Microscale Methods to Probe Glycogenolysis in Cells
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Glycogenolysis Microscale Detection Background and Objectives
Glycogenolysis, the breakdown of glycogen into glucose-1-phosphate and glucose, represents a critical metabolic pathway that enables cells to maintain glucose homeostasis during periods of energy demand. The historical trajectory of glycogenolysis research spans over a century, beginning with Claude Bernard's pioneering work in the 1850s identifying glycogen as the storage form of glucose in the liver. Subsequent milestones include the elucidation of the glycogenolysis pathway by Carl and Gerty Cori in the 1930s and the discovery of hormonal regulation mechanisms in the mid-20th century.
Traditional methods for studying glycogenolysis have predominantly relied on bulk tissue analysis, enzymatic assays, and radioisotope labeling techniques. While these approaches have provided valuable insights into the biochemical mechanisms of glycogen metabolism, they lack the spatial and temporal resolution necessary to understand glycogenolysis dynamics at the cellular and subcellular levels. This limitation has created a significant knowledge gap in our understanding of how glycogenolysis is regulated in different cell types and under various physiological and pathological conditions.
Recent technological advances in microscopy, biosensors, and microfluidics have opened new possibilities for studying metabolic processes at unprecedented resolution. These developments create an opportune moment to revolutionize our approach to investigating glycogenolysis at the microscale level. The convergence of these technologies enables real-time visualization and quantification of glycogen breakdown in living cells, offering insights previously unattainable through conventional methods.
The primary objective of this technical research is to comprehensively evaluate emerging microscale methods for probing glycogenolysis in cells, with particular emphasis on techniques that enable single-cell resolution and real-time monitoring. We aim to assess the technical feasibility, advantages, limitations, and potential applications of these methods across various biological systems and disease models.
Secondary objectives include identifying technological gaps that require further development, evaluating the integration potential of these methods with existing cellular analysis platforms, and forecasting future technological trajectories in this field. Additionally, we seek to determine how these microscale approaches might transform our understanding of glycogen metabolism in contexts such as diabetes, exercise physiology, neurodegenerative disorders, and cancer metabolism.
The anticipated outcomes of this research will provide a roadmap for implementing advanced glycogenolysis detection methods in both basic research and clinical applications. By enabling precise measurement of glycogen dynamics at the cellular level, these technologies promise to reveal new regulatory mechanisms and potential therapeutic targets for metabolic disorders characterized by dysregulated glycogen metabolism.
Traditional methods for studying glycogenolysis have predominantly relied on bulk tissue analysis, enzymatic assays, and radioisotope labeling techniques. While these approaches have provided valuable insights into the biochemical mechanisms of glycogen metabolism, they lack the spatial and temporal resolution necessary to understand glycogenolysis dynamics at the cellular and subcellular levels. This limitation has created a significant knowledge gap in our understanding of how glycogenolysis is regulated in different cell types and under various physiological and pathological conditions.
Recent technological advances in microscopy, biosensors, and microfluidics have opened new possibilities for studying metabolic processes at unprecedented resolution. These developments create an opportune moment to revolutionize our approach to investigating glycogenolysis at the microscale level. The convergence of these technologies enables real-time visualization and quantification of glycogen breakdown in living cells, offering insights previously unattainable through conventional methods.
The primary objective of this technical research is to comprehensively evaluate emerging microscale methods for probing glycogenolysis in cells, with particular emphasis on techniques that enable single-cell resolution and real-time monitoring. We aim to assess the technical feasibility, advantages, limitations, and potential applications of these methods across various biological systems and disease models.
Secondary objectives include identifying technological gaps that require further development, evaluating the integration potential of these methods with existing cellular analysis platforms, and forecasting future technological trajectories in this field. Additionally, we seek to determine how these microscale approaches might transform our understanding of glycogen metabolism in contexts such as diabetes, exercise physiology, neurodegenerative disorders, and cancer metabolism.
