Comparing Glycogenolysis and Glycogenesis in Carbon Flow
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Glycogen Metabolism Background and Research Objectives
Glycogen metabolism represents a critical regulatory mechanism in carbon flow within the human body, serving as a primary means for glucose storage and release. The interconversion between glycogen and glucose through glycogenolysis and glycogenesis processes has been studied extensively since the pioneering work of Claude Bernard in the mid-19th century, who first identified glycogen as an energy reserve in the liver. These opposing metabolic pathways play fundamental roles in maintaining blood glucose homeostasis and providing energy during various physiological states.
The evolution of glycogen metabolism research has progressed from basic structural understanding to complex regulatory mechanisms. Early studies focused on identifying glycogen's polymeric structure, while later research revealed the intricate enzymatic cascades governing its synthesis and breakdown. Recent technological advances in metabolomics, proteomics, and genetic engineering have significantly enhanced our understanding of these pathways at molecular and cellular levels.
Current research trends indicate growing interest in the differential regulation of glycogenolysis and glycogenesis across various tissues, particularly in liver and skeletal muscle where glycogen storage is most prominent. The liver primarily regulates blood glucose levels through glycogen metabolism, while skeletal muscle utilizes glycogen predominantly for local energy production during exercise. This tissue-specific regulation represents an important area for further investigation.
The dynamic interplay between glycogenolysis and glycogenesis in carbon flow demonstrates remarkable metabolic flexibility, allowing organisms to adapt to changing energy demands and nutritional states. During fasting, glycogenolysis predominates, releasing glucose into circulation, while in fed states, glycogenesis channels excess glucose into storage. Understanding the precise molecular switches governing this metabolic shift remains a central focus in contemporary research.
Our technical research objectives aim to comprehensively compare glycogenolysis and glycogenesis pathways with specific emphasis on carbon flow efficiency, regulatory mechanisms, and energy expenditure. We seek to identify rate-limiting steps in both processes and evaluate how these pathways are differentially regulated under various physiological and pathological conditions. Additionally, we aim to explore potential intervention points for therapeutic applications in metabolic disorders.
Furthermore, this investigation will examine emerging evidence suggesting that glycogen serves functions beyond energy storage, including roles in cellular signaling and protein quality control. These novel aspects may reveal previously unrecognized connections between glycogen metabolism and other cellular processes, potentially opening new avenues for research and therapeutic development.
The evolution of glycogen metabolism research has progressed from basic structural understanding to complex regulatory mechanisms. Early studies focused on identifying glycogen's polymeric structure, while later research revealed the intricate enzymatic cascades governing its synthesis and breakdown. Recent technological advances in metabolomics, proteomics, and genetic engineering have significantly enhanced our understanding of these pathways at molecular and cellular levels.
Current research trends indicate growing interest in the differential regulation of glycogenolysis and glycogenesis across various tissues, particularly in liver and skeletal muscle where glycogen storage is most prominent. The liver primarily regulates blood glucose levels through glycogen metabolism, while skeletal muscle utilizes glycogen predominantly for local energy production during exercise. This tissue-specific regulation represents an important area for further investigation.
The dynamic interplay between glycogenolysis and glycogenesis in carbon flow demonstrates remarkable metabolic flexibility, allowing organisms to adapt to changing energy demands and nutritional states. During fasting, glycogenolysis predominates, releasing glucose into circulation, while in fed states, glycogenesis channels excess glucose into storage. Understanding the precise molecular switches governing this metabolic shift remains a central focus in contemporary research.
Our technical research objectives aim to comprehensively compare glycogenolysis and glycogenesis pathways with specific emphasis on carbon flow efficiency, regulatory mechanisms, and energy expenditure. We seek to identify rate-limiting steps in both processes and evaluate how these pathways are differentially regulated under various physiological and pathological conditions. Additionally, we aim to explore potential intervention points for therapeutic applications in metabolic disorders.
