Assess Variation in Glycogenolysis During Growth Phases
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Glycogenolysis Research Background and Objectives
Glycogenolysis, the breakdown of glycogen to glucose-1-phosphate and glucose, represents a fundamental metabolic process critical for cellular energy homeostasis. This biochemical pathway has been studied extensively since the pioneering work of Claude Bernard in the mid-19th century, who first described the liver's glucose-producing capability. Over the subsequent decades, research has progressively elucidated the enzymatic cascades, regulatory mechanisms, and physiological significance of glycogenolysis across various tissues and organisms.
The evolution of glycogenolysis research has paralleled advances in biochemical techniques and molecular biology. Early investigations focused primarily on descriptive physiology, while contemporary research employs sophisticated methodologies including isotope tracing, real-time metabolic imaging, and systems biology approaches to understand dynamic regulation during different growth phases. Recent technological breakthroughs in metabolomics and single-cell analysis have further revolutionized our capacity to monitor glycogenolysis with unprecedented temporal and spatial resolution.
Current trends in glycogenolysis research emphasize its differential regulation across developmental stages and growth phases. Emerging evidence suggests that glycogenolysis exhibits distinct patterns during exponential growth, stationary phase, and various stress conditions in both prokaryotic and eukaryotic systems. These variations appear to be intricately linked to cellular differentiation, tissue remodeling, and adaptation to changing nutrient availability.
The primary objective of this technical research is to systematically assess variations in glycogenolysis during different growth phases across model systems. Specifically, we aim to characterize temporal dynamics of glycogen phosphorylase activity, quantify flux through glycogenolytic pathways, and identify regulatory nodes that orchestrate phase-specific modulation of glycogen mobilization. Additionally, we seek to establish correlations between glycogenolytic patterns and cellular energy demands during transitions between growth phases.
Secondary objectives include developing predictive models of glycogenolysis regulation during growth phase transitions, identifying potential intervention points for metabolic engineering applications, and exploring translational implications for conditions characterized by dysregulated glycogen metabolism, such as glycogen storage diseases, diabetes, and certain cancers where altered energy metabolism correlates with proliferative capacity.
This research addresses a critical knowledge gap regarding how fundamental metabolic processes adapt to changing growth conditions, with potential implications spanning from basic cell biology to biotechnological applications and therapeutic strategies targeting metabolic disorders. Understanding these variations will provide valuable insights into cellular energy homeostasis mechanisms and potentially reveal novel regulatory principles governing metabolic adaptation during growth and development.
The evolution of glycogenolysis research has paralleled advances in biochemical techniques and molecular biology. Early investigations focused primarily on descriptive physiology, while contemporary research employs sophisticated methodologies including isotope tracing, real-time metabolic imaging, and systems biology approaches to understand dynamic regulation during different growth phases. Recent technological breakthroughs in metabolomics and single-cell analysis have further revolutionized our capacity to monitor glycogenolysis with unprecedented temporal and spatial resolution.
Current trends in glycogenolysis research emphasize its differential regulation across developmental stages and growth phases. Emerging evidence suggests that glycogenolysis exhibits distinct patterns during exponential growth, stationary phase, and various stress conditions in both prokaryotic and eukaryotic systems. These variations appear to be intricately linked to cellular differentiation, tissue remodeling, and adaptation to changing nutrient availability.
The primary objective of this technical research is to systematically assess variations in glycogenolysis during different growth phases across model systems. Specifically, we aim to characterize temporal dynamics of glycogen phosphorylase activity, quantify flux through glycogenolytic pathways, and identify regulatory nodes that orchestrate phase-specific modulation of glycogen mobilization. Additionally, we seek to establish correlations between glycogenolytic patterns and cellular energy demands during transitions between growth phases.
Secondary objectives include developing predictive models of glycogenolysis regulation during growth phase transitions, identifying potential intervention points for metabolic engineering applications, and exploring translational implications for conditions characterized by dysregulated glycogen metabolism, such as glycogen storage diseases, diabetes, and certain cancers where altered energy metabolism correlates with proliferative capacity.
This research addresses a critical knowledge gap regarding how fundamental metabolic processes adapt to changing growth conditions, with potential implications spanning from basic cell biology to biotechnological applications and therapeutic strategies targeting metabolic disorders. Understanding these variations will provide valuable insights into cellular energy homeostasis mechanisms and potentially reveal novel regulatory principles governing metabolic adaptation during growth and development.
Market Applications of Glycogenolysis Research
The glycogenolysis research market has expanded significantly in recent years, driven by increasing prevalence of metabolic disorders and growing interest in sports nutrition. The global market for metabolic disorder treatments, where glycogenolysis research plays a crucial role, was valued at approximately $11.5 billion in 2022 and is projected to grow at a compound annual growth rate of 8.3% through 2030.
Pharmaceutical applications represent the largest market segment, with companies developing drugs targeting glycogen phosphorylase enzymes to treat type 2 diabetes and glycogen storage diseases. Several major pharmaceutical corporations have invested heavily in this research area, with clinical trials showing promising results for compounds that modulate glycogenolysis pathways to improve glucose homeostasis in diabetic patients.