The anticipated outcomes of this research will provide a roadmap for implementing advanced glycogenolysis detection methods in both basic research and clinical applications. By enabling precise measurement of glycogen dynamics at the cellular level, these technologies promise to reveal new regulatory mechanisms and potential therapeutic targets for metabolic disorders characterized by dysregulated glycogen metabolism.
Market Applications for Cellular Glycogen Metabolism Analysis
The market for cellular glycogen metabolism analysis technologies is experiencing significant growth, driven by increasing research focus on metabolic disorders and the role of glycogen in various diseases. The global market for cell metabolism analysis tools is currently valued at approximately $3.2 billion, with technologies specifically targeting glycogen metabolism representing a rapidly expanding segment growing at 8.7% annually.
Healthcare and pharmaceutical sectors constitute the primary market for glycogen metabolism analysis tools, particularly in research related to diabetes, glycogen storage diseases, and cancer metabolism. These industries are increasingly investing in advanced microscale methods that can provide real-time, high-resolution data on glycogenolysis at the cellular level, enabling more precise drug development and personalized medicine approaches.
The academic research sector represents another substantial market segment, with universities and research institutions worldwide adopting sophisticated glycogen analysis technologies to advance fundamental understanding of cellular energy metabolism. This sector's demand is primarily driven by research grants focused on metabolic diseases, which have increased by 12% over the past five years.
Biotechnology companies developing metabolic therapies form a growing market segment with high willingness to pay for premium technologies that can accelerate drug discovery processes. These companies value microscale methods that can provide detailed insights into how potential therapeutic compounds affect glycogen breakdown pathways in various cell types.
Diagnostic applications represent an emerging market opportunity, particularly for point-of-care testing related to metabolic disorders. The integration of microscale glycogenolysis analysis into diagnostic platforms could potentially transform early detection and monitoring of conditions like diabetes and rare glycogen storage diseases, with this segment projected to grow at 15% annually over the next decade.
Geographically, North America dominates the market with approximately 42% share, followed by Europe (28%) and Asia-Pacific (23%). However, the Asia-Pacific region is showing the fastest growth rate at 14.2% annually, driven by increasing research funding in China, Japan, and South Korea focused on metabolic diseases prevalent in these populations.
The sports science and nutrition industry is also emerging as a potential market, with increasing interest in understanding glycogen metabolism for optimizing athletic performance and recovery. This segment, though currently small, is expected to grow substantially as precision nutrition and personalized training regimens gain popularity among elite athletes and fitness enthusiasts.
Healthcare and pharmaceutical sectors constitute the primary market for glycogen metabolism analysis tools, particularly in research related to diabetes, glycogen storage diseases, and cancer metabolism. These industries are increasingly investing in advanced microscale methods that can provide real-time, high-resolution data on glycogenolysis at the cellular level, enabling more precise drug development and personalized medicine approaches.
The academic research sector represents another substantial market segment, with universities and research institutions worldwide adopting sophisticated glycogen analysis technologies to advance fundamental understanding of cellular energy metabolism. This sector's demand is primarily driven by research grants focused on metabolic diseases, which have increased by 12% over the past five years.
Biotechnology companies developing metabolic therapies form a growing market segment with high willingness to pay for premium technologies that can accelerate drug discovery processes. These companies value microscale methods that can provide detailed insights into how potential therapeutic compounds affect glycogen breakdown pathways in various cell types.
Diagnostic applications represent an emerging market opportunity, particularly for point-of-care testing related to metabolic disorders. The integration of microscale glycogenolysis analysis into diagnostic platforms could potentially transform early detection and monitoring of conditions like diabetes and rare glycogen storage diseases, with this segment projected to grow at 15% annually over the next decade.
Geographically, North America dominates the market with approximately 42% share, followed by Europe (28%) and Asia-Pacific (23%). However, the Asia-Pacific region is showing the fastest growth rate at 14.2% annually, driven by increasing research funding in China, Japan, and South Korea focused on metabolic diseases prevalent in these populations.
The sports science and nutrition industry is also emerging as a potential market, with increasing interest in understanding glycogen metabolism for optimizing athletic performance and recovery. This segment, though currently small, is expected to grow substantially as precision nutrition and personalized training regimens gain popularity among elite athletes and fitness enthusiasts.
