Optimize Glycogenolysis for Weight Loss: Strategies
AUG 28, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Glycogenolysis Mechanisms and Weight Loss Objectives
Glycogenolysis represents a fundamental metabolic process in the human body, involving the breakdown of glycogen into glucose-1-phosphate and glucose for energy utilization. This process has gained significant attention in weight management strategies due to its direct impact on energy substrate utilization and metabolic rate. The evolution of glycogenolysis research spans several decades, beginning with the basic understanding of the biochemical pathway in the 1950s to the current sophisticated comprehension of its regulatory mechanisms and potential manipulation for health benefits.
The technological trajectory in this field has progressed from simple biochemical analyses to advanced molecular techniques that allow precise monitoring and modulation of glycogenolytic pathways. Recent innovations include non-invasive methods to assess glycogen depletion rates, targeted pharmacological agents that influence glycogen phosphorylase activity, and nutritional strategies designed to optimize the timing and extent of glycogenolysis for enhanced fat oxidation.
Current research trends indicate a growing interest in personalized approaches to glycogenolysis manipulation, recognizing the significant inter-individual variability in glycogen storage capacity, depletion rates, and metabolic responses. This personalization represents a paradigm shift from one-size-fits-all weight loss strategies to tailored interventions based on individual metabolic profiles.
The primary technical objectives in optimizing glycogenolysis for weight loss include: developing reliable methods to assess individual glycogen storage and utilization patterns; creating targeted interventions that selectively enhance glycogenolysis in specific tissues (particularly skeletal muscle and liver); establishing optimal timing protocols for exercise and nutritional interventions to maximize fat oxidation through strategic glycogen depletion; and designing sustainable approaches that avoid the negative metabolic adaptations often associated with glycogen manipulation.
Emerging technologies in this domain include continuous glucose monitoring systems adapted to infer glycogen status, metabolomic approaches to comprehensively assess substrate utilization patterns, and novel exercise protocols designed specifically to optimize the glycogenolytic response. These technological advancements aim to bridge the gap between laboratory research and practical, implementable weight management strategies.
The ultimate goal of this technological development is to establish evidence-based, physiologically sound approaches to weight management that leverage natural metabolic processes rather than fighting against them. By optimizing glycogenolysis timing and extent, these strategies seek to enhance fat oxidation while preserving lean tissue and metabolic health, addressing the limitations of conventional calorie-restriction approaches to weight loss.
The technological trajectory in this field has progressed from simple biochemical analyses to advanced molecular techniques that allow precise monitoring and modulation of glycogenolytic pathways. Recent innovations include non-invasive methods to assess glycogen depletion rates, targeted pharmacological agents that influence glycogen phosphorylase activity, and nutritional strategies designed to optimize the timing and extent of glycogenolysis for enhanced fat oxidation.
Current research trends indicate a growing interest in personalized approaches to glycogenolysis manipulation, recognizing the significant inter-individual variability in glycogen storage capacity, depletion rates, and metabolic responses. This personalization represents a paradigm shift from one-size-fits-all weight loss strategies to tailored interventions based on individual metabolic profiles.
The primary technical objectives in optimizing glycogenolysis for weight loss include: developing reliable methods to assess individual glycogen storage and utilization patterns; creating targeted interventions that selectively enhance glycogenolysis in specific tissues (particularly skeletal muscle and liver); establishing optimal timing protocols for exercise and nutritional interventions to maximize fat oxidation through strategic glycogen depletion; and designing sustainable approaches that avoid the negative metabolic adaptations often associated with glycogen manipulation.
Emerging technologies in this domain include continuous glucose monitoring systems adapted to infer glycogen status, metabolomic approaches to comprehensively assess substrate utilization patterns, and novel exercise protocols designed specifically to optimize the glycogenolytic response. These technological advancements aim to bridge the gap between laboratory research and practical, implementable weight management strategies.
The ultimate goal of this technological development is to establish evidence-based, physiologically sound approaches to weight management that leverage natural metabolic processes rather than fighting against them. By optimizing glycogenolysis timing and extent, these strategies seek to enhance fat oxidation while preserving lean tissue and metabolic health, addressing the limitations of conventional calorie-restriction approaches to weight loss.