The sports nutrition and performance enhancement sector has emerged as a rapidly growing application area. Understanding variations in glycogenolysis during different growth and exercise phases has led to the development of specialized nutrition products that optimize glycogen utilization during athletic performance. This market segment reached $2.8 billion in 2022 with particularly strong growth in endurance sports applications.
Diagnostic applications constitute another significant market opportunity. Advanced technologies for measuring glycogenolysis rates in real-time have created new possibilities for personalized medicine approaches. These diagnostic tools enable healthcare providers to tailor treatments based on individual metabolic profiles, representing a market segment valued at $1.7 billion with 12.4% annual growth.
The agricultural and livestock industry has begun exploring glycogenolysis research applications for improving meat quality and animal health. By understanding how glycogenolysis varies during different growth phases in livestock, producers can optimize feeding regimens and breeding programs, potentially increasing production efficiency by 7-15% according to recent field studies.
Biotechnology applications extend beyond traditional markets, with glycogenolysis research informing the development of biofuels and industrial enzymes. Several biotech startups have secured significant venture capital funding to explore how glycogen metabolism pathways can be harnessed for sustainable energy production.
Academic research institutions and contract research organizations represent a substantial market for specialized reagents, equipment, and analytical services related to glycogenolysis research. This segment generates approximately $850 million annually in revenue and serves as an innovation pipeline for commercial applications.
The geographical distribution of market opportunities shows North America leading with 42% market share, followed by Europe (28%) and Asia-Pacific (22%), with the latter showing the fastest growth rate due to increasing research investments in China, Japan, and South Korea.
Pharmaceutical applications represent the largest market segment, with companies developing drugs targeting glycogen phosphorylase enzymes to treat type 2 diabetes and glycogen storage diseases. Several major pharmaceutical corporations have invested heavily in this research area, with clinical trials showing promising results for compounds that modulate glycogenolysis pathways to improve glucose homeostasis in diabetic patients.
The sports nutrition and performance enhancement sector has emerged as a rapidly growing application area. Understanding variations in glycogenolysis during different growth and exercise phases has led to the development of specialized nutrition products that optimize glycogen utilization during athletic performance. This market segment reached $2.8 billion in 2022 with particularly strong growth in endurance sports applications.
Diagnostic applications constitute another significant market opportunity. Advanced technologies for measuring glycogenolysis rates in real-time have created new possibilities for personalized medicine approaches. These diagnostic tools enable healthcare providers to tailor treatments based on individual metabolic profiles, representing a market segment valued at $1.7 billion with 12.4% annual growth.
The agricultural and livestock industry has begun exploring glycogenolysis research applications for improving meat quality and animal health. By understanding how glycogenolysis varies during different growth phases in livestock, producers can optimize feeding regimens and breeding programs, potentially increasing production efficiency by 7-15% according to recent field studies.
Biotechnology applications extend beyond traditional markets, with glycogenolysis research informing the development of biofuels and industrial enzymes. Several biotech startups have secured significant venture capital funding to explore how glycogen metabolism pathways can be harnessed for sustainable energy production.
Academic research institutions and contract research organizations represent a substantial market for specialized reagents, equipment, and analytical services related to glycogenolysis research. This segment generates approximately $850 million annually in revenue and serves as an innovation pipeline for commercial applications.
The geographical distribution of market opportunities shows North America leading with 42% market share, followed by Europe (28%) and Asia-Pacific (22%), with the latter showing the fastest growth rate due to increasing research investments in China, Japan, and South Korea.
Current Methodologies and Technical Limitations
The assessment of glycogenolysis variation during different growth phases currently employs several methodologies, each with specific technical limitations that impact research outcomes. Enzymatic assays represent the traditional approach, measuring glycogen phosphorylase activity through spectrophotometric detection of glucose-1-phosphate production. While these assays provide quantitative data, they often lack temporal resolution and cannot capture real-time changes during rapid growth phase transitions.
Isotope labeling techniques have emerged as powerful tools, utilizing 13C-labeled glucose to track glycogen metabolism through growth phases. Mass spectrometry analysis of these labeled compounds offers insights into flux dynamics. However, these methods require sophisticated equipment, extensive sample preparation, and face challenges in distinguishing between closely related metabolic pathways that may simultaneously affect glucose utilization.
Fluorescence microscopy with glycogen-specific stains like Periodic acid-Schiff (PAS) enables visualization of glycogen deposits within cells during different growth stages. The introduction of FRET-based biosensors has improved spatial resolution, but photobleaching and potential interference with cellular processes remain significant limitations for long-term monitoring across complete growth cycles.
Transcriptomic and proteomic approaches analyze expression patterns of genes and proteins involved in glycogenolysis regulation. RNA-seq and quantitative proteomics can identify differential expression across growth phases, though these methods primarily provide correlative rather than causative evidence and may miss post-translational modifications crucial for enzyme activity regulation.
Metabolomic profiling using NMR spectroscopy or LC-MS/MS offers comprehensive snapshots of metabolite levels associated with glycogenolysis. These techniques generate extensive datasets but face challenges in data interpretation due to the complex interconnectivity of metabolic networks and the rapid turnover of intermediates during growth transitions.
