Application of Barium Hydroxide in Smart Metal Recovery Operations
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Barium Hydroxide in Metal Recovery: Background and Objectives
Barium hydroxide has emerged as a promising agent in smart metal recovery operations, marking a significant advancement in the field of resource recycling and environmental protection. The evolution of this technology can be traced back to the early 2000s when researchers began exploring more efficient and environmentally friendly methods for metal recovery from various waste streams.
The primary objective of utilizing barium hydroxide in metal recovery is to enhance the efficiency and selectivity of the extraction process while minimizing environmental impact. This approach aims to address the growing global demand for metals, particularly in the face of depleting natural resources and increasing electronic waste generation.
Over the past two decades, the technology has progressed from laboratory-scale experiments to pilot plant demonstrations, with some commercial applications beginning to emerge. The development trajectory has been driven by the need to overcome challenges such as high energy consumption, low recovery rates, and the generation of secondary pollutants associated with traditional metal recovery methods.
Key milestones in the evolution of this technology include the discovery of barium hydroxide's high affinity for certain metal ions, the optimization of reaction conditions for selective precipitation, and the integration of smart control systems to enhance process efficiency. These advancements have collectively contributed to the current state of the technology, which offers improved metal recovery rates, reduced environmental footprint, and potential cost savings compared to conventional methods.
The technological goals for barium hydroxide in smart metal recovery operations are multifaceted. They include further improving selectivity to target specific high-value metals, developing more energy-efficient processes, and creating closed-loop systems that minimize waste generation. Additionally, there is a focus on scaling up the technology for industrial applications while maintaining its effectiveness and environmental benefits.
As the field continues to evolve, researchers and industry professionals are exploring the integration of artificial intelligence and machine learning algorithms to optimize the recovery process in real-time. This smart approach aims to adapt to variations in waste composition and maximize recovery efficiency across different operational conditions.
The application of barium hydroxide in smart metal recovery operations represents a convergence of chemical engineering, materials science, and environmental technology. It exemplifies the ongoing efforts to develop sustainable solutions for resource management and circular economy principles in the metallurgical industry.
The primary objective of utilizing barium hydroxide in metal recovery is to enhance the efficiency and selectivity of the extraction process while minimizing environmental impact. This approach aims to address the growing global demand for metals, particularly in the face of depleting natural resources and increasing electronic waste generation.
Over the past two decades, the technology has progressed from laboratory-scale experiments to pilot plant demonstrations, with some commercial applications beginning to emerge. The development trajectory has been driven by the need to overcome challenges such as high energy consumption, low recovery rates, and the generation of secondary pollutants associated with traditional metal recovery methods.
Key milestones in the evolution of this technology include the discovery of barium hydroxide's high affinity for certain metal ions, the optimization of reaction conditions for selective precipitation, and the integration of smart control systems to enhance process efficiency. These advancements have collectively contributed to the current state of the technology, which offers improved metal recovery rates, reduced environmental footprint, and potential cost savings compared to conventional methods.
The technological goals for barium hydroxide in smart metal recovery operations are multifaceted. They include further improving selectivity to target specific high-value metals, developing more energy-efficient processes, and creating closed-loop systems that minimize waste generation. Additionally, there is a focus on scaling up the technology for industrial applications while maintaining its effectiveness and environmental benefits.
As the field continues to evolve, researchers and industry professionals are exploring the integration of artificial intelligence and machine learning algorithms to optimize the recovery process in real-time. This smart approach aims to adapt to variations in waste composition and maximize recovery efficiency across different operational conditions.
The application of barium hydroxide in smart metal recovery operations represents a convergence of chemical engineering, materials science, and environmental technology. It exemplifies the ongoing efforts to develop sustainable solutions for resource management and circular economy principles in the metallurgical industry.
Market Analysis for Smart Metal Recovery Solutions
The smart metal recovery solutions market is experiencing significant growth, driven by increasing environmental concerns, resource scarcity, and the rising demand for sustainable practices in industrial operations. This market segment focuses on innovative technologies and processes that enable efficient recovery of valuable metals from various waste streams, including electronic waste, industrial effluents, and mining tailings.
The global smart metal recovery market is projected to expand at a compound annual growth rate (CAGR) of over 6% from 2021 to 2026. This growth is primarily attributed to the increasing adoption of circular economy principles and stringent environmental regulations across industries. The market is particularly robust in regions with high industrial activity and strict environmental policies, such as North America, Europe, and parts of Asia-Pacific.