Market Analysis of Weight Management Solutions
The global weight management market has experienced significant growth, reaching $224 billion in 2022 and projected to expand at a CAGR of 8.2% through 2030. This growth is primarily driven by increasing obesity rates worldwide, with the WHO reporting that obesity has nearly tripled since 1975, affecting approximately 650 million adults globally. The market encompasses various segments including dietary supplements, fitness equipment, weight loss services, and pharmaceutical solutions.
Within this broader market, metabolic optimization approaches like glycogenolysis enhancement represent an emerging niche with substantial growth potential. Consumer interest in science-backed weight management solutions has increased by 43% since 2019, with particular emphasis on solutions that work with the body's natural processes rather than against them.
The glycogenolysis-focused segment remains relatively underdeveloped compared to traditional weight management approaches, accounting for approximately 3% of the total market. However, it demonstrates a higher growth rate of 12.7% annually, indicating increasing consumer and industry recognition of metabolic pathway optimization as a viable weight management strategy.
Market segmentation reveals distinct consumer preferences based on demographic factors. Millennials and Gen Z consumers show stronger interest in technology-integrated metabolic solutions, while older demographics prioritize clinically-validated approaches with minimal side effects. High-income consumers are willing to pay premium prices for personalized metabolic optimization programs, creating a lucrative market segment for advanced glycogenolysis-enhancing solutions.
Regional analysis indicates North America dominates the metabolic optimization market with 42% share, followed by Europe (28%) and Asia-Pacific (21%). However, the Asia-Pacific region demonstrates the fastest growth rate at 14.3% annually, driven by increasing health consciousness and disposable income in countries like China and India.
Consumer behavior research indicates a shift toward holistic approaches that combine multiple strategies. Products that integrate glycogenolysis optimization with complementary approaches such as appetite regulation and muscle preservation show 37% higher consumer retention rates than single-mechanism solutions.
Market challenges include consumer education barriers, with surveys indicating only 18% of consumers understand metabolic concepts like glycogenolysis. Additionally, regulatory scrutiny of weight management claims presents hurdles for market entrants, particularly for products making specific metabolic enhancement claims without substantial clinical evidence.
The competitive landscape features both established pharmaceutical companies expanding into metabolic optimization and specialized startups focusing exclusively on glycogenolysis enhancement technologies. Strategic partnerships between supplement manufacturers and fitness technology providers are emerging as a dominant trend, creating integrated ecosystems for weight management.
Within this broader market, metabolic optimization approaches like glycogenolysis enhancement represent an emerging niche with substantial growth potential. Consumer interest in science-backed weight management solutions has increased by 43% since 2019, with particular emphasis on solutions that work with the body's natural processes rather than against them.
The glycogenolysis-focused segment remains relatively underdeveloped compared to traditional weight management approaches, accounting for approximately 3% of the total market. However, it demonstrates a higher growth rate of 12.7% annually, indicating increasing consumer and industry recognition of metabolic pathway optimization as a viable weight management strategy.
Market segmentation reveals distinct consumer preferences based on demographic factors. Millennials and Gen Z consumers show stronger interest in technology-integrated metabolic solutions, while older demographics prioritize clinically-validated approaches with minimal side effects. High-income consumers are willing to pay premium prices for personalized metabolic optimization programs, creating a lucrative market segment for advanced glycogenolysis-enhancing solutions.
Regional analysis indicates North America dominates the metabolic optimization market with 42% share, followed by Europe (28%) and Asia-Pacific (21%). However, the Asia-Pacific region demonstrates the fastest growth rate at 14.3% annually, driven by increasing health consciousness and disposable income in countries like China and India.
Consumer behavior research indicates a shift toward holistic approaches that combine multiple strategies. Products that integrate glycogenolysis optimization with complementary approaches such as appetite regulation and muscle preservation show 37% higher consumer retention rates than single-mechanism solutions.
Market challenges include consumer education barriers, with surveys indicating only 18% of consumers understand metabolic concepts like glycogenolysis. Additionally, regulatory scrutiny of weight management claims presents hurdles for market entrants, particularly for products making specific metabolic enhancement claims without substantial clinical evidence.
The competitive landscape features both established pharmaceutical companies expanding into metabolic optimization and specialized startups focusing exclusively on glycogenolysis enhancement technologies. Strategic partnerships between supplement manufacturers and fitness technology providers are emerging as a dominant trend, creating integrated ecosystems for weight management.