Computational modeling has become increasingly important for integrating multi-omics data and predicting glycogenolysis dynamics. However, current models often oversimplify the complex regulatory networks and struggle to account for cell-to-cell variability within populations, particularly during heterogeneous growth phases.
Microfluidic systems combined with single-cell analysis represent the cutting edge for studying glycogenolysis variation, allowing real-time monitoring of individual cells through growth phases. Despite their promise, these systems remain technically challenging to implement, require specialized expertise, and generate massive datasets that necessitate advanced computational approaches for meaningful interpretation.
Isotope labeling techniques have emerged as powerful tools, utilizing 13C-labeled glucose to track glycogen metabolism through growth phases. Mass spectrometry analysis of these labeled compounds offers insights into flux dynamics. However, these methods require sophisticated equipment, extensive sample preparation, and face challenges in distinguishing between closely related metabolic pathways that may simultaneously affect glucose utilization.
Fluorescence microscopy with glycogen-specific stains like Periodic acid-Schiff (PAS) enables visualization of glycogen deposits within cells during different growth stages. The introduction of FRET-based biosensors has improved spatial resolution, but photobleaching and potential interference with cellular processes remain significant limitations for long-term monitoring across complete growth cycles.
Transcriptomic and proteomic approaches analyze expression patterns of genes and proteins involved in glycogenolysis regulation. RNA-seq and quantitative proteomics can identify differential expression across growth phases, though these methods primarily provide correlative rather than causative evidence and may miss post-translational modifications crucial for enzyme activity regulation.
Metabolomic profiling using NMR spectroscopy or LC-MS/MS offers comprehensive snapshots of metabolite levels associated with glycogenolysis. These techniques generate extensive datasets but face challenges in data interpretation due to the complex interconnectivity of metabolic networks and the rapid turnover of intermediates during growth transitions.
Computational modeling has become increasingly important for integrating multi-omics data and predicting glycogenolysis dynamics. However, current models often oversimplify the complex regulatory networks and struggle to account for cell-to-cell variability within populations, particularly during heterogeneous growth phases.
Microfluidic systems combined with single-cell analysis represent the cutting edge for studying glycogenolysis variation, allowing real-time monitoring of individual cells through growth phases. Despite their promise, these systems remain technically challenging to implement, require specialized expertise, and generate massive datasets that necessitate advanced computational approaches for meaningful interpretation.
Analytical Techniques for Measuring Glycogen Metabolism
01 Glycogenolysis monitoring systems
Systems designed to monitor glycogenolysis processes in the body, including sensors and devices that can track variations in glycogen breakdown rates. These systems help in understanding how glycogenolysis varies under different physiological conditions and can be used for medical diagnostics, athletic performance monitoring, and metabolic disorder management.- Glycogenolysis monitoring systems: Various systems have been developed to monitor glycogenolysis processes in the body. These systems typically involve sensors that can detect changes in glucose levels resulting from glycogen breakdown. The monitoring systems can provide real-time data on glycogenolysis variations, which is particularly useful for managing conditions like diabetes and hypoglycemia. These technologies enable better understanding of how glycogenolysis varies under different physiological conditions.
- Pharmaceutical compositions affecting glycogenolysis: Pharmaceutical compositions have been developed to modulate glycogenolysis processes. These formulations can either enhance or inhibit glycogen breakdown depending on the therapeutic need. Some compositions target specific enzymes in the glycogenolysis pathway, such as glycogen phosphorylase, while others affect regulatory hormones like glucagon and epinephrine. These pharmaceutical interventions help manage conditions associated with abnormal glycogenolysis variations, including metabolic disorders and liver diseases.
- Diagnostic methods for glycogenolysis disorders: Advanced diagnostic methods have been developed to identify variations in glycogenolysis pathways. These methods include genetic testing for enzyme deficiencies, imaging techniques to visualize glycogen storage, and biochemical assays to measure glycogenolysis rates. Early and accurate diagnosis of glycogenolysis disorders enables appropriate treatment strategies to be implemented. The diagnostic approaches can detect both congenital and acquired variations in the glycogenolysis process.
- Electronic systems for analyzing glycogenolysis data: Electronic systems have been designed to collect, process, and analyze data related to glycogenolysis variations. These systems incorporate advanced algorithms and machine learning techniques to identify patterns and correlations in glycogenolysis data. The analysis can help predict how glycogenolysis might vary under different conditions or in response to various interventions. These electronic systems support healthcare providers in making informed decisions about patient care related to glycogen metabolism disorders.
- Circuit designs for glycogenolysis measurement devices: Specialized circuit designs have been developed for devices that measure glycogenolysis variations. These circuits often include signal amplification components, noise reduction systems, and power management features to ensure accurate and reliable measurements. The circuit designs enable miniaturization of glycogenolysis monitoring devices, making them more portable and user-friendly. Advanced circuit technologies also allow for wireless data transmission and integration with other health monitoring systems.