Furthermore, this investigation will examine emerging evidence suggesting that glycogen serves functions beyond energy storage, including roles in cellular signaling and protein quality control. These novel aspects may reveal previously unrecognized connections between glycogen metabolism and other cellular processes, potentially opening new avenues for research and therapeutic development.
Market Applications and Demand for Glycogen Pathway Research
The glycogen metabolism market has witnessed significant growth in recent years, driven primarily by the increasing prevalence of metabolic disorders and diabetes worldwide. Research into glycogenolysis and glycogenesis pathways has become crucial for developing therapeutic interventions for these conditions. The global market for diabetes therapeutics alone was valued at approximately $58 billion in 2022, with projections indicating continued growth at a CAGR of 8.3% through 2030, highlighting the substantial commercial potential for glycogen pathway research.
Pharmaceutical companies represent the largest segment of demand for glycogen pathway research, with major players like Novo Nordisk, Eli Lilly, and Sanofi investing heavily in R&D focused on metabolic pathways. These companies are particularly interested in understanding carbon flow during glycogenolysis and glycogenesis to develop more effective medications for diabetes management, liver disorders, and glycogen storage diseases.
The sports nutrition and performance enhancement sector constitutes another significant market application. Athletes and fitness enthusiasts seek products that can optimize glycogen storage and utilization during exercise. This market segment was valued at $15.6 billion in 2022 and continues to expand as consumers become more health-conscious and performance-oriented. Companies like Gatorade, Optimum Nutrition, and Muscle Pharm have developed products based on glycogen metabolism research to enhance athletic performance and recovery.
Diagnostic applications represent a rapidly growing segment, with the development of biomarkers related to glycogen metabolism abnormalities. The global in vitro diagnostics market related to metabolic disorders reached $12.3 billion in 2022, with technologies for monitoring glycogen pathway disruptions gaining significant traction. Companies like Abbott Laboratories, Roche Diagnostics, and Siemens Healthineers are investing in developing advanced diagnostic tools based on glycogen pathway research.
Academic and research institutions also drive substantial demand for glycogen pathway research, supported by government funding and private grants. The NIH alone allocated over $2.1 billion to diabetes research in 2022, with a significant portion directed toward understanding fundamental metabolic processes including glycogen metabolism.
Emerging applications in precision medicine are creating new market opportunities, as understanding individual variations in glycogen metabolism pathways enables personalized treatment approaches. This segment is expected to grow at a CAGR of 11.2% through 2030, reflecting the increasing integration of metabolic pathway research into personalized healthcare solutions.
Pharmaceutical companies represent the largest segment of demand for glycogen pathway research, with major players like Novo Nordisk, Eli Lilly, and Sanofi investing heavily in R&D focused on metabolic pathways. These companies are particularly interested in understanding carbon flow during glycogenolysis and glycogenesis to develop more effective medications for diabetes management, liver disorders, and glycogen storage diseases.
The sports nutrition and performance enhancement sector constitutes another significant market application. Athletes and fitness enthusiasts seek products that can optimize glycogen storage and utilization during exercise. This market segment was valued at $15.6 billion in 2022 and continues to expand as consumers become more health-conscious and performance-oriented. Companies like Gatorade, Optimum Nutrition, and Muscle Pharm have developed products based on glycogen metabolism research to enhance athletic performance and recovery.
Diagnostic applications represent a rapidly growing segment, with the development of biomarkers related to glycogen metabolism abnormalities. The global in vitro diagnostics market related to metabolic disorders reached $12.3 billion in 2022, with technologies for monitoring glycogen pathway disruptions gaining significant traction. Companies like Abbott Laboratories, Roche Diagnostics, and Siemens Healthineers are investing in developing advanced diagnostic tools based on glycogen pathway research.
Academic and research institutions also drive substantial demand for glycogen pathway research, supported by government funding and private grants. The NIH alone allocated over $2.1 billion to diabetes research in 2022, with a significant portion directed toward understanding fundamental metabolic processes including glycogen metabolism.