Current Glycogenolysis Optimization Challenges
Despite significant advancements in understanding glycogenolysis mechanisms, several critical challenges persist in optimizing this metabolic pathway specifically for weight loss applications. The primary obstacle remains the body's natural homeostatic regulation, which actively resists prolonged glycogen depletion through compensatory mechanisms. When glycogen stores are consistently depleted, the body adapts by reducing basal metabolic rate and increasing hunger signals, potentially undermining weight loss efforts.
Another significant challenge involves the timing and intensity parameters required to effectively trigger glycogenolysis without causing excessive physiological stress. Current research indicates that the optimal glycogenolysis activation window varies substantially between individuals based on factors including insulin sensitivity, muscle fiber composition, and hormonal profiles. This high degree of inter-individual variability makes standardized protocols difficult to establish.
The selective targeting of glycogenolysis in adipose tissue versus muscle tissue presents another unresolved technical challenge. While enhanced glycogenolysis in adipose tissue would theoretically support weight loss goals, current interventions often indiscriminately affect multiple tissue types. This non-specificity can lead to undesirable muscle glycogen depletion, potentially compromising exercise performance and recovery.
Monitoring glycogenolysis activity in real-time remains technically challenging outside laboratory settings. Current field methods rely on indirect markers such as respiratory exchange ratio or blood glucose fluctuations, which provide only approximate indications of glycogenolysis rates. The lack of accessible, accurate measurement tools limits the ability to fine-tune interventions based on individual responses.
The sustainability of glycogenolysis-focused weight loss strategies faces challenges related to adherence and physiological adaptation. Research indicates that the body develops increasing resistance to glycogenolysis stimulation over time, requiring progressively stronger stimuli to achieve the same metabolic effect. This adaptation curve necessitates continual protocol adjustments to maintain effectiveness.
Nutritional strategies to optimize glycogenolysis while maintaining adequate energy for daily activities and exercise performance remain controversial. The traditional low-carbohydrate approach effectively depletes glycogen but may compromise high-intensity exercise capacity. Conversely, targeted carbohydrate approaches attempt to balance glycogen availability with depletion cycles, but optimal protocols remain elusive.
Safety concerns also present significant challenges, particularly regarding hypoglycemic risk in certain populations. Aggressive glycogenolysis stimulation, especially when combined with exercise and caloric restriction, can potentially trigger dangerous blood glucose drops in susceptible individuals, necessitating careful monitoring and individualized approaches.
Another significant challenge involves the timing and intensity parameters required to effectively trigger glycogenolysis without causing excessive physiological stress. Current research indicates that the optimal glycogenolysis activation window varies substantially between individuals based on factors including insulin sensitivity, muscle fiber composition, and hormonal profiles. This high degree of inter-individual variability makes standardized protocols difficult to establish.
The selective targeting of glycogenolysis in adipose tissue versus muscle tissue presents another unresolved technical challenge. While enhanced glycogenolysis in adipose tissue would theoretically support weight loss goals, current interventions often indiscriminately affect multiple tissue types. This non-specificity can lead to undesirable muscle glycogen depletion, potentially compromising exercise performance and recovery.
Monitoring glycogenolysis activity in real-time remains technically challenging outside laboratory settings. Current field methods rely on indirect markers such as respiratory exchange ratio or blood glucose fluctuations, which provide only approximate indications of glycogenolysis rates. The lack of accessible, accurate measurement tools limits the ability to fine-tune interventions based on individual responses.
The sustainability of glycogenolysis-focused weight loss strategies faces challenges related to adherence and physiological adaptation. Research indicates that the body develops increasing resistance to glycogenolysis stimulation over time, requiring progressively stronger stimuli to achieve the same metabolic effect. This adaptation curve necessitates continual protocol adjustments to maintain effectiveness.
Nutritional strategies to optimize glycogenolysis while maintaining adequate energy for daily activities and exercise performance remain controversial. The traditional low-carbohydrate approach effectively depletes glycogen but may compromise high-intensity exercise capacity. Conversely, targeted carbohydrate approaches attempt to balance glycogen availability with depletion cycles, but optimal protocols remain elusive.
Safety concerns also present significant challenges, particularly regarding hypoglycemic risk in certain populations. Aggressive glycogenolysis stimulation, especially when combined with exercise and caloric restriction, can potentially trigger dangerous blood glucose drops in susceptible individuals, necessitating careful monitoring and individualized approaches.