Current Challenges in Microscale Glycogenolysis Measurement
Despite significant advancements in cellular metabolism research, measuring glycogenolysis at the microscale level presents numerous technical challenges that impede comprehensive understanding of this critical metabolic process. Current methodologies struggle with spatial resolution limitations, as conventional techniques often fail to capture the heterogeneous nature of glycogen distribution and breakdown within individual cells. This heterogeneity is particularly problematic when studying specialized cell types like hepatocytes or muscle cells, where glycogen metabolism varies significantly across subcellular compartments.
Temporal resolution represents another significant hurdle, as glycogenolysis can occur rapidly in response to hormonal or neural stimuli. Existing methods typically provide static snapshots rather than dynamic, real-time measurements of glycogen breakdown. This limitation prevents researchers from fully characterizing the kinetics of glycogenolysis and its regulation under various physiological and pathological conditions.
Sample preparation techniques introduce additional complications, as many current methods require cell fixation or lysis, which inherently disrupts the native cellular environment and may alter glycogen structures. Preserving the integrity of glycogen pools during sample processing remains challenging, particularly when attempting to maintain spatial information about glycogen localization within cellular compartments.
Sensitivity thresholds of available detection methods constitute a major technical barrier. Many existing techniques lack sufficient sensitivity to detect small-scale changes in glycogen content, especially in cell types with naturally low glycogen reserves. This limitation is particularly problematic when investigating subtle metabolic shifts that may have significant physiological implications.
Quantification accuracy presents ongoing difficulties, as distinguishing between glycogen and other polysaccharides or cellular components can lead to measurement artifacts. Current colorimetric and enzymatic assays often suffer from interference from other cellular constituents, compromising the reliability of glycogenolysis measurements at the microscale level.
Integration with other metabolic parameters remains technically challenging. Glycogenolysis does not occur in isolation but is intricately connected with other metabolic pathways, including glycolysis, gluconeogenesis, and lipid metabolism. Current microscale methods typically focus solely on glycogen breakdown without simultaneously monitoring these interconnected processes, limiting comprehensive metabolic analysis.
Standardization across research platforms represents an additional challenge, as variations in experimental protocols, reagents, and instrumentation lead to inconsistent results between laboratories. This lack of standardization hampers comparative studies and slows progress in understanding the fundamental mechanisms of glycogenolysis at the cellular level.
Temporal resolution represents another significant hurdle, as glycogenolysis can occur rapidly in response to hormonal or neural stimuli. Existing methods typically provide static snapshots rather than dynamic, real-time measurements of glycogen breakdown. This limitation prevents researchers from fully characterizing the kinetics of glycogenolysis and its regulation under various physiological and pathological conditions.
Sample preparation techniques introduce additional complications, as many current methods require cell fixation or lysis, which inherently disrupts the native cellular environment and may alter glycogen structures. Preserving the integrity of glycogen pools during sample processing remains challenging, particularly when attempting to maintain spatial information about glycogen localization within cellular compartments.
Sensitivity thresholds of available detection methods constitute a major technical barrier. Many existing techniques lack sufficient sensitivity to detect small-scale changes in glycogen content, especially in cell types with naturally low glycogen reserves. This limitation is particularly problematic when investigating subtle metabolic shifts that may have significant physiological implications.
Quantification accuracy presents ongoing difficulties, as distinguishing between glycogen and other polysaccharides or cellular components can lead to measurement artifacts. Current colorimetric and enzymatic assays often suffer from interference from other cellular constituents, compromising the reliability of glycogenolysis measurements at the microscale level.
Integration with other metabolic parameters remains technically challenging. Glycogenolysis does not occur in isolation but is intricately connected with other metabolic pathways, including glycolysis, gluconeogenesis, and lipid metabolism. Current microscale methods typically focus solely on glycogen breakdown without simultaneously monitoring these interconnected processes, limiting comprehensive metabolic analysis.