Key drivers of market demand include the growing emphasis on resource conservation, the need for cost-effective metal recovery processes, and the potential for significant economic benefits through the reclamation of valuable metals. Industries such as electronics manufacturing, automotive, and mining are major contributors to the demand for smart metal recovery solutions.
The application of barium hydroxide in smart metal recovery operations represents a niche but promising segment within this market. Barium hydroxide's unique properties make it particularly effective in selective metal precipitation and separation processes, offering advantages in terms of efficiency and environmental compatibility compared to traditional recovery methods.
Market trends indicate a growing interest in integrated solutions that combine multiple technologies for comprehensive metal recovery. This includes the integration of chemical processes, such as those involving barium hydroxide, with advanced physical separation techniques and digital monitoring systems. Such integrated approaches are gaining traction due to their ability to improve recovery rates and reduce operational costs.
The market for barium hydroxide-based smart metal recovery solutions is expected to see increased adoption in regions with stringent environmental regulations and a strong focus on circular economy practices. Developed economies in North America and Europe are likely to be early adopters, while emerging economies in Asia and South America present significant growth opportunities as they strengthen their environmental policies and industrial waste management practices.
Challenges in the market include the need for significant initial investments in technology and infrastructure, as well as the complexity of implementing these solutions across diverse industrial settings. However, the long-term economic and environmental benefits are expected to outweigh these initial hurdles, driving continued market expansion and innovation in smart metal recovery technologies.
The global smart metal recovery market is projected to expand at a compound annual growth rate (CAGR) of over 6% from 2021 to 2026. This growth is primarily attributed to the increasing adoption of circular economy principles and stringent environmental regulations across industries. The market is particularly robust in regions with high industrial activity and strict environmental policies, such as North America, Europe, and parts of Asia-Pacific.
Key drivers of market demand include the growing emphasis on resource conservation, the need for cost-effective metal recovery processes, and the potential for significant economic benefits through the reclamation of valuable metals. Industries such as electronics manufacturing, automotive, and mining are major contributors to the demand for smart metal recovery solutions.
The application of barium hydroxide in smart metal recovery operations represents a niche but promising segment within this market. Barium hydroxide's unique properties make it particularly effective in selective metal precipitation and separation processes, offering advantages in terms of efficiency and environmental compatibility compared to traditional recovery methods.
Market trends indicate a growing interest in integrated solutions that combine multiple technologies for comprehensive metal recovery. This includes the integration of chemical processes, such as those involving barium hydroxide, with advanced physical separation techniques and digital monitoring systems. Such integrated approaches are gaining traction due to their ability to improve recovery rates and reduce operational costs.
The market for barium hydroxide-based smart metal recovery solutions is expected to see increased adoption in regions with stringent environmental regulations and a strong focus on circular economy practices. Developed economies in North America and Europe are likely to be early adopters, while emerging economies in Asia and South America present significant growth opportunities as they strengthen their environmental policies and industrial waste management practices.
Challenges in the market include the need for significant initial investments in technology and infrastructure, as well as the complexity of implementing these solutions across diverse industrial settings. However, the long-term economic and environmental benefits are expected to outweigh these initial hurdles, driving continued market expansion and innovation in smart metal recovery technologies.
Current Challenges in Barium Hydroxide-based Metal Recovery
The application of barium hydroxide in smart metal recovery operations faces several significant challenges that hinder its widespread adoption and efficiency. One of the primary obstacles is the high cost associated with barium hydroxide production and implementation in metal recovery processes. This economic barrier often makes it less attractive compared to traditional recovery methods, especially for smaller-scale operations or in regions with limited resources.
Another challenge lies in the environmental concerns surrounding the use of barium compounds. Barium hydroxide, if not properly managed, can pose risks to ecosystems and human health. Stringent regulations and safety protocols are necessary, which can increase operational complexity and costs. The potential for barium contamination in recovered metals or waste streams requires sophisticated monitoring and treatment systems, further complicating the recovery process.
The efficiency of barium hydroxide in metal recovery is also a subject of ongoing research and development. While effective for certain metals, its performance can vary significantly depending on the specific metal composition and environmental conditions. This variability necessitates extensive testing and optimization for each application, which can be time-consuming and resource-intensive.