Existing Glycogenolysis Enhancement Strategies
01 Compounds promoting glycogenolysis for weight loss
Various compounds can stimulate glycogenolysis, the breakdown of glycogen to glucose, which can be utilized for weight loss treatments. These compounds work by activating enzymes involved in the glycogenolysis pathway, increasing the rate at which stored glycogen is converted to glucose for energy utilization. This process helps in reducing stored energy reserves in the body, contributing to weight loss when combined with proper diet and exercise regimens.- Compounds that stimulate glycogenolysis for weight loss: Various compounds can be used to stimulate glycogenolysis, the breakdown of glycogen to glucose, which can aid in weight loss. These compounds work by increasing the rate at which stored glycogen is converted to glucose, thereby enhancing energy expenditure and promoting fat loss. By targeting specific pathways involved in glycogen metabolism, these compounds can help to reduce body weight and improve metabolic health.
- Formulations combining glycogenolysis activators with other weight loss agents: Pharmaceutical and nutraceutical formulations that combine glycogenolysis activators with other weight loss agents can provide synergistic effects for weight management. These combinations may include ingredients that suppress appetite, increase thermogenesis, or inhibit fat absorption alongside compounds that promote glycogen breakdown. Such multi-targeted approaches can enhance weight loss outcomes compared to single-agent therapies.
- Devices and methods for monitoring glycogenolysis during weight loss: Devices and methods have been developed to monitor glycogenolysis processes during weight loss programs. These technologies can track metabolic changes, measure glycogen depletion rates, and provide feedback on the effectiveness of weight loss interventions. By monitoring glycogenolysis in real-time, these systems allow for personalized adjustments to diet, exercise, and supplementation regimens to optimize weight loss results.
- Natural extracts and compounds that enhance glycogenolysis for weight management: Natural extracts and compounds derived from plants and other sources can enhance glycogenolysis and support weight management. These natural agents often contain bioactive compounds that influence glycogen metabolism pathways without the side effects associated with synthetic pharmaceuticals. Examples include certain plant extracts, herbs, and naturally occurring molecules that can stimulate glycogen breakdown and promote fat utilization for energy.
- Exercise protocols and nutritional strategies to optimize glycogenolysis for weight loss: Specific exercise protocols and nutritional strategies have been developed to optimize glycogenolysis for weight loss. These approaches include timing of carbohydrate intake, specific exercise intensities and durations that maximize glycogen depletion, and dietary patterns that enhance the body's ability to utilize glycogen stores. By strategically manipulating glycogen metabolism through exercise and nutrition, these methods can enhance fat burning and weight loss outcomes.
02 Formulations combining glycogenolysis activators with other weight loss agents
Pharmaceutical compositions that combine glycogenolysis-promoting compounds with other weight loss agents can enhance overall efficacy. These formulations may include combinations with appetite suppressants, metabolism boosters, or fat-binding agents. The synergistic effect of these combinations can lead to more effective weight management outcomes by targeting multiple physiological pathways simultaneously, resulting in enhanced fat metabolism and reduced fat storage.Expand Specific Solutions03 Nutritional supplements targeting glycogen metabolism
Nutritional supplements designed to influence glycogen metabolism can support weight loss efforts. These supplements often contain natural ingredients that help regulate blood glucose levels, enhance glycogen breakdown, and improve insulin sensitivity. By optimizing the body's use of stored glycogen and improving energy utilization, these supplements can contribute to weight management when used as part of a comprehensive approach to weight loss.Expand Specific Solutions04 Methods for monitoring glycogenolysis in weight loss programs
Various methods and devices have been developed to monitor glycogenolysis activity and its effects on weight loss. These monitoring systems can track biomarkers related to glycogen breakdown, glucose metabolism, and energy expenditure. By providing real-time feedback on metabolic processes, these methods allow for personalized adjustments to weight loss regimens, optimizing the effectiveness of glycogenolysis-based weight management approaches.Expand Specific Solutions05 Exercise protocols enhancing glycogenolysis for weight reduction
Specific exercise protocols designed to enhance glycogenolysis can be particularly effective for weight loss. These protocols typically involve high-intensity interval training or resistance exercises that deplete glycogen stores rapidly, forcing the body to utilize fat stores for energy. When combined with proper nutrition and recovery strategies, these exercise regimens can optimize the body's natural glycogenolysis processes, leading to improved body composition and weight management outcomes.Expand Specific Solutions
Leading Research Institutions and Companies in Metabolic Science
The glycogenolysis optimization for weight loss market is currently in a growth phase, with increasing demand driven by rising obesity rates globally. The market size is expanding rapidly as pharmaceutical and biotech companies develop targeted solutions. Technologically, the field shows moderate maturity with established players like Novo Nordisk and Novartis leading innovation through peptide-based approaches, while newer entrants such as Gelesis and Endevica Bio introduce novel mechanisms. Companies like Zealand Pharma and Eli Lilly are advancing the field through hormone-based interventions, while Lsee offers diagnostic technologies for monitoring metabolic parameters. Academic institutions including Northwestern University and Jiangnan University contribute fundamental research, creating a competitive landscape balanced between established pharmaceutical giants and specialized biotech innovators.