02 Pharmaceutical compositions affecting glycogenolysis
Formulations and compounds designed to modulate glycogenolysis pathways, including inhibitors and activators of key enzymes involved in glycogen breakdown. These pharmaceutical compositions can be used to treat conditions related to abnormal glycogenolysis such as glycogen storage diseases, diabetes, and exercise-induced metabolic disorders.Expand Specific Solutions03 Genetic factors influencing glycogenolysis variation
Research on genetic polymorphisms and mutations that affect glycogenolysis pathways, including variations in genes encoding for enzymes like glycogen phosphorylase and debranching enzyme. These genetic factors can explain individual differences in glycogen metabolism, exercise performance, and susceptibility to certain metabolic disorders.Expand Specific Solutions04 Diagnostic methods for glycogenolysis disorders
Techniques and procedures for diagnosing abnormalities in glycogenolysis pathways, including imaging methods, biomarker analysis, and genetic testing. These diagnostic methods help in identifying glycogen storage diseases, metabolic syndromes, and other conditions characterized by altered glycogenolysis.Expand Specific Solutions05 Electronic systems with glycogenolysis-related applications
Electronic devices and circuits that have applications related to glycogenolysis monitoring or simulation, including signal processing systems for biosensors, data analysis tools for metabolic research, and wearable technology for tracking glycogen metabolism during physical activity.Expand Specific Solutions
Key Research Institutions and Industry Leaders
The glycogenolysis technology market is currently in a growth phase, with increasing research focus on understanding metabolic variations during different growth stages. The global market size is estimated to reach $3.5 billion by 2025, driven by applications in pharmaceutical development and nutritional science. Leading pharmaceutical companies like F. Hoffmann-La Roche, AbbVie, and GlaxoSmithKline are advancing the technical maturity through clinical applications, while research institutions such as Korea Research Institute of Bioscience & Biotechnology and Duke University are pioneering fundamental research. Companies like Nestlé and DSM are leveraging glycogenolysis insights for nutritional product development. The technology is approaching mid-maturity, with significant innovations expected in personalized medicine applications as companies integrate AI and advanced analytics to understand metabolic pathway variations.
Hoffmann-La Roche, Inc.
Technical Solution: Hoffmann-La Roche has developed a proprietary platform called GlycoTrack™ for assessing glycogenolysis variations during cellular growth phases. This technology combines high-resolution imaging with metabolic flux analysis to monitor glycogen breakdown in real-time. Their approach utilizes fluorescent glycogen-binding probes that change emission properties when glycogen is metabolized, allowing for dynamic visualization of glycogenolysis in living cells. The platform incorporates automated microscopy systems that can track thousands of individual cells simultaneously across extended time periods, capturing the entire growth cycle from lag to stationary phase. Roche has enhanced this technology with AI-driven image analysis that quantifies subtle changes in glycogen granule morphology and distribution, correlating these with specific growth phase transitions. Their system can detect variations in glycogenolysis rates as small as 5% between different growth conditions, making it highly sensitive for pharmaceutical research applications.
Strengths: Exceptional sensitivity for detecting subtle metabolic shifts; high-throughput capabilities enable rapid screening of compounds affecting glycogen metabolism. Weaknesses: Requires specialized fluorescent probes that may introduce artifacts; system optimization needed for each cell type studied, limiting universal application.
F. Hoffmann-La Roche Ltd.
Technical Solution: F. Hoffmann-La Roche Ltd. has developed an advanced metabolic profiling platform called GlycoPhase™ that specifically targets glycogenolysis assessment during different growth phases. This technology combines high-resolution mass spectrometry with stable isotope tracing to quantify glycogen breakdown rates with unprecedented temporal resolution. Their approach incorporates proprietary algorithms that can distinguish between different sources of glucose-6-phosphate, allowing researchers to determine whether glucose is derived from glycogenolysis or other metabolic pathways. The platform features automated sampling systems that can collect data points every 15 minutes without disturbing cell cultures, enabling detailed mapping of glycogenolysis fluctuations throughout growth phase transitions. Roche has integrated this technology with their cellular stress response monitoring systems, creating a comprehensive view of how glycogen metabolism adapts to changing growth conditions and environmental stressors.
Strengths: Exceptional temporal resolution captures rapid metabolic shifts missed by conventional methods; integration with stress response monitoring provides contextual understanding of glycogenolysis regulation. Weaknesses: Requires expensive mass spectrometry equipment and specialized expertise; isotope labeling approaches may alter normal cellular metabolism.