Furthermore, the selectivity of barium hydroxide in complex metal mixtures remains a challenge. In real-world scenarios, metal-containing waste streams often comprise multiple elements, and achieving high purity in the recovered metals using barium hydroxide alone can be difficult. This limitation often requires additional separation and purification steps, increasing process complexity and cost.
The scalability of barium hydroxide-based recovery systems presents another hurdle. While effective in laboratory settings or small-scale operations, scaling up these processes for industrial applications introduces new challenges in terms of equipment design, process control, and waste management. The need for specialized equipment and expertise can limit the adoption of this technology in diverse industrial settings.
Lastly, the regeneration and recycling of barium hydroxide within the recovery process pose technical challenges. Efficient regeneration is crucial for the economic viability and sustainability of the process. However, achieving high regeneration rates without significant loss or degradation of the barium hydroxide remains a complex task, often requiring advanced chemical engineering solutions.
Another challenge lies in the environmental concerns surrounding the use of barium compounds. Barium hydroxide, if not properly managed, can pose risks to ecosystems and human health. Stringent regulations and safety protocols are necessary, which can increase operational complexity and costs. The potential for barium contamination in recovered metals or waste streams requires sophisticated monitoring and treatment systems, further complicating the recovery process.
The efficiency of barium hydroxide in metal recovery is also a subject of ongoing research and development. While effective for certain metals, its performance can vary significantly depending on the specific metal composition and environmental conditions. This variability necessitates extensive testing and optimization for each application, which can be time-consuming and resource-intensive.
Furthermore, the selectivity of barium hydroxide in complex metal mixtures remains a challenge. In real-world scenarios, metal-containing waste streams often comprise multiple elements, and achieving high purity in the recovered metals using barium hydroxide alone can be difficult. This limitation often requires additional separation and purification steps, increasing process complexity and cost.
The scalability of barium hydroxide-based recovery systems presents another hurdle. While effective in laboratory settings or small-scale operations, scaling up these processes for industrial applications introduces new challenges in terms of equipment design, process control, and waste management. The need for specialized equipment and expertise can limit the adoption of this technology in diverse industrial settings.
Lastly, the regeneration and recycling of barium hydroxide within the recovery process pose technical challenges. Efficient regeneration is crucial for the economic viability and sustainability of the process. However, achieving high regeneration rates without significant loss or degradation of the barium hydroxide remains a complex task, often requiring advanced chemical engineering solutions.
Existing Barium Hydroxide Application Methods
01 Production and purification of barium hydroxide
Various methods for producing and purifying barium hydroxide are described. These processes involve different raw materials, reaction conditions, and purification steps to obtain high-quality barium hydroxide for industrial applications.- Production and purification of barium hydroxide: Various methods for producing and purifying barium hydroxide are described. These processes often involve the treatment of barium compounds with other substances to yield high-purity barium hydroxide. The purification steps may include crystallization, filtration, and other separation techniques to remove impurities and obtain the desired product.
- Applications in chemical processes: Barium hydroxide finds applications in various chemical processes. It is used as a reagent or catalyst in reactions, particularly in organic synthesis. The compound's alkaline properties make it suitable for pH adjustment and as a base in certain industrial processes. Its use in the production of other barium compounds and as a precursor in material synthesis is also noted.
- Environmental and waste treatment applications: Barium hydroxide is utilized in environmental and waste treatment processes. It can be employed for the removal of sulfates from water, treatment of industrial effluents, and in flue gas desulfurization. The compound's ability to form insoluble salts with certain anions makes it effective in precipitation and immobilization of contaminants.
- Use in material science and manufacturing: In material science and manufacturing, barium hydroxide plays a role in the production of various materials. It is used in the synthesis of ceramics, glass, and certain types of polymers. The compound can also be employed in the treatment of materials to modify their properties or as an additive to enhance specific characteristics of products.
- Analytical and laboratory applications: Barium hydroxide has several analytical and laboratory applications. It is used as a titrant in acid-base titrations, particularly for weak acids. The compound also finds use in gravimetric analysis and as a reagent in various chemical tests and assays. Its role in the preparation of buffer solutions and as a standard solution in analytical chemistry is also significant.