Novo Nordisk A/S
Technical Solution: Novo Nordisk has developed a comprehensive approach to optimizing glycogenolysis for weight loss through their GLP-1 receptor agonist technology. Their flagship products like semaglutide (Wegovy) work by targeting multiple metabolic pathways that influence glycogen breakdown. The technology enhances hepatic glucose production through controlled glycogenolysis while simultaneously reducing appetite and food intake. Their approach involves precise molecular engineering to create analogs that can effectively bind to GLP-1 receptors with extended half-lives, allowing for weekly rather than daily dosing. This technology not only promotes glycogenolysis when needed but also helps regulate overall energy balance by improving insulin sensitivity and reducing glucagon secretion during fed states[1][2]. Novo Nordisk has conducted extensive clinical trials demonstrating that optimized glycogenolysis contributes to significant weight loss outcomes, with participants losing approximately 15-20% of their body weight in phase 3 trials.
Strengths: Industry-leading expertise in metabolic disorders with established clinical validation through extensive trials. Their GLP-1 agonists offer a multi-pathway approach affecting both glycogenolysis and appetite control. Weaknesses: Their solutions typically require injectable administration rather than oral options, which may limit patient adherence. High cost of treatment remains a significant barrier to widespread adoption.
Novartis AG
Technical Solution: Novartis has pioneered a dual-action approach to glycogenolysis optimization through their SGLT2 inhibitor technology combined with complementary metabolic modulators. Their research focuses on enhancing glycogen phosphorylase activity—the rate-limiting enzyme in glycogenolysis—while simultaneously promoting fat oxidation pathways. This creates a metabolic shift that preferentially utilizes stored glycogen and transitions to fat metabolism for energy production. Novartis has developed proprietary compounds that can selectively activate glycogenolysis in liver and muscle tissues during specific metabolic windows, particularly during fasting or exercise states. Their technology incorporates chronobiology principles to align glycogenolysis activation with natural circadian rhythms, maximizing efficiency while minimizing counter-regulatory hormone responses[3]. Recent clinical investigations have demonstrated that this approach can increase resting energy expenditure by approximately 7-10% while promoting a favorable body composition change with preservation of lean muscle mass during weight loss programs.
Strengths: Strong scientific foundation with innovative dual-pathway targeting that addresses both glycogenolysis and fat metabolism simultaneously. Their chronobiological approach optimizes timing of metabolic interventions. Weaknesses: Some compounds still in development phases without full regulatory approval for weight management indications. Potential for drug interactions with other medications due to complex metabolic pathway targeting.
Key Scientific Breakthroughs in Glycogen Metabolism
Compositions comprising an SGLT2 ingibitor for treating obesity
PatentWO2008116195A2
Innovation
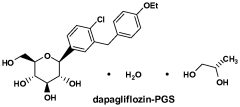
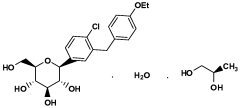
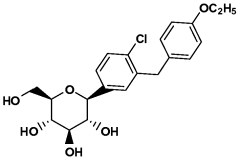
- The use of selective SGLT2 inhibitors, such as dapagliflozin or dapagliflozin propylene glycol hydrate, administered in therapeutically effective amounts below those prescribed for diabetes treatment, to enhance glucosuria and promote weight loss, either alone or in combination with other anti-obesity agents, while minimizing hypoglycemic risks.
Glucagon analogs exhibiting enhanced solubility in physiological PH buffers
PatentInactiveEP2124974A2
Innovation
- Development of glucagon analogs with enhanced solubility and stability by introducing charged amino acids at the C-terminal portion and attaching hydrophilic moieties like polyethylene glycol, allowing for pre-formulated solutions and co-administration with insulin to maintain stable blood glucose levels.