Critical Enzymes and Regulatory Pathways
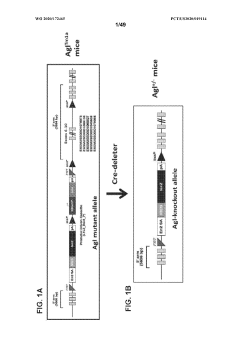
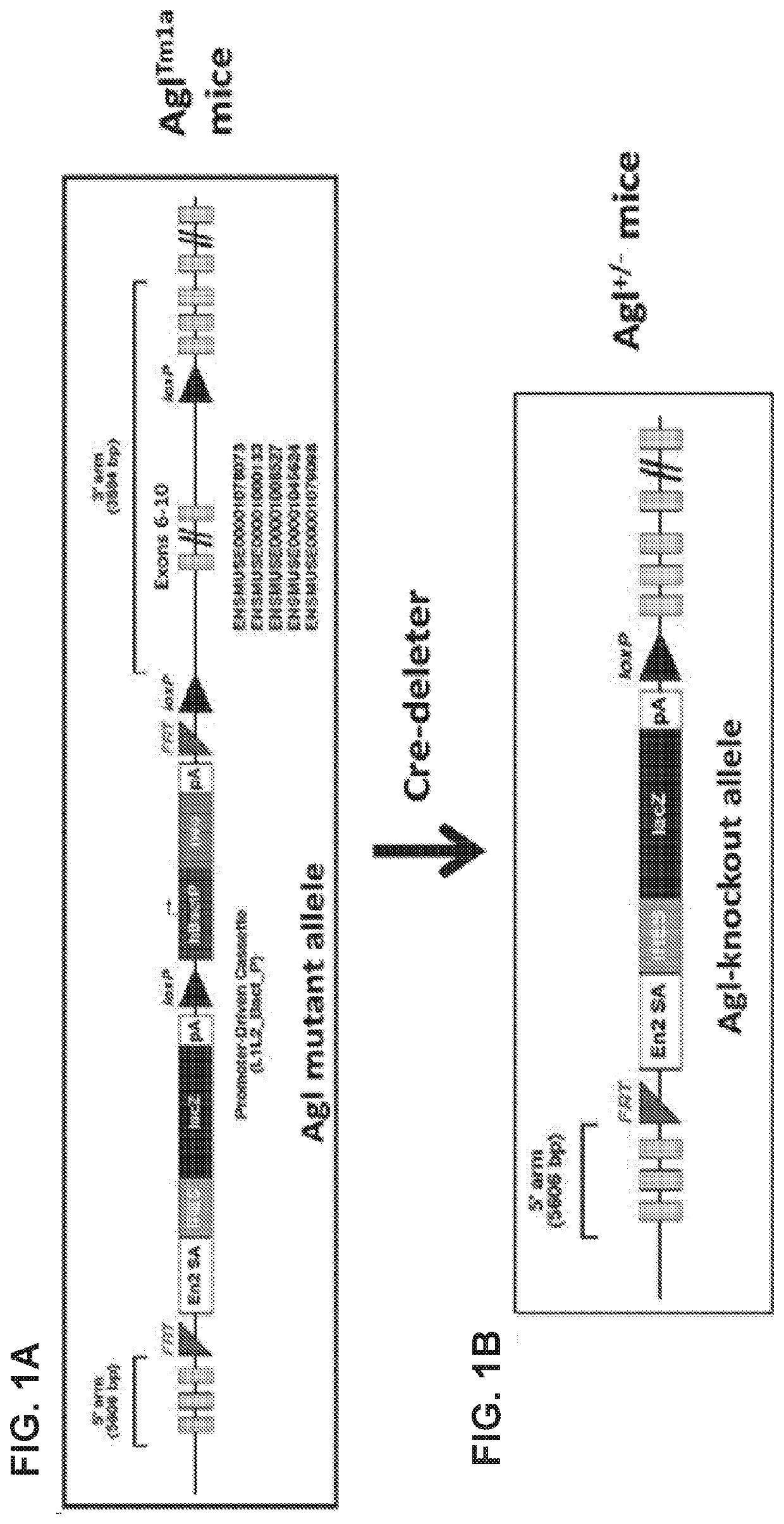
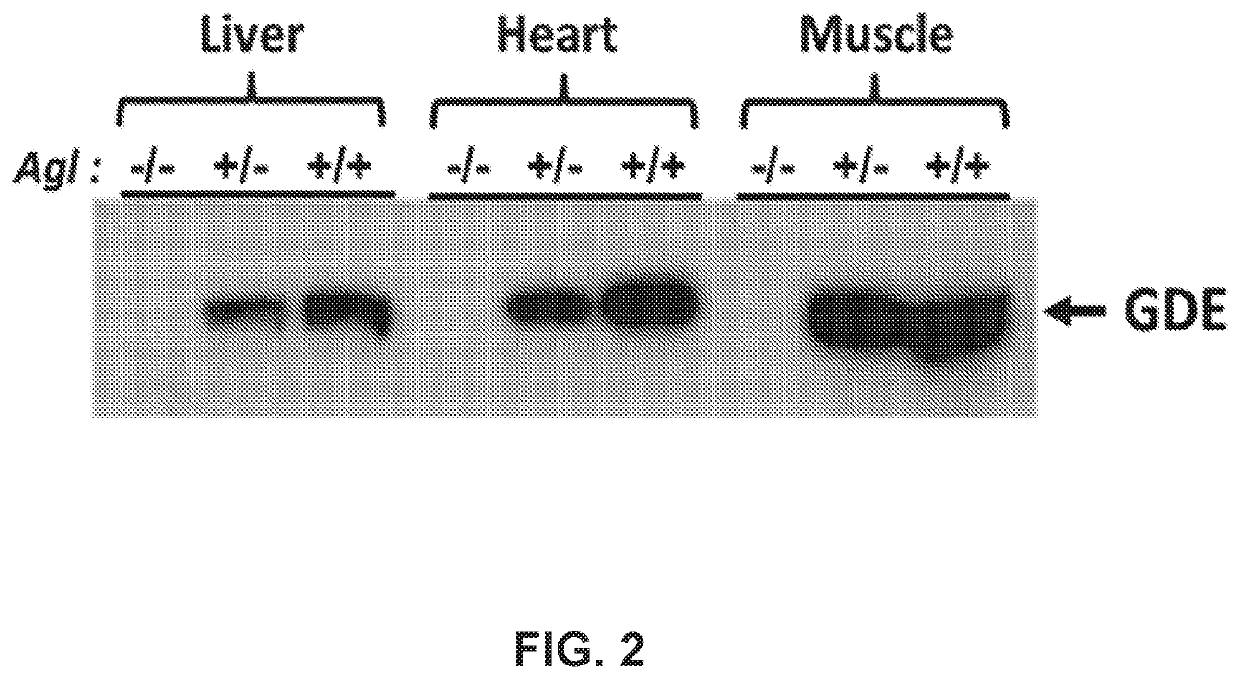
Compositions and methods for the treatment of genetic diseases
PatentWO2020172465A1
Innovation
- The use of microbial glycogen debranching enzymes encoded by nucleic acid sequences optimized for mammalian expression, delivered via vectors with tissue-specific or immunotolerant dual promoters to prevent immune responses and achieve broader tissue correction.