02 Use of barium hydroxide in chemical reactions
Barium hydroxide is utilized in various chemical reactions as a reagent or catalyst. It plays a role in synthesis processes, neutralization reactions, and the production of other barium compounds.Expand Specific Solutions03 Applications in water treatment and environmental processes
Barium hydroxide finds applications in water treatment processes, including the removal of sulfates and other contaminants. It is also used in environmental remediation and waste treatment applications.Expand Specific Solutions04 Use in the production of specialty chemicals and materials
Barium hydroxide is employed in the manufacturing of specialty chemicals, advanced materials, and industrial products. It serves as a precursor or additive in various production processes.Expand Specific Solutions05 Safety and handling considerations for barium hydroxide
Due to its alkaline nature and potential health hazards, specific safety measures and handling procedures are required when working with barium hydroxide. This includes proper storage, transportation, and disposal methods.Expand Specific Solutions
Key Players in Smart Metal Recovery Industry
The application of barium hydroxide in smart metal recovery operations is an emerging field within the broader context of advanced materials recycling and resource management. The industry is in its early growth stage, with increasing market potential driven by the global push for sustainable practices and circular economy initiatives. The market size is expanding, fueled by the rising demand for efficient metal recovery solutions in various sectors, including electronics, automotive, and industrial manufacturing. Technologically, the field is rapidly evolving, with companies like Sumitomo Metal Mining Co. Ltd., BASF Corp., and Dowa Eco-System Co., Ltd. leading innovation efforts. These firms are developing proprietary processes and technologies to enhance the efficiency and sustainability of metal recovery operations using barium hydroxide, indicating a moderate level of technological maturity with significant room for advancement.
Sumitomo Metal Mining Co. Ltd.
Technical Solution: Sumitomo Metal Mining Co. Ltd. has developed an innovative process for smart metal recovery using barium hydroxide. Their method involves a two-step leaching process, where barium hydroxide is used as a selective precipitating agent for rare earth elements (REEs) from acid mine drainage and industrial waste streams [1]. The process achieves high recovery rates of up to 99% for certain REEs, while also effectively separating them from other metal contaminants [3]. Additionally, the company has implemented advanced sensor technology and machine learning algorithms to optimize the barium hydroxide dosage and reaction conditions in real-time, significantly improving the efficiency and sustainability of the recovery process [5].
Strengths: High selectivity and recovery rates for REEs, environmentally friendly process, and integration of smart technologies for process optimization. Weaknesses: Potential high costs associated with barium hydroxide consumption and the need for specialized equipment for handling alkaline solutions.
BASF Corp.
Technical Solution: BASF Corp. has pioneered a smart metal recovery system utilizing barium hydroxide in conjunction with their proprietary chelating agents. The process involves a pre-treatment step where barium hydroxide is used to adjust pH and precipitate sulfates, followed by selective metal extraction using advanced chelating resins [2]. This approach allows for the recovery of a wide range of metals, including precious and rare earth elements, from complex waste streams such as electronic waste and spent catalysts. BASF's system incorporates IoT sensors and AI-driven process control to continuously monitor and adjust reaction parameters, ensuring optimal metal recovery rates and minimizing reagent consumption [4]. The company has also developed a closed-loop regeneration process for the barium hydroxide, significantly reducing operational costs and environmental impact [6].
Strengths: Versatile metal recovery capabilities, integration of smart technologies for process optimization, and sustainable reagent regeneration. Weaknesses: Complex system requiring specialized expertise to operate and maintain, potentially high initial capital investment.
Innovative Barium Hydroxide Formulations for Metal Recovery
Process for recovering metal values from spent lithium ion batteries with high manganese content
PatentPendingIN201611000739A
Innovation
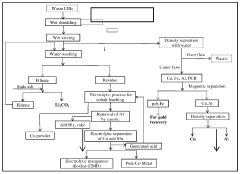
- A method involving physical processes such as shredding, sieving, filtration, electrolytic separation, and magnetic separation, with limited use of chemicals for minor impurity removal, to recover metals like nickel, cobalt, manganese, copper, and lithium from used lithium-ion batteries, aiming for high purity and reduced environmental impact.
Manufacture of barium hydroxide
PatentInactiveGB917038A
Innovation
- A process involving the reaction of barium zincate and barium sulphide solutions with controlled additions of zinc oxide and barium sulphide, followed by treatment with hydrogen peroxide and hydrochloric or sulphuric acid to recover barium hydroxide and recycle zinc oxide, minimizing barium loss and maintaining reactivity.