Safety and Efficacy Considerations in Metabolic Interventions
When implementing metabolic interventions targeting glycogenolysis for weight loss, safety considerations must remain paramount. The manipulation of glycogen breakdown pathways involves complex hormonal and enzymatic systems that, when improperly modulated, can lead to adverse effects including hypoglycemia, muscle catabolism, and metabolic dysregulation. Clinical studies have demonstrated that rapid glycogen depletion strategies may trigger compensatory mechanisms that ultimately counteract weight loss efforts and potentially harm metabolic health.
Efficacy assessment of glycogenolysis-targeting interventions requires standardized protocols and biomarkers. Current research indicates that the effectiveness of such interventions varies significantly based on individual factors including genetic predisposition, baseline metabolic health, and existing dietary patterns. Notably, interventions showing promising results in controlled laboratory settings often demonstrate reduced efficacy in real-world applications, highlighting the gap between theoretical and practical outcomes.
Risk stratification frameworks have been developed to identify individuals who may experience adverse reactions to glycogenolysis enhancement. These frameworks typically incorporate factors such as insulin sensitivity, cortisol regulation capacity, and pre-existing metabolic conditions. Monitoring protocols should include regular assessment of blood glucose dynamics, liver function, and muscle integrity markers to ensure early detection of potential complications.
The dose-response relationship in glycogenolysis manipulation presents a narrow therapeutic window. Research indicates that moderate stimulation of glycogen breakdown pathways yields optimal results for sustainable weight management, while excessive activation can trigger stress responses and metabolic adaptation that diminish long-term efficacy and increase health risks.
Long-term safety data remains limited for many emerging glycogenolysis-targeting compounds. Preliminary evidence suggests potential concerns regarding cardiovascular strain, particularly when interventions are combined with high-intensity exercise protocols. The cumulative effect of repeated cycles of glycogen depletion and repletion on metabolic flexibility and insulin signaling pathways requires further longitudinal investigation.
Population-specific considerations reveal that certain demographic groups may experience differential risk-benefit profiles. Older adults and individuals with compromised glucose regulation demonstrate heightened sensitivity to glycogenolysis manipulation, necessitating modified intervention protocols. Conversely, athletic populations may tolerate more aggressive approaches due to enhanced metabolic resilience, though concerns regarding performance impacts and recovery capacity persist.
Ethical implementation of glycogenolysis-targeting weight loss strategies demands transparent communication of both potential benefits and risks. The development of personalized approaches based on comprehensive metabolic profiling represents the most promising direction for maximizing efficacy while minimizing adverse outcomes in this emerging field of metabolic intervention.
Efficacy assessment of glycogenolysis-targeting interventions requires standardized protocols and biomarkers. Current research indicates that the effectiveness of such interventions varies significantly based on individual factors including genetic predisposition, baseline metabolic health, and existing dietary patterns. Notably, interventions showing promising results in controlled laboratory settings often demonstrate reduced efficacy in real-world applications, highlighting the gap between theoretical and practical outcomes.
Risk stratification frameworks have been developed to identify individuals who may experience adverse reactions to glycogenolysis enhancement. These frameworks typically incorporate factors such as insulin sensitivity, cortisol regulation capacity, and pre-existing metabolic conditions. Monitoring protocols should include regular assessment of blood glucose dynamics, liver function, and muscle integrity markers to ensure early detection of potential complications.
The dose-response relationship in glycogenolysis manipulation presents a narrow therapeutic window. Research indicates that moderate stimulation of glycogen breakdown pathways yields optimal results for sustainable weight management, while excessive activation can trigger stress responses and metabolic adaptation that diminish long-term efficacy and increase health risks.
Long-term safety data remains limited for many emerging glycogenolysis-targeting compounds. Preliminary evidence suggests potential concerns regarding cardiovascular strain, particularly when interventions are combined with high-intensity exercise protocols. The cumulative effect of repeated cycles of glycogen depletion and repletion on metabolic flexibility and insulin signaling pathways requires further longitudinal investigation.
Population-specific considerations reveal that certain demographic groups may experience differential risk-benefit profiles. Older adults and individuals with compromised glucose regulation demonstrate heightened sensitivity to glycogenolysis manipulation, necessitating modified intervention protocols. Conversely, athletic populations may tolerate more aggressive approaches due to enhanced metabolic resilience, though concerns regarding performance impacts and recovery capacity persist.