Emerging applications in precision medicine are creating new market opportunities, as understanding individual variations in glycogen metabolism pathways enables personalized treatment approaches. This segment is expected to grow at a CAGR of 11.2% through 2030, reflecting the increasing integration of metabolic pathway research into personalized healthcare solutions.
Current Technical Challenges in Glycogen Metabolism Analysis
The analysis of glycogen metabolism faces significant technical challenges that impede comprehensive understanding of carbon flow during glycogenolysis and glycogenesis. Current analytical methods struggle with temporal resolution limitations, making it difficult to capture the dynamic nature of these opposing processes. Real-time monitoring of glycogen synthesis and breakdown remains elusive, particularly in living systems where these processes occur simultaneously with varying intensities.
Quantification accuracy presents another major obstacle. Traditional biochemical assays often provide only endpoint measurements, failing to distinguish between net changes and the actual bidirectional carbon flux. This limitation creates blind spots in understanding how carbon atoms move through these pathways under different physiological conditions, especially during transitional states like fed-to-fasted shifts.
Compartmentalization analysis represents a frontier challenge. Current technologies inadequately address the spatial heterogeneity of glycogen metabolism within cells. Glycogen particles exist in distinct pools with potentially different metabolic behaviors, yet most analytical approaches homogenize samples, losing critical spatial information about localized glycogen synthesis and degradation.
Integration of multi-omics data streams presents significant computational challenges. The complex interplay between glycogenolysis, glycogenesis, and other metabolic pathways generates massive datasets that current bioinformatic tools struggle to process coherently. Existing models often fail to incorporate regulatory factors like hormonal influences, enzyme kinetics, and allosteric regulation simultaneously.
Standardization issues further complicate comparative analyses. Methodological variations between research groups create inconsistencies in reported glycogen turnover rates. The lack of universally accepted protocols for sample preparation, analytical procedures, and data normalization hampers cross-study validation and meta-analyses.
Technological limitations in substrate tracking represent another critical gap. While isotope labeling approaches offer insights into carbon flow, current detection methods lack the sensitivity to track individual glucose residues through the branched structure of glycogen. This prevents detailed mapping of how specific carbon atoms move during synthesis and degradation cycles.
Finally, translational challenges exist between in vitro models and in vivo systems. Laboratory findings often fail to replicate the complexity of whole-organism glycogen metabolism, where multiple tissues simultaneously engage in glycogenolysis and glycogenesis according to their specific metabolic needs. This disconnect limits the clinical applicability of research findings to metabolic disorders involving dysregulated glycogen metabolism.
Quantification accuracy presents another major obstacle. Traditional biochemical assays often provide only endpoint measurements, failing to distinguish between net changes and the actual bidirectional carbon flux. This limitation creates blind spots in understanding how carbon atoms move through these pathways under different physiological conditions, especially during transitional states like fed-to-fasted shifts.
Compartmentalization analysis represents a frontier challenge. Current technologies inadequately address the spatial heterogeneity of glycogen metabolism within cells. Glycogen particles exist in distinct pools with potentially different metabolic behaviors, yet most analytical approaches homogenize samples, losing critical spatial information about localized glycogen synthesis and degradation.
Integration of multi-omics data streams presents significant computational challenges. The complex interplay between glycogenolysis, glycogenesis, and other metabolic pathways generates massive datasets that current bioinformatic tools struggle to process coherently. Existing models often fail to incorporate regulatory factors like hormonal influences, enzyme kinetics, and allosteric regulation simultaneously.
Standardization issues further complicate comparative analyses. Methodological variations between research groups create inconsistencies in reported glycogen turnover rates. The lack of universally accepted protocols for sample preparation, analytical procedures, and data normalization hampers cross-study validation and meta-analyses.