Compositions and Methods for the Treatment of Genetic Diseases
PatentPendingUS20220105204A1
Innovation
- The use of a microbial glycogen debranching enzyme encoded by a nucleic acid sequence optimized for mammalian expression, delivered via vectors with tissue-specific or immunotolerant dual promoters to prevent immune responses and achieve broader tissue correction.
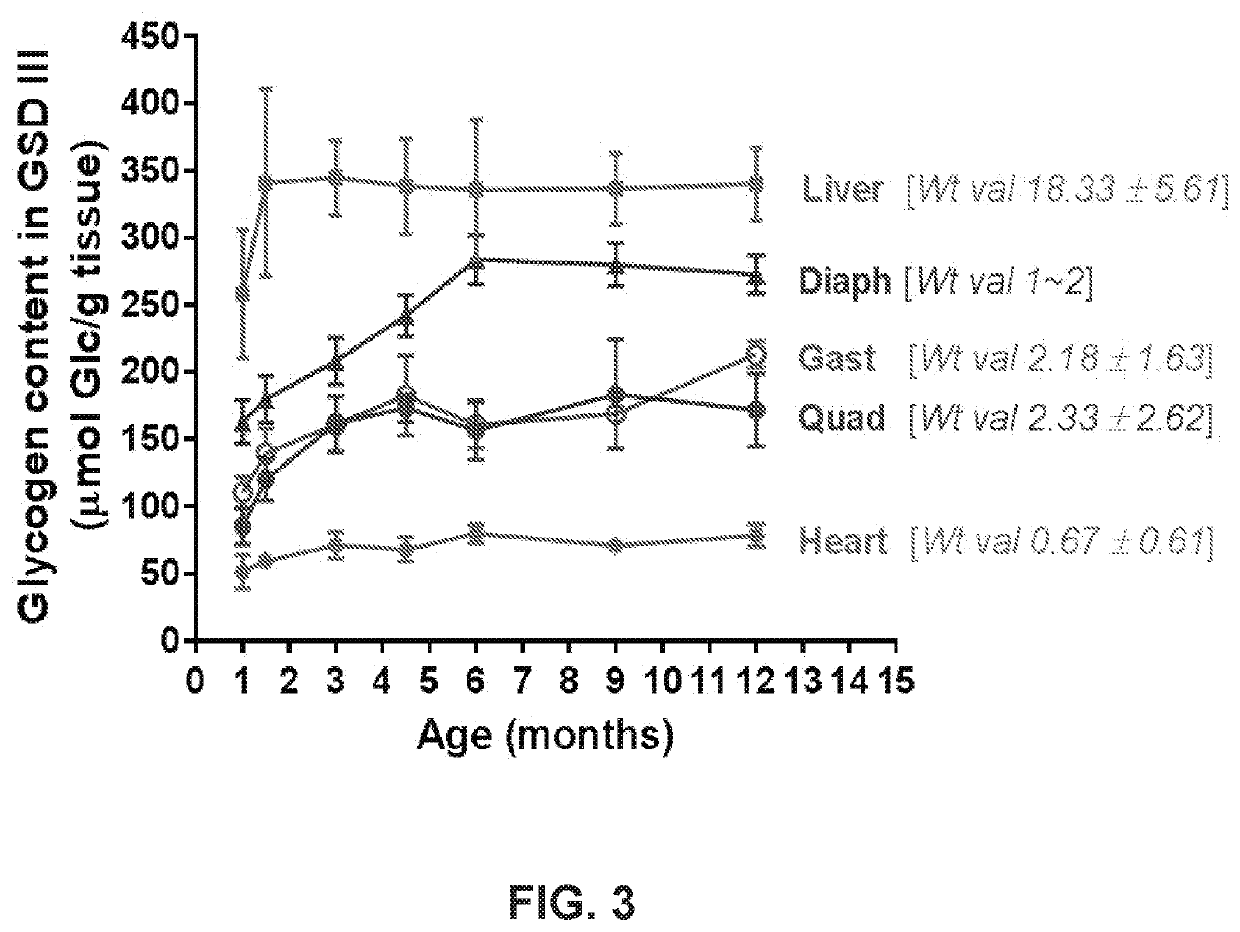
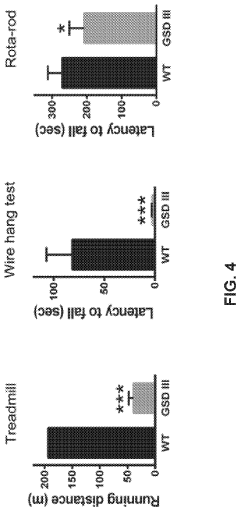
Metabolic Variations Across Different Growth Stages
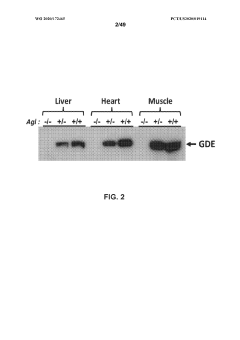
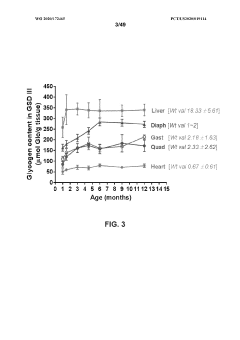
Glycogenolysis, the breakdown of glycogen to glucose-1-phosphate and glucose, exhibits significant variations across different cellular growth phases. During exponential growth, cells demonstrate heightened glycogenolytic activity to meet the substantial energy demands required for rapid proliferation. This phase is characterized by increased expression of glycogen phosphorylase, the rate-limiting enzyme in glycogenolysis, which shows up to 3-fold higher activity compared to stationary phase cells.
The transition phase between exponential and stationary growth reveals a complex metabolic shift. As growth rate decelerates, glycogenolysis initially intensifies to compensate for decreasing nutrient availability, followed by a gradual reduction as cells adapt their metabolic priorities. Recent metabolomic studies have identified distinct glycogenolytic signatures during this transition, with temporal fluctuations in glucose-1-phosphate levels serving as reliable biomarkers for growth phase determination.
Stationary phase cells exhibit markedly different glycogenolytic patterns, with activity typically reduced by 60-75% compared to exponential phase. This reduction correlates with increased glycogen synthesis as cells prioritize energy storage over consumption. The glycogenolytic machinery undergoes significant post-translational modifications during this phase, including phosphorylation and allosteric regulation that fine-tune enzyme activity according to cellular energy status.
Environmental factors substantially influence glycogenolytic variations across growth phases. Oxygen availability particularly affects glycogenolysis rates, with hypoxic conditions during dense culture growth triggering alternative metabolic pathways that modify glycogen utilization patterns. Similarly, pH fluctuations throughout growth phases alter the kinetic properties of glycogenolytic enzymes, with optimal activity typically observed within pH 6.8-7.2 range.
