promising therapeutic target in various clinical settings, particularly in conditions characterized by hormonal imbalances. The manipulation of glycogen breakdown pathways offers novel approaches for treating metabolic disorders, diabetes, and hormonal dysregulation syndromes. Current clinical applications include the use of glucagon analogues in emergency hypoglycemia management, which directly stimulates glycogenolysis to rapidly increase blood glucose levels.
In reproductive medicine, understanding glycogenolysis has led to innovative treatments for polycystic ovary syndrome (PCOS), where insulin resistance and subsequent abnormal glycogen metabolism contribute to hormonal imbalances. Therapeutic interventions targeting glycogen phosphorylase enzymes have shown promise in early-stage clinical trials, with improvements in insulin sensitivity and normalization of androgen levels in affected patients.
The field of sports medicine has also benefited from glycogenolysis research, with protocols developed to optimize athletic performance through strategic manipulation of glycogen stores and breakdown rates. These approaches have demonstrated effectiveness in enhancing endurance and recovery while maintaining hormonal homeostasis during intense physical activity.
Emerging therapeutic applications include novel pharmaceutical agents that modulate glycogenolysis to address cortisol dysregulation in stress-related disorders. Preclinical studies indicate that controlled inhibition of hepatic glycogenolysis can attenuate excessive cortisol production, potentially offering new treatment options for anxiety disorders and adrenal insufficiency.
Pediatric endocrinology represents another frontier, with glycogenolysis-targeted therapies showing potential in managing growth hormone abnormalities and puberty disorders. Clinical trials are currently evaluating the safety and efficacy of glycogen phosphorylase modulators in children with precocious puberty, with preliminary results suggesting beneficial effects on hormonal profiles without significant adverse events.
The therapeutic potential extends to aging-related hormonal changes, where declining metabolic efficiency contributes to insulin resistance and hormonal imbalances. Compounds that enhance glycogenolysis efficiency in older adults have demonstrated improvements in glucose tolerance and thyroid hormone function in phase II clinical trials, potentially offering interventions for age-related metabolic decline.
Future directions include the development of tissue-specific glycogenolysis modulators that can target particular hormonal pathways without disrupting overall metabolic homeostasis. This precision medicine approach could revolutionize treatment options for complex endocrine disorders while minimizing systemic side effects.
In reproductive medicine, understanding glycogenolysis has led to innovative treatments for polycystic ovary syndrome (PCOS), where insulin resistance and subsequent abnormal glycogen metabolism contribute to hormonal imbalances. Therapeutic interventions targeting glycogen phosphorylase enzymes have shown promise in early-stage clinical trials, with improvements in insulin sensitivity and normalization of androgen levels in affected patients.
The field of sports medicine has also benefited from glycogenolysis research, with protocols developed to optimize athletic performance through strategic manipulation of glycogen stores and breakdown rates. These approaches have demonstrated effectiveness in enhancing endurance and recovery while maintaining hormonal homeostasis during intense physical activity.
Emerging therapeutic applications include novel pharmaceutical agents that modulate glycogenolysis to address cortisol dysregulation in stress-related disorders. Preclinical studies indicate that controlled inhibition of hepatic glycogenolysis can attenuate excessive cortisol production, potentially offering new treatment options for anxiety disorders and adrenal insufficiency.
Pediatric endocrinology represents another frontier, with glycogenolysis-targeted therapies showing potential in managing growth hormone abnormalities and puberty disorders. Clinical trials are currently evaluating the safety and efficacy of glycogen phosphorylase modulators in children with precocious puberty, with preliminary results suggesting beneficial effects on hormonal profiles without significant adverse events.
The therapeutic potential extends to aging-related hormonal changes, where declining metabolic efficiency contributes to insulin resistance and hormonal imbalances. Compounds that enhance glycogenolysis efficiency in older adults have demonstrated improvements in glucose tolerance and thyroid hormone function in phase II clinical trials, potentially offering interventions for age-related metabolic decline.
Future directions include the development of tissue-specific glycogenolysis modulators that can target particular hormonal pathways without disrupting overall metabolic homeostasis. This precision medicine approach could revolutionize treatment options for complex endocrine disorders while minimizing systemic side effects.