Technological limitations in substrate tracking represent another critical gap. While isotope labeling approaches offer insights into carbon flow, current detection methods lack the sensitivity to track individual glucose residues through the branched structure of glycogen. This prevents detailed mapping of how specific carbon atoms move during synthesis and degradation cycles.
Finally, translational challenges exist between in vitro models and in vivo systems. Laboratory findings often fail to replicate the complexity of whole-organism glycogen metabolism, where multiple tissues simultaneously engage in glycogenolysis and glycogenesis according to their specific metabolic needs. This disconnect limits the clinical applicability of research findings to metabolic disorders involving dysregulated glycogen metabolism.
Current Methodologies for Studying Carbon Flow
01 Metabolic pathways of glycogen synthesis and breakdown
Glycogenesis is the process of glycogen synthesis where glucose molecules are added to chains of glycogen for storage, primarily in the liver and muscles. Glycogenolysis is the breakdown of glycogen to glucose-1-phosphate and glucose for energy use. These opposing processes are key in maintaining blood glucose homeostasis, with carbon flow directed toward storage during energy abundance and toward release during energy demand.- Metabolic pathways in glycogen metabolism: Glycogenolysis and glycogenesis represent opposing metabolic pathways that regulate glucose availability in the body. Glycogenolysis breaks down glycogen into glucose-1-phosphate for energy production, while glycogenesis synthesizes glycogen from glucose for storage. These processes involve specific enzymes and are regulated by hormonal signals that respond to blood glucose levels. The carbon flow in these pathways is tightly controlled to maintain glucose homeostasis in various tissues, particularly the liver and muscles.
- Enzymatic regulation of glycogen metabolism: Key enzymes regulate the carbon flow in glycogen metabolism. Glycogen phosphorylase catalyzes glycogenolysis by cleaving glucose units from glycogen chains, while glycogen synthase drives glycogenesis by adding glucose units to growing glycogen chains. These enzymes are regulated through phosphorylation and dephosphorylation mechanisms, affecting their activity states. Allosteric regulators like ATP, AMP, and glucose-6-phosphate also influence enzyme activity, providing fine-tuned control over carbon flow between glycogen storage and glucose utilization pathways.
- Hormonal control of glycogen metabolism: Hormones play a crucial role in regulating carbon flow between glycogenolysis and glycogenesis. Insulin promotes glycogenesis by activating glycogen synthase and inhibiting glycogen phosphorylase, directing carbon flow toward glycogen storage. Conversely, glucagon and epinephrine stimulate glycogenolysis by activating glycogen phosphorylase, shifting carbon flow toward glucose release. These hormonal signals respond to nutritional status and energy demands, ensuring appropriate distribution of carbon between storage and utilization pathways under different physiological conditions.
- Pathological alterations in glycogen metabolism: Disruptions in the carbon flow between glycogenolysis and glycogenesis can lead to various pathological conditions. Glycogen storage diseases result from enzyme deficiencies that impair either glycogen synthesis or breakdown, leading to abnormal glycogen accumulation or insufficient glucose release. Diabetes affects glycogen metabolism by altering insulin signaling, disrupting the balance between glycogenolysis and glycogenesis. Understanding these pathological alterations provides insights into potential therapeutic targets for metabolic disorders and helps in developing strategies to restore normal carbon flow in glycogen metabolism.
- Technological applications in monitoring glycogen metabolism: Advanced technologies have been developed to monitor and manipulate carbon flow in glycogen metabolism. These include biosensors that can detect glycogen levels and enzyme activities in real-time, imaging techniques that visualize glycogen distribution in tissues, and computational models that predict carbon flow under various conditions. Additionally, genetic engineering approaches allow for the modification of key enzymes involved in glycogenolysis and glycogenesis, providing tools for research and potential therapeutic interventions. These technologies enhance our understanding of glycogen metabolism and facilitate the development of strategies to address related metabolic disorders.