Interestingly, cell-to-cell heterogeneity in glycogenolysis increases dramatically during late exponential and stationary phases. Single-cell metabolomic analyses reveal subpopulations with distinct glycogenolytic profiles, suggesting specialized metabolic roles within the culture. This heterogeneity appears to serve as a survival strategy, allowing population-level resilience through metabolic diversification.
Recent advances in real-time metabolic monitoring have enabled dynamic tracking of glycogenolytic flux throughout growth phases. These technologies have revealed previously undetected oscillatory patterns in glycogen breakdown, particularly during nutrient-limited conditions, suggesting sophisticated regulatory mechanisms that coordinate glycogenolysis with other metabolic pathways to optimize resource utilization across different growth stages.
The transition phase between exponential and stationary growth reveals a complex metabolic shift. As growth rate decelerates, glycogenolysis initially intensifies to compensate for decreasing nutrient availability, followed by a gradual reduction as cells adapt their metabolic priorities. Recent metabolomic studies have identified distinct glycogenolytic signatures during this transition, with temporal fluctuations in glucose-1-phosphate levels serving as reliable biomarkers for growth phase determination.
Stationary phase cells exhibit markedly different glycogenolytic patterns, with activity typically reduced by 60-75% compared to exponential phase. This reduction correlates with increased glycogen synthesis as cells prioritize energy storage over consumption. The glycogenolytic machinery undergoes significant post-translational modifications during this phase, including phosphorylation and allosteric regulation that fine-tune enzyme activity according to cellular energy status.
Environmental factors substantially influence glycogenolytic variations across growth phases. Oxygen availability particularly affects glycogenolysis rates, with hypoxic conditions during dense culture growth triggering alternative metabolic pathways that modify glycogen utilization patterns. Similarly, pH fluctuations throughout growth phases alter the kinetic properties of glycogenolytic enzymes, with optimal activity typically observed within pH 6.8-7.2 range.
Interestingly, cell-to-cell heterogeneity in glycogenolysis increases dramatically during late exponential and stationary phases. Single-cell metabolomic analyses reveal subpopulations with distinct glycogenolytic profiles, suggesting specialized metabolic roles within the culture. This heterogeneity appears to serve as a survival strategy, allowing population-level resilience through metabolic diversification.