Standardization across research platforms represents an additional challenge, as variations in experimental protocols, reagents, and instrumentation lead to inconsistent results between laboratories. This lack of standardization hampers comparative studies and slows progress in understanding the fundamental mechanisms of glycogenolysis at the cellular level.
Established Microscale Methods for Glycogen Breakdown Analysis
01 Microscale probes for material analysis
Microscale probes are used for analyzing materials at the micro and nano level. These probes can detect physical properties, chemical compositions, and structural characteristics of various materials. The technology enables high-resolution imaging and measurement of surface properties, allowing researchers to understand material behavior at microscopic scales. These methods often incorporate specialized sensors that can detect minute changes in the sample properties.- Microscale probe technologies for material analysis: Advanced microscale probe technologies enable precise analysis of material properties at the micro and nano levels. These probes utilize various physical principles to characterize surfaces, measure mechanical properties, and detect specific chemical or biological markers. The technologies incorporate specialized sensors and detection mechanisms that can operate at microscopic scales with high sensitivity and resolution, allowing for detailed examination of material characteristics in research and industrial applications.
- Microfluidic systems for sample handling and analysis: Microfluidic systems provide platforms for handling and analyzing microscale samples with high precision. These systems incorporate channels, chambers, and other structures at the microscale to manipulate small volumes of fluids. They enable efficient sample preparation, separation, and detection processes for various analytical applications. The integration of microfluidic components with detection systems allows for automated and high-throughput analysis of biological and chemical samples with minimal reagent consumption.
- Microscale sensing and detection methods: Innovative microscale sensing and detection methods provide high-sensitivity analysis capabilities for various applications. These methods utilize specialized transduction mechanisms to convert physical, chemical, or biological signals into measurable outputs. The technologies incorporate advanced signal processing techniques to enhance detection limits and improve measurement accuracy. Microscale sensors can be designed for specific target analytes and integrated into portable or automated systems for field or laboratory use.
- Nanofabrication techniques for microscale probes: Specialized nanofabrication techniques enable the creation of precise microscale probe structures with tailored properties. These manufacturing methods combine advanced lithography, deposition, etching, and assembly processes to create functional probe components at the micro and nano scales. The fabrication approaches allow for control over probe geometry, material composition, and surface properties to optimize performance for specific applications. Integration of multiple materials and functional elements enhances the capabilities of the resulting probe systems.
- Acoustic and vibrational microscale probe methods: Acoustic and vibrational techniques provide unique capabilities for microscale analysis through mechanical interactions with samples. These methods utilize controlled sound waves or mechanical vibrations to probe material properties and structures at microscopic scales. The technologies can measure elasticity, density, and other mechanical characteristics without destructive sample preparation. Advanced signal processing and instrumentation enable high-resolution mapping of sample properties based on their acoustic or vibrational responses.
02 Microscale probe fabrication techniques
Various fabrication techniques are employed to create microscale probes with specific functionalities. These include micromachining, lithography, and deposition methods to produce probes with precise dimensions and properties. The fabrication processes often involve creating cantilevers, tips, or arrays of sensors that can interact with samples at the microscale. Advanced materials and composites are used to enhance the sensitivity and durability of these probes.Expand Specific Solutions03 Microscale methods for biological applications
Microscale methods and probes are applied in biological research for cell analysis, DNA sequencing, and protein detection. These techniques allow for minimally invasive sampling and analysis of biological materials. The methods often involve microfluidic systems that can handle small volumes of biological samples, enabling high-throughput screening and analysis. These approaches provide insights into cellular processes and molecular interactions at unprecedented resolution.Expand Specific Solutions04 Acoustic and vibrational microscale probe systems
Acoustic and vibrational technologies are utilized in microscale probe systems to detect and analyze material properties. These systems use sound waves or mechanical vibrations to interact with samples and measure their responses. The technology enables non-destructive testing and characterization of materials at the microscale. These methods are particularly useful for analyzing mechanical properties, defects, and structural integrity of various materials and components.Expand Specific Solutions05 Integrated microscale probe systems for environmental monitoring
Integrated microscale probe systems are developed for environmental monitoring applications. These systems combine multiple sensing modalities to detect pollutants, pathogens, or other environmental parameters. The technology enables real-time monitoring and analysis of environmental samples in field conditions. These systems often incorporate data processing capabilities to provide immediate analysis results, making them valuable tools for environmental research and protection efforts.Expand Specific Solutions
Leading Research Groups and Companies in Glycogenolysis Detection
The glycogenolysis probe technology market is in an early growth phase, characterized by significant research activity but limited commercial applications. Current market size remains modest but shows promising expansion potential as cellular metabolism research gains importance in disease understanding. Technical maturity varies across approaches, with academic institutions (MIT, University of California, Arizona State University) leading fundamental research while specialized biotechnology companies (Momenta Pharmaceuticals, bitBiome) develop practical applications. Pharmaceutical companies (Hoffmann-La Roche, Incyte, GlaxoSmithKline) are investing in this field to enhance drug discovery capabilities. Medical device manufacturers (ARKRAY, Azbil) are exploring integration of these microscale methods into diagnostic platforms, indicating growing cross-sector interest in this emerging technology.