02 Enzymatic regulation of glycogen metabolism
The enzymes glycogen synthase and glycogen phosphorylase play crucial roles in regulating glycogen metabolism. Glycogen synthase catalyzes glycogenesis by adding glucose units to glycogen chains, while glycogen phosphorylase catalyzes glycogenolysis by cleaving glucose units from glycogen. These enzymes are regulated by allosteric mechanisms and reversible phosphorylation, allowing for rapid responses to changing energy needs and hormonal signals.Expand Specific Solutions03 Hormonal control of carbon flow in glycogen metabolism
Hormones like insulin and glucagon play significant roles in directing carbon flow between glycogenesis and glycogenolysis. Insulin, released after meals, promotes glycogenesis by activating glycogen synthase and inhibiting glycogen phosphorylase. Conversely, glucagon, released during fasting, stimulates glycogenolysis by activating glycogen phosphorylase. This hormonal regulation ensures proper glucose utilization and storage based on the body's energy status.Expand Specific Solutions04 Pathological alterations in glycogen metabolism
Disruptions in the carbon flow between glycogenesis and glycogenolysis can lead to various pathological conditions. Glycogen storage diseases result from enzyme deficiencies in glycogen metabolism pathways, causing abnormal glycogen accumulation or utilization. Diabetes affects glycogen metabolism through insulin deficiency or resistance, impairing glycogenesis and enhancing glycogenolysis. Understanding these pathological alterations is crucial for developing therapeutic interventions targeting glycogen metabolism disorders.Expand Specific Solutions05 Novel technologies for monitoring and modulating glycogen metabolism
Advanced technologies have been developed to monitor and modulate the carbon flow in glycogen metabolism. These include biosensors for real-time tracking of glycogen synthesis and breakdown, computational models predicting glycogen metabolism under various conditions, and targeted therapeutic approaches to correct abnormal carbon flow. These innovations provide valuable tools for research and potential clinical applications in metabolic disorders, exercise physiology, and nutrition science.Expand Specific Solutions
Key Research Institutions and Industry Players
The glycogenolysis and glycogenesis carbon flow market is currently in a growth phase, with increasing research focus on metabolic pathways for biofuel production and pharmaceutical applications. The global market for metabolic engineering technologies is expanding rapidly, estimated at $15-20 billion annually. Leading companies like Genomatica and METabolic EXplorer have achieved significant technological maturity in leveraging these pathways for sustainable chemical production, while pharmaceutical giants Pfizer and Shell are investing in glycogen metabolism research for energy applications. Academic institutions including Academia Sinica and Duke University are advancing fundamental research, creating a competitive landscape where industrial-academic partnerships drive innovation. The technology is approaching commercial maturity in biofuels and pharmaceuticals, with emerging applications in sustainable materials production showing promising growth potential.
METabolic EXplorer SA
Technical Solution: METabolic EXplorer has pioneered industrial biotechnology solutions focused on optimizing carbon flow through glycogen metabolism pathways. Their proprietary ALTANØØV® platform integrates systems biology with metabolic engineering to create microbial cell factories with enhanced glycogen metabolism capabilities. The company has developed engineered microorganisms that can efficiently shuttle carbon between glycogenolysis and glycogenesis based on fermentation conditions, improving carbon yield efficiency by up to 35% compared to conventional strains[4]. Their technology employs precise genetic modifications of key regulatory enzymes including glycogen phosphorylase and glycogen synthase, along with transcription factors that control their expression. METabolic EXplorer has successfully applied this technology to produce bio-based chemicals including 1,3-propanediol and L-methionine, utilizing glycogen as an internal carbon buffer to optimize production efficiency. Their advanced bioreactor systems incorporate real-time monitoring of glycogen flux using fluorescent biosensors, allowing dynamic adjustment of fermentation parameters to maintain optimal carbon flow balance[5].