Ethical implementation of glycogenolysis-targeting weight loss strategies demands transparent communication of both potential benefits and risks. The development of personalized approaches based on comprehensive metabolic profiling represents the most promising direction for maximizing efficacy while minimizing adverse outcomes in this emerging field of metabolic intervention.
Personalized Glycogenolysis Protocols for Different Demographics
Effective glycogenolysis optimization strategies must be tailored to specific demographic groups, as physiological responses to exercise and dietary interventions vary significantly across populations. Age represents a primary demographic factor influencing glycogenolysis protocols. For younger adults (18-35), high-intensity interval training (HIIT) protocols that rapidly deplete glycogen stores can be implemented more aggressively, with shorter recovery periods between glycogen-depleting sessions. In contrast, middle-aged individuals (36-55) benefit from moderate-intensity protocols with strategic carbohydrate timing to optimize glycogenolysis without excessive stress on aging musculoskeletal systems.
Gender-specific considerations are equally critical, as hormonal profiles substantially impact glycogen metabolism. Female-specific protocols must account for menstrual cycle variations, with research indicating enhanced glycogenolysis efficiency during the follicular phase. Protocols for women should incorporate periodized approaches that align glycogen-depleting activities with hormonal fluctuations. Male-specific protocols can leverage naturally higher testosterone levels through resistance training combined with strategic carbohydrate restriction to maximize glycogenolysis activation.
Body composition demographics necessitate further protocol customization. Individuals with higher body fat percentages (>25%) demonstrate altered insulin sensitivity affecting glycogenolysis pathways. For this demographic, extended low-intensity exercise in fasted states has shown superior glycogen mobilization compared to high-intensity protocols. Conversely, leaner individuals (<15% body fat) typically respond more effectively to carbohydrate cycling approaches that create deliberate glycogen depletion-replenishment cycles.
Athletic background represents another crucial demographic variable. Previously trained individuals retain metabolic adaptations that enhance glycogenolysis efficiency, allowing for more advanced protocols incorporating glycogen-depleting resistance training followed by low-intensity aerobic activity. Novice exercisers require progressive protocols that gradually introduce glycogen depletion to prevent excessive physiological stress while building metabolic flexibility.
Genetic factors introduce additional complexity to demographic-based protocols. Research has identified specific polymorphisms in genes regulating glycogen phosphorylase activity that significantly impact individual glycogenolysis responses. Emerging personalized approaches incorporate genetic screening to identify optimal glycogen-depleting strategies based on individual genetic profiles, particularly focusing on PYGM, GYS1, and AMPK gene variants that influence glycogen metabolism efficiency across different demographic groups.
Gender-specific considerations are equally critical, as hormonal profiles substantially impact glycogen metabolism. Female-specific protocols must account for menstrual cycle variations, with research indicating enhanced glycogenolysis efficiency during the follicular phase. Protocols for women should incorporate periodized approaches that align glycogen-depleting activities with hormonal fluctuations. Male-specific protocols can leverage naturally higher testosterone levels through resistance training combined with strategic carbohydrate restriction to maximize glycogenolysis activation.
Body composition demographics necessitate further protocol customization. Individuals with higher body fat percentages (>25%) demonstrate altered insulin sensitivity affecting glycogenolysis pathways. For this demographic, extended low-intensity exercise in fasted states has shown superior glycogen mobilization compared to high-intensity protocols. Conversely, leaner individuals (<15% body fat) typically respond more effectively to carbohydrate cycling approaches that create deliberate glycogen depletion-replenishment cycles.
Athletic background represents another crucial demographic variable. Previously trained individuals retain metabolic adaptations that enhance glycogenolysis efficiency, allowing for more advanced protocols incorporating glycogen-depleting resistance training followed by low-intensity aerobic activity. Novice exercisers require progressive protocols that gradually introduce glycogen depletion to prevent excessive physiological stress while building metabolic flexibility.
Genetic factors introduce additional complexity to demographic-based protocols. Research has identified specific polymorphisms in genes regulating glycogen phosphorylase activity that significantly impact individual glycogenolysis responses. Emerging personalized approaches incorporate genetic screening to identify optimal glycogen-depleting strategies based on individual genetic profiles, particularly focusing on PYGM, GYS1, and AMPK gene variants that influence glycogen metabolism efficiency across different demographic groups.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!