The Regents of the University of California
Technical Solution: UC researchers have pioneered microscale fluorescence-based biosensors for glycogenolysis monitoring in living cells. Their approach utilizes genetically encoded FRET-based sensors that specifically bind to glycogen-derived glucose-1-phosphate, enabling real-time visualization of glycogenolysis with subcellular resolution. These sensors are engineered with optimized binding domains derived from glycogen phosphorylase and feature ratiometric fluorescence properties that minimize artifacts from variations in sensor concentration or optical path length. The UC system incorporates microfluidic delivery systems that allow precise temporal control of hormonal stimuli while maintaining cells in physiologically relevant conditions. Their latest iterations include sensors with improved dynamic range (>400% change in FRET ratio) and sensitivity (Kd ~5 μM for glucose-1-phosphate), enabling detection of subtle changes in glycogenolysis rates.
Strengths: Non-invasive monitoring in living cells; excellent subcellular spatial resolution; compatibility with conventional fluorescence microscopy equipment. Weaknesses: Potential interference from other metabolites; requires genetic modification of target cells; limited to fluorescence-accessible tissues and cell types.
Massachusetts Institute of Technology
Technical Solution: MIT has developed advanced microfluidic platforms for real-time monitoring of glycogenolysis at the cellular level. Their approach combines PDMS-based microfluidic devices with integrated electrochemical sensors that can detect glucose release from individual cells with high temporal resolution. The system incorporates specialized microchannels (10-50 μm) that allow precise control of cellular microenvironments while enabling simultaneous optical imaging and electrochemical detection. MIT researchers have further enhanced this technology by implementing machine learning algorithms that can identify patterns in glycogen metabolism across heterogeneous cell populations, achieving detection limits in the nanomolar range for glucose and related metabolites. This integrated approach allows for multiplexed analysis of glycogenolysis dynamics in response to various stimuli.
Strengths: Exceptional spatial and temporal resolution; integration of multiple detection modalities; high-throughput capability for analyzing numerous cells simultaneously. Weaknesses: Complex fabrication process; requires specialized expertise for operation; relatively high cost compared to conventional methods.
Key Technologies in Single-Cell Glycogenolysis Measurement
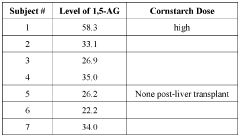
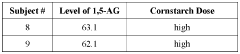
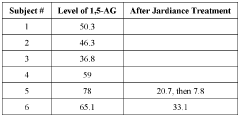
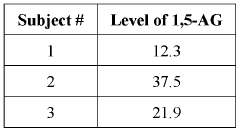
Methods of detecting and using biomarkers for glycogen storage diseases
PatentWO2022061290A1
Innovation
- A method involving the use of biosamples to detect GSD biomarkers, particularly 1,5-anhydroglucitol (1,5-AG), to diagnose, treat, and monitor the efficacy of treatments in subjects with GSD, potentially combined with other therapies like enzyme replacement therapy, gene therapy, or substrate reduction therapy.