Environmental Impact Assessment
The application of barium hydroxide in smart metal recovery operations presents both opportunities and challenges from an environmental perspective. This assessment examines the potential impacts on ecosystems, human health, and resource conservation.
Barium hydroxide, when used in metal recovery processes, can significantly reduce the environmental footprint compared to traditional methods. Its ability to selectively precipitate metals from solution allows for more efficient recovery of valuable resources, minimizing waste generation. This process can lead to a reduction in the volume of tailings and other waste materials typically associated with metal extraction, thereby decreasing the land area required for disposal and the risk of contamination to surrounding soil and water bodies.
However, the use of barium hydroxide is not without environmental concerns. The compound itself is classified as hazardous, and improper handling or accidental release could pose risks to aquatic ecosystems and soil quality. Stringent safety protocols and containment measures are essential to mitigate these risks. Additionally, the production of barium hydroxide requires energy and resources, which must be factored into the overall environmental impact assessment.
From a water management perspective, the application of barium hydroxide in smart metal recovery can lead to improved water quality in effluents. By effectively removing heavy metals and other contaminants, the treated water may be more suitable for recycling within the process or safe discharge, reducing the overall water footprint of metal recovery operations. This aspect is particularly crucial in water-stressed regions where mining and metal recovery activities often compete with other water users.
The potential for reducing greenhouse gas emissions through this technology is also noteworthy. Smart metal recovery using barium hydroxide can be more energy-efficient than conventional methods, potentially lowering the carbon footprint of metal production. Furthermore, by enabling the recovery of metals from low-grade ores or waste streams that were previously uneconomical to process, this technology could extend the life of existing mineral resources, reducing the need for new mining activities and their associated environmental impacts.
Long-term environmental monitoring will be crucial to fully understand the impacts of this technology. While initial assessments suggest potential benefits, the cumulative effects of prolonged use of barium hydroxide in metal recovery operations must be carefully studied. This includes examining potential bioaccumulation in food chains, long-term soil and water quality changes, and any unforeseen ecological interactions.
In conclusion, the environmental impact assessment of barium hydroxide in smart metal recovery operations reveals a complex balance of potential benefits and risks. While offering promising solutions for resource efficiency and waste reduction, careful management and ongoing research are necessary to ensure that the environmental advantages outweigh any potential negative impacts.
Barium hydroxide, when used in metal recovery processes, can significantly reduce the environmental footprint compared to traditional methods. Its ability to selectively precipitate metals from solution allows for more efficient recovery of valuable resources, minimizing waste generation. This process can lead to a reduction in the volume of tailings and other waste materials typically associated with metal extraction, thereby decreasing the land area required for disposal and the risk of contamination to surrounding soil and water bodies.
However, the use of barium hydroxide is not without environmental concerns. The compound itself is classified as hazardous, and improper handling or accidental release could pose risks to aquatic ecosystems and soil quality. Stringent safety protocols and containment measures are essential to mitigate these risks. Additionally, the production of barium hydroxide requires energy and resources, which must be factored into the overall environmental impact assessment.
From a water management perspective, the application of barium hydroxide in smart metal recovery can lead to improved water quality in effluents. By effectively removing heavy metals and other contaminants, the treated water may be more suitable for recycling within the process or safe discharge, reducing the overall water footprint of metal recovery operations. This aspect is particularly crucial in water-stressed regions where mining and metal recovery activities often compete with other water users.
The potential for reducing greenhouse gas emissions through this technology is also noteworthy. Smart metal recovery using barium hydroxide can be more energy-efficient than conventional methods, potentially lowering the carbon footprint of metal production. Furthermore, by enabling the recovery of metals from low-grade ores or waste streams that were previously uneconomical to process, this technology could extend the life of existing mineral resources, reducing the need for new mining activities and their associated environmental impacts.
Long-term environmental monitoring will be crucial to fully understand the impacts of this technology. While initial assessments suggest potential benefits, the cumulative effects of prolonged use of barium hydroxide in metal recovery operations must be carefully studied. This includes examining potential bioaccumulation in food chains, long-term soil and water quality changes, and any unforeseen ecological interactions.