Recent advances in real-time metabolic monitoring have enabled dynamic tracking of glycogenolytic flux throughout growth phases. These technologies have revealed previously undetected oscillatory patterns in glycogen breakdown, particularly during nutrient-limited conditions, suggesting sophisticated regulatory mechanisms that coordinate glycogenolysis with other metabolic pathways to optimize resource utilization across different growth stages.
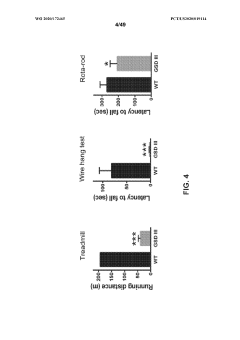
Implications for Nutritional and Medical Interventions
Understanding the variations in glycogenolysis during different growth phases has significant implications for developing targeted nutritional and medical interventions. The dynamic nature of glycogen metabolism throughout developmental stages presents unique opportunities for therapeutic approaches tailored to specific life phases.
For pediatric populations, insights into glycogenolysis patterns can inform optimized nutrition protocols that support proper growth and development. Children experiencing rapid growth phases require carefully balanced carbohydrate intake to maintain adequate glycogen reserves while supporting increased energy demands. Nutritional interventions designed with awareness of age-specific glycogenolysis rates may help prevent hypoglycemic episodes in children with metabolic disorders and improve athletic performance in young athletes.
In adolescence, when hormonal fluctuations significantly impact glycogen metabolism, targeted nutritional strategies can help manage energy availability during critical developmental windows. This knowledge is particularly valuable for addressing adolescent insulin resistance patterns and supporting metabolic health during this transitional period. Nutritional timing strategies based on glycogenolysis patterns may enhance recovery from physical activity and support cognitive function during intensive academic periods.
For adult populations, understanding phase-specific glycogenolysis variations enables the development of personalized medical interventions for metabolic disorders. Pharmaceutical approaches targeting specific enzymes in the glycogenolysis pathway can be optimized based on age-related changes in enzyme activity and regulation. This precision medicine approach may improve treatment outcomes for conditions like glycogen storage diseases and diabetes.
In aging populations, compensating for reduced glycogenolysis efficiency through tailored nutritional interventions may help maintain metabolic flexibility and prevent age-related metabolic decline. Supplementation strategies addressing specific enzymatic deficiencies that emerge with age could potentially mitigate some aspects of metabolic aging. Additionally, exercise prescriptions calibrated to age-specific glycogen utilization patterns may optimize health benefits while minimizing risks.
The therapeutic potential extends to critical care settings, where understanding growth phase variations in glycogenolysis can inform glucose management protocols for patients of different ages. This knowledge may improve outcomes in conditions requiring precise glycemic control, such as traumatic brain injury, sepsis, and post-surgical recovery, by accounting for age-specific responses to metabolic stress.
Future medical interventions may leverage emerging technologies like continuous glucose monitoring combined with artificial intelligence to deliver real-time, personalized recommendations based on individual glycogenolysis patterns across different life stages. Such approaches promise to transform management of both acute and chronic metabolic conditions through unprecedented precision in matching interventions to physiological needs.
For pediatric populations, insights into glycogenolysis patterns can inform optimized nutrition protocols that support proper growth and development. Children experiencing rapid growth phases require carefully balanced carbohydrate intake to maintain adequate glycogen reserves while supporting increased energy demands. Nutritional interventions designed with awareness of age-specific glycogenolysis rates may help prevent hypoglycemic episodes in children with metabolic disorders and improve athletic performance in young athletes.
In adolescence, when hormonal fluctuations significantly impact glycogen metabolism, targeted nutritional strategies can help manage energy availability during critical developmental windows. This knowledge is particularly valuable for addressing adolescent insulin resistance patterns and supporting metabolic health during this transitional period. Nutritional timing strategies based on glycogenolysis patterns may enhance recovery from physical activity and support cognitive function during intensive academic periods.
For adult populations, understanding phase-specific glycogenolysis variations enables the development of personalized medical interventions for metabolic disorders. Pharmaceutical approaches targeting specific enzymes in the glycogenolysis pathway can be optimized based on age-related changes in enzyme activity and regulation. This precision medicine approach may improve treatment outcomes for conditions like glycogen storage diseases and diabetes.
In aging populations, compensating for reduced glycogenolysis efficiency through tailored nutritional interventions may help maintain metabolic flexibility and prevent age-related metabolic decline. Supplementation strategies addressing specific enzymatic deficiencies that emerge with age could potentially mitigate some aspects of metabolic aging. Additionally, exercise prescriptions calibrated to age-specific glycogen utilization patterns may optimize health benefits while minimizing risks.
The therapeutic potential extends to critical care settings, where understanding growth phase variations in glycogenolysis can inform glucose management protocols for patients of different ages. This knowledge may improve outcomes in conditions requiring precise glycemic control, such as traumatic brain injury, sepsis, and post-surgical recovery, by accounting for age-specific responses to metabolic stress.
Future medical interventions may leverage emerging technologies like continuous glucose monitoring combined with artificial intelligence to deliver real-time, personalized recommendations based on individual glycogenolysis patterns across different life stages. Such approaches promise to transform management of both acute and chronic metabolic conditions through unprecedented precision in matching interventions to physiological needs.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!