Compositions and methods for the treatment of genetic diseases
PatentWO2020172465A1
Innovation
- The use of microbial glycogen debranching enzymes encoded by nucleic acid sequences optimized for mammalian expression, delivered via vectors with tissue-specific or immunotolerant dual promoters to prevent immune responses and achieve broader tissue correction.
Bioethical Implications of Cellular Metabolism Probing
The advancement of microscale methods to probe glycogenolysis in cells raises significant bioethical considerations that must be addressed as this technology evolves. The ability to monitor cellular metabolism at unprecedented resolution provides valuable insights into disease mechanisms but simultaneously creates complex ethical dilemmas regarding privacy, consent, and potential applications.
The collection of cellular metabolic data through these microscale methods generates highly personalized biological information that may reveal predispositions to certain conditions or diseases. This raises fundamental questions about ownership of biological data and the right to privacy. Researchers and clinicians must navigate the delicate balance between scientific advancement and protecting individuals from potential discrimination based on their metabolic profiles, particularly as these technologies become more accessible and widespread.
Informed consent frameworks require substantial reconsideration in light of these emerging technologies. Traditional consent models may prove inadequate when dealing with the extensive and detailed cellular information that glycogenolysis probing can generate. The potential for secondary discoveries—findings beyond the original research scope—creates ethical challenges regarding disclosure obligations and the management of incidental findings that may have significant implications for patients.
The application of these technologies across diverse populations introduces questions of justice and equitable access. If advanced metabolic probing becomes standard in healthcare, ensuring that all demographic groups benefit equally becomes an ethical imperative. Historical patterns of healthcare disparities suggest vigilance is needed to prevent new technologies from exacerbating existing inequalities in healthcare delivery and outcomes.
Dual-use concerns also emerge as these technologies develop. While primarily designed for medical research and diagnostics, the detailed metabolic information obtained could potentially be misused for non-medical purposes, including surveillance or discrimination. Establishing robust governance frameworks that prevent misuse while encouraging beneficial applications represents a significant bioethical challenge.
The long-term implications of routine cellular metabolism monitoring extend to questions about human identity and autonomy. As our understanding of metabolic processes becomes increasingly deterministic, society must grapple with the philosophical tension between biological determinism and human agency. Preserving the concept of individual autonomy while acknowledging the influence of cellular processes on behavior and health outcomes requires careful ethical consideration.
The collection of cellular metabolic data through these microscale methods generates highly personalized biological information that may reveal predispositions to certain conditions or diseases. This raises fundamental questions about ownership of biological data and the right to privacy. Researchers and clinicians must navigate the delicate balance between scientific advancement and protecting individuals from potential discrimination based on their metabolic profiles, particularly as these technologies become more accessible and widespread.
Informed consent frameworks require substantial reconsideration in light of these emerging technologies. Traditional consent models may prove inadequate when dealing with the extensive and detailed cellular information that glycogenolysis probing can generate. The potential for secondary discoveries—findings beyond the original research scope—creates ethical challenges regarding disclosure obligations and the management of incidental findings that may have significant implications for patients.
The application of these technologies across diverse populations introduces questions of justice and equitable access. If advanced metabolic probing becomes standard in healthcare, ensuring that all demographic groups benefit equally becomes an ethical imperative. Historical patterns of healthcare disparities suggest vigilance is needed to prevent new technologies from exacerbating existing inequalities in healthcare delivery and outcomes.
Dual-use concerns also emerge as these technologies develop. While primarily designed for medical research and diagnostics, the detailed metabolic information obtained could potentially be misused for non-medical purposes, including surveillance or discrimination. Establishing robust governance frameworks that prevent misuse while encouraging beneficial applications represents a significant bioethical challenge.
The long-term implications of routine cellular metabolism monitoring extend to questions about human identity and autonomy. As our understanding of metabolic processes becomes increasingly deterministic, society must grapple with the philosophical tension between biological determinism and human agency. Preserving the concept of individual autonomy while acknowledging the influence of cellular processes on behavior and health outcomes requires careful ethical consideration.