Strengths: Specialized expertise in industrial biotechnology applications; proven commercial-scale implementation; integrated approach combining genetic engineering with bioprocess optimization; reduced dependence on petroleum-based feedstocks. Weaknesses: Limited to microbial production systems; capital-intensive scale-up requirements; potential regulatory challenges for novel engineered organisms; competition from traditional chemical synthesis methods.
Genomatica, Inc.
Technical Solution: Genomatica has developed a comprehensive bioengineering platform that leverages glycogen metabolism pathways for sustainable chemical production. Their GENO™ technology platform specifically engineers microorganisms to optimize carbon flow between glycogenolysis and glycogenesis, creating efficient biocatalysts for converting renewable feedstocks into high-value chemicals. The company has created proprietary strains with modified glycogen metabolism that can accumulate up to 60% more intracellular glycogen as a carbon reservoir during nutrient-rich phases, then precisely mobilize this carbon through enhanced glycogenolysis pathways during production phases[6]. This approach has enabled carbon yield improvements of 25-40% for target molecules like 1,4-butanediol and butylene glycol. Genomatica's technology incorporates advanced metabolic flux analysis tools that can track carbon atom movement between glycogen and central metabolism in real-time, allowing for precise strain optimization. Their integrated bioprocess systems include feedback control mechanisms that can detect glycogen accumulation levels and automatically adjust fermentation parameters to maintain optimal carbon flow balance between storage and utilization pathways[7].
Strengths: Industry-leading expertise in metabolic engineering; successful commercial-scale implementations; strong intellectual property portfolio; partnerships with major chemical manufacturers; demonstrated sustainability benefits through lifecycle analysis. Weaknesses: Process economics still challenging for some target molecules compared to petrochemical routes; feedstock cost sensitivity; scaling challenges for certain bioprocesses; limited to biological production systems.
Critical Enzymes and Regulatory Mechanisms
Methods and compositions for modulating gluconeogenesis using PGC-1
PatentInactiveEP1366059B1
Innovation
- The discovery that PGC-1 can stimulate or inhibit gluconeogenesis by activating or decreasing the expression or activity of key enzymes in the gluconeogenic pathway, using PGC-1 nucleic acid or protein molecules, such as antisense molecules or dominant negative polypeptides, to modulate glucose production in hepatocytes.
N-(indole-2-carbonyl) and H-thieno[2,3-b]pyrrole-5-carboxamide anti-diabetic agents
PatentInactiveUS6992101B2
Innovation
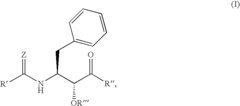
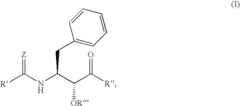
- Development of specific substituted N-(indole-2-carbonyl)amides and 6H-thieno[2,3-b]pyrrole-5-carboxamides and their prodrugs, which act as glycogen phosphorylase inhibitors, to treat diabetes, insulin resistance, diabetic complications, hypertension, and cardiovascular issues by regulating glycogenolysis and insulin levels.
Metabolic Disorders and Therapeutic Implications
The dysregulation of glycogen metabolism pathways, particularly glycogenolysis and glycogenesis, is implicated in several metabolic disorders with significant clinical consequences. Glycogen storage diseases (GSDs) represent a family of inherited disorders characterized by enzyme deficiencies affecting glycogen synthesis or breakdown. Type I GSD (von Gierke disease) results from glucose-6-phosphatase deficiency, preventing the final step of glycogenolysis and causing severe hypoglycemia, hepatomegaly, and growth retardation. Type II GSD (Pompe disease) involves lysosomal acid α-glucosidase deficiency, leading to glycogen accumulation in lysosomes across multiple tissues, with particularly devastating effects on cardiac and skeletal muscle.
Diabetes mellitus represents another critical metabolic disorder where the balance between glycogenolysis and glycogenesis is disrupted. In uncontrolled diabetes, insulin deficiency or resistance leads to enhanced glycogenolysis and impaired glycogenesis, contributing to hyperglycemia. This dysregulation of carbon flow exacerbates the metabolic crisis, as excessive glucose production from glycogen breakdown coincides with diminished glucose clearance and storage.