In conclusion, the environmental impact assessment of barium hydroxide in smart metal recovery operations reveals a complex balance of potential benefits and risks. While offering promising solutions for resource efficiency and waste reduction, careful management and ongoing research are necessary to ensure that the environmental advantages outweigh any potential negative impacts.
Economic Feasibility Analysis
The economic feasibility of applying barium hydroxide in smart metal recovery operations is a critical consideration for industries seeking to optimize their resource management and environmental sustainability. This analysis examines the financial implications, potential returns on investment, and overall economic viability of implementing barium hydroxide-based technologies in metal recovery processes.
Initial capital expenditure for integrating barium hydroxide systems into existing metal recovery operations can be substantial. This includes costs associated with equipment procurement, installation, and necessary modifications to current infrastructure. However, these upfront investments must be weighed against the long-term economic benefits and operational efficiencies that can be achieved.
One of the primary economic advantages of using barium hydroxide in metal recovery is its high efficiency in precipitating and recovering valuable metals from waste streams. This increased recovery rate translates directly into higher yields of marketable metals, potentially offsetting the initial implementation costs. Furthermore, the process's ability to selectively recover specific metals can lead to higher-purity end products, which often command premium prices in the market.
Operational costs associated with barium hydroxide usage in metal recovery are generally competitive when compared to traditional methods. The reagent itself is relatively inexpensive and widely available, contributing to lower ongoing material costs. Additionally, the process typically requires less energy input than alternative recovery methods, potentially resulting in reduced utility expenses over time.
Environmental compliance is another factor that significantly impacts the economic feasibility of this technology. As regulatory pressures increase globally, industries are facing stricter guidelines and potential fines for improper waste management. Barium hydroxide-based metal recovery can help companies meet or exceed environmental standards, potentially avoiding costly penalties and improving their corporate image, which may indirectly contribute to market value.
The scalability of barium hydroxide applications in metal recovery operations also enhances its economic viability. The technology can be adapted to various scales of operation, from small-scale specialized recovery units to large industrial complexes. This flexibility allows businesses to tailor their investments according to their specific needs and financial capabilities, optimizing the return on investment across different operational scales.
In conclusion, while the initial investment in barium hydroxide-based smart metal recovery systems may be significant, the long-term economic benefits, including increased metal recovery rates, operational efficiencies, and environmental compliance, present a compelling case for its economic feasibility. As with any technological investment, a detailed cost-benefit analysis specific to each operation's unique circumstances is essential for making informed decisions on implementation.
Initial capital expenditure for integrating barium hydroxide systems into existing metal recovery operations can be substantial. This includes costs associated with equipment procurement, installation, and necessary modifications to current infrastructure. However, these upfront investments must be weighed against the long-term economic benefits and operational efficiencies that can be achieved.
One of the primary economic advantages of using barium hydroxide in metal recovery is its high efficiency in precipitating and recovering valuable metals from waste streams. This increased recovery rate translates directly into higher yields of marketable metals, potentially offsetting the initial implementation costs. Furthermore, the process's ability to selectively recover specific metals can lead to higher-purity end products, which often command premium prices in the market.
Operational costs associated with barium hydroxide usage in metal recovery are generally competitive when compared to traditional methods. The reagent itself is relatively inexpensive and widely available, contributing to lower ongoing material costs. Additionally, the process typically requires less energy input than alternative recovery methods, potentially resulting in reduced utility expenses over time.
Environmental compliance is another factor that significantly impacts the economic feasibility of this technology. As regulatory pressures increase globally, industries are facing stricter guidelines and potential fines for improper waste management. Barium hydroxide-based metal recovery can help companies meet or exceed environmental standards, potentially avoiding costly penalties and improving their corporate image, which may indirectly contribute to market value.
The scalability of barium hydroxide applications in metal recovery operations also enhances its economic viability. The technology can be adapted to various scales of operation, from small-scale specialized recovery units to large industrial complexes. This flexibility allows businesses to tailor their investments according to their specific needs and financial capabilities, optimizing the return on investment across different operational scales.
In conclusion, while the initial investment in barium hydroxide-based smart metal recovery systems may be significant, the long-term economic benefits, including increased metal recovery rates, operational efficiencies, and environmental compliance, present a compelling case for its economic feasibility. As with any technological investment, a detailed cost-benefit analysis specific to each operation's unique circumstances is essential for making informed decisions on implementation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!