Standardization and Validation Protocols for Glycogenolysis Assays
The standardization and validation of glycogenolysis assays at the microscale level represents a critical challenge in cellular metabolism research. Current protocols exhibit significant variability across laboratories, hampering reproducibility and cross-study comparisons. Establishing robust standardization frameworks requires addressing multiple technical parameters that influence assay performance.
Primary considerations for standardization include cell preparation protocols, where factors such as cell density, passage number, and culture conditions significantly impact glycogen metabolism baseline measurements. Research indicates that cells between passages 3-10 typically demonstrate optimal consistency in glycogenolysis responses, while higher passages may exhibit altered metabolic profiles that confound results interpretation.
Sample preparation represents another critical variable requiring standardization. The timing between cell harvesting and analysis must be precisely controlled, as glycogen degradation continues ex vivo. Protocols should specify maximum allowable delays (ideally under 30 minutes) and standardized temperature conditions during processing to minimize artifactual glycogenolysis.
Reagent quality and preparation protocols demand rigorous validation. Enzyme preparations used in glycogenolysis assays, particularly phosphorylase kinase and glycogen phosphorylase, require activity verification prior to experimental use. Batch-to-batch variations in commercial reagents necessitate internal calibration standards to normalize results across experiments.
Instrument calibration protocols constitute an essential component of standardization efforts. Microscale fluorescence-based glycogenolysis assays require regular calibration using defined glucose standards to establish detection limits and linear response ranges. Validation data suggests optimal performance when calibration curves are prepared fresh for each experimental session rather than relying on stored calibration data.
Statistical validation frameworks must address the inherent variability in cellular responses. Minimum replicate numbers (n≥6 for cell-based assays) should be established, along with acceptance criteria for control samples. Statistical power calculations specific to microscale glycogenolysis measurements can guide experimental design to ensure meaningful conclusions.
Inter-laboratory validation represents the gold standard for protocol robustness. Emerging consortium efforts are establishing reference cell lines and standardized glycogenolysis challenge protocols that can serve as benchmarks across research groups. These validation networks facilitate protocol refinement through comparative analysis of methodological variations and their impact on measurement outcomes.
Primary considerations for standardization include cell preparation protocols, where factors such as cell density, passage number, and culture conditions significantly impact glycogen metabolism baseline measurements. Research indicates that cells between passages 3-10 typically demonstrate optimal consistency in glycogenolysis responses, while higher passages may exhibit altered metabolic profiles that confound results interpretation.
Sample preparation represents another critical variable requiring standardization. The timing between cell harvesting and analysis must be precisely controlled, as glycogen degradation continues ex vivo. Protocols should specify maximum allowable delays (ideally under 30 minutes) and standardized temperature conditions during processing to minimize artifactual glycogenolysis.
Reagent quality and preparation protocols demand rigorous validation. Enzyme preparations used in glycogenolysis assays, particularly phosphorylase kinase and glycogen phosphorylase, require activity verification prior to experimental use. Batch-to-batch variations in commercial reagents necessitate internal calibration standards to normalize results across experiments.
Instrument calibration protocols constitute an essential component of standardization efforts. Microscale fluorescence-based glycogenolysis assays require regular calibration using defined glucose standards to establish detection limits and linear response ranges. Validation data suggests optimal performance when calibration curves are prepared fresh for each experimental session rather than relying on stored calibration data.
Statistical validation frameworks must address the inherent variability in cellular responses. Minimum replicate numbers (n≥6 for cell-based assays) should be established, along with acceptance criteria for control samples. Statistical power calculations specific to microscale glycogenolysis measurements can guide experimental design to ensure meaningful conclusions.
Inter-laboratory validation represents the gold standard for protocol robustness. Emerging consortium efforts are establishing reference cell lines and standardized glycogenolysis challenge protocols that can serve as benchmarks across research groups. These validation networks facilitate protocol refinement through comparative analysis of methodological variations and their impact on measurement outcomes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!