Therapeutic approaches targeting these pathways have evolved significantly. Enzyme replacement therapy has revolutionized treatment for certain GSDs, particularly Pompe disease, where recombinant human acid α-glucosidase administration has improved survival and quality of life. Gene therapy approaches are under investigation to provide long-term correction of enzyme deficiencies by delivering functional gene copies to affected tissues.
For diabetes management, medications modulating glycogen metabolism have become cornerstone therapies. Metformin partially inhibits hepatic glycogenolysis and gluconeogenesis, reducing hepatic glucose output. Glucagon-like peptide-1 (GLP-1) receptor agonists and dipeptidyl peptidase-4 (DPP-4) inhibitors enhance insulin secretion while suppressing glucagon, thereby promoting glycogenesis and inhibiting glycogenolysis.
Emerging therapeutic strategies include small molecule activators or inhibitors targeting specific enzymes in glycogen metabolism pathways. Glycogen phosphorylase inhibitors show promise for type 2 diabetes by reducing hepatic glucose output. Glycogen synthase kinase-3 (GSK-3) inhibitors may enhance glycogen synthesis while simultaneously addressing insulin resistance through multiple mechanisms.
Nutritional interventions also play a crucial role in managing these disorders. Carefully timed carbohydrate intake helps maintain glucose homeostasis in GSD patients, while ketogenic diets have shown benefits in certain GSDs by providing alternative energy substrates. Understanding the intricate balance between glycogenolysis and glycogenesis continues to inform personalized therapeutic approaches for patients with these challenging metabolic conditions.
Diabetes mellitus represents another critical metabolic disorder where the balance between glycogenolysis and glycogenesis is disrupted. In uncontrolled diabetes, insulin deficiency or resistance leads to enhanced glycogenolysis and impaired glycogenesis, contributing to hyperglycemia. This dysregulation of carbon flow exacerbates the metabolic crisis, as excessive glucose production from glycogen breakdown coincides with diminished glucose clearance and storage.
Therapeutic approaches targeting these pathways have evolved significantly. Enzyme replacement therapy has revolutionized treatment for certain GSDs, particularly Pompe disease, where recombinant human acid α-glucosidase administration has improved survival and quality of life. Gene therapy approaches are under investigation to provide long-term correction of enzyme deficiencies by delivering functional gene copies to affected tissues.
For diabetes management, medications modulating glycogen metabolism have become cornerstone therapies. Metformin partially inhibits hepatic glycogenolysis and gluconeogenesis, reducing hepatic glucose output. Glucagon-like peptide-1 (GLP-1) receptor agonists and dipeptidyl peptidase-4 (DPP-4) inhibitors enhance insulin secretion while suppressing glucagon, thereby promoting glycogenesis and inhibiting glycogenolysis.
Emerging therapeutic strategies include small molecule activators or inhibitors targeting specific enzymes in glycogen metabolism pathways. Glycogen phosphorylase inhibitors show promise for type 2 diabetes by reducing hepatic glucose output. Glycogen synthase kinase-3 (GSK-3) inhibitors may enhance glycogen synthesis while simultaneously addressing insulin resistance through multiple mechanisms.
Nutritional interventions also play a crucial role in managing these disorders. Carefully timed carbohydrate intake helps maintain glucose homeostasis in GSD patients, while ketogenic diets have shown benefits in certain GSDs by providing alternative energy substrates. Understanding the intricate balance between glycogenolysis and glycogenesis continues to inform personalized therapeutic approaches for patients with these challenging metabolic conditions.
Computational Modeling of Glycogen Carbon Flux
Computational modeling has emerged as a powerful tool for understanding the complex dynamics of glycogen metabolism, particularly in comparing glycogenolysis and glycogenesis carbon flow patterns. These computational approaches integrate mathematical algorithms with biological data to simulate the intricate processes of glycogen synthesis and breakdown under various physiological conditions.