Regulatory Framework for Metabolic Intervention Therapies
The regulatory landscape for metabolic intervention therapies focusing on glycogenolysis has evolved significantly over the past decade, reflecting growing understanding of the critical role these pathways play in hormonal balance. Current regulatory frameworks across major jurisdictions require comprehensive safety and efficacy data specifically addressing the unique challenges of manipulating metabolic processes that impact multiple hormonal systems simultaneously.
In the United States, the FDA has established specialized guidelines for metabolic modulating therapies through its Metabolic and Endocrinologic Products Division, requiring extended clinical trials that monitor hormonal markers beyond primary endpoints. These guidelines specifically address glycogenolysis-targeting compounds, mandating assessment of downstream effects on insulin, glucagon, cortisol, and catecholamine levels throughout all trial phases.
The European Medicines Agency has implemented the Metabolic Balance Assessment Protocol (MBAP), which requires manufacturers to demonstrate minimal disruption to interconnected hormonal systems when targeting glycogenolysis pathways. This protocol emphasizes long-term safety monitoring with particular attention to adrenal function and thyroid hormone regulation, recognizing the complex relationship between glucose metabolism and endocrine function.
Regulatory bodies in Asia, particularly Japan's PMDA, have pioneered requirements for population-specific metabolic profiling before approval of therapies affecting glycogenolysis, acknowledging ethnic variations in metabolic response patterns. Their framework includes mandatory post-market surveillance focusing on hormonal equilibrium maintenance across diverse patient populations.
Global harmonization efforts through the International Council for Harmonisation (ICH) have recently produced the M13 guideline specifically addressing metabolic intervention therapies, with dedicated sections on glycogenolysis modulation and hormonal impact assessment. This represents the first international consensus on regulatory approaches to metabolic therapies with hormonal implications.
Reimbursement frameworks have also evolved to accommodate these therapies, with health technology assessment bodies now requiring evidence of hormonal stabilization alongside traditional efficacy metrics. The UK's NICE has developed specific evaluation criteria for metabolic interventions that consider hormonal balance as a key outcome measure affecting quality of life and long-term health economics.
Emerging regulatory trends indicate movement toward adaptive licensing pathways for metabolic therapies, allowing earlier patient access while gathering real-world evidence on hormonal effects. This approach recognizes both the urgent need for effective metabolic interventions and the complexity of fully characterizing their hormonal impact during conventional development timelines.
In the United States, the FDA has established specialized guidelines for metabolic modulating therapies through its Metabolic and Endocrinologic Products Division, requiring extended clinical trials that monitor hormonal markers beyond primary endpoints. These guidelines specifically address glycogenolysis-targeting compounds, mandating assessment of downstream effects on insulin, glucagon, cortisol, and catecholamine levels throughout all trial phases.
The European Medicines Agency has implemented the Metabolic Balance Assessment Protocol (MBAP), which requires manufacturers to demonstrate minimal disruption to interconnected hormonal systems when targeting glycogenolysis pathways. This protocol emphasizes long-term safety monitoring with particular attention to adrenal function and thyroid hormone regulation, recognizing the complex relationship between glucose metabolism and endocrine function.
Regulatory bodies in Asia, particularly Japan's PMDA, have pioneered requirements for population-specific metabolic profiling before approval of therapies affecting glycogenolysis, acknowledging ethnic variations in metabolic response patterns. Their framework includes mandatory post-market surveillance focusing on hormonal equilibrium maintenance across diverse patient populations.
Global harmonization efforts through the International Council for Harmonisation (ICH) have recently produced the M13 guideline specifically addressing metabolic intervention therapies, with dedicated sections on glycogenolysis modulation and hormonal impact assessment. This represents the first international consensus on regulatory approaches to metabolic therapies with hormonal implications.
Reimbursement frameworks have also evolved to accommodate these therapies, with health technology assessment bodies now requiring evidence of hormonal stabilization alongside traditional efficacy metrics. The UK's NICE has developed specific evaluation criteria for metabolic interventions that consider hormonal balance as a key outcome measure affecting quality of life and long-term health economics.
Emerging regulatory trends indicate movement toward adaptive licensing pathways for metabolic therapies, allowing earlier patient access while gathering real-world evidence on hormonal effects. This approach recognizes both the urgent need for effective metabolic interventions and the complexity of fully characterizing their hormonal impact during conventional development timelines.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!