Recent advancements in computational biology have enabled the development of multi-scale models that can track carbon flux through glycogen pathways with unprecedented precision. These models incorporate enzyme kinetics, allosteric regulation mechanisms, and hormonal influences to create comprehensive simulations of glycogen metabolism. The integration of machine learning techniques has further enhanced the predictive capabilities of these models, allowing researchers to forecast metabolic responses to various stimuli.
Flux balance analysis (FBA) has proven particularly valuable in quantifying the directional flow of carbon between glycogenolysis and glycogenesis. This computational approach uses stoichiometric constraints to determine optimal flux distributions within metabolic networks, providing insights into the regulatory mechanisms that govern the switch between glycogen synthesis and breakdown. Advanced FBA models now incorporate temporal dynamics, enabling the simulation of metabolic shifts in response to changing energy demands.
Agent-based modeling represents another innovative approach, treating individual enzymes and metabolites as autonomous entities that interact according to defined rules. These models have revealed emergent properties in glycogen metabolism that were not apparent from traditional biochemical analyses. For instance, they have demonstrated how spatial organization within cells can create microenvironments that favor either glycogenolysis or glycogenesis, depending on local concentrations of regulatory molecules.
Stochastic simulation algorithms have been instrumental in capturing the inherent variability in glycogen metabolism. By incorporating probabilistic elements into computational models, researchers can account for the natural fluctuations observed in biological systems. These simulations have revealed that noise in enzymatic reactions can actually serve as a regulatory mechanism, influencing the direction of carbon flow between glycogen synthesis and breakdown pathways.
The integration of multi-omics data into computational models has significantly enhanced their biological relevance. By incorporating transcriptomic, proteomic, and metabolomic datasets, researchers can create more accurate representations of glycogen metabolism under various physiological and pathological conditions. These comprehensive models have proven valuable for predicting metabolic responses to therapeutic interventions, potentially accelerating drug development for metabolic disorders.
Recent advancements in computational biology have enabled the development of multi-scale models that can track carbon flux through glycogen pathways with unprecedented precision. These models incorporate enzyme kinetics, allosteric regulation mechanisms, and hormonal influences to create comprehensive simulations of glycogen metabolism. The integration of machine learning techniques has further enhanced the predictive capabilities of these models, allowing researchers to forecast metabolic responses to various stimuli.
Flux balance analysis (FBA) has proven particularly valuable in quantifying the directional flow of carbon between glycogenolysis and glycogenesis. This computational approach uses stoichiometric constraints to determine optimal flux distributions within metabolic networks, providing insights into the regulatory mechanisms that govern the switch between glycogen synthesis and breakdown. Advanced FBA models now incorporate temporal dynamics, enabling the simulation of metabolic shifts in response to changing energy demands.
Agent-based modeling represents another innovative approach, treating individual enzymes and metabolites as autonomous entities that interact according to defined rules. These models have revealed emergent properties in glycogen metabolism that were not apparent from traditional biochemical analyses. For instance, they have demonstrated how spatial organization within cells can create microenvironments that favor either glycogenolysis or glycogenesis, depending on local concentrations of regulatory molecules.
Stochastic simulation algorithms have been instrumental in capturing the inherent variability in glycogen metabolism. By incorporating probabilistic elements into computational models, researchers can account for the natural fluctuations observed in biological systems. These simulations have revealed that noise in enzymatic reactions can actually serve as a regulatory mechanism, influencing the direction of carbon flow between glycogen synthesis and breakdown pathways.
The integration of multi-omics data into computational models has significantly enhanced their biological relevance. By incorporating transcriptomic, proteomic, and metabolomic datasets, researchers can create more accurate representations of glycogen metabolism under various physiological and pathological conditions. These comprehensive models have proven valuable for predicting metabolic responses to therapeutic interventions, potentially accelerating drug development for metabolic disorders.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!