Application of Perchloric Acid in Electrochemical Cells for Energy Storage
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perchloric Acid in Energy Storage: Background and Objectives
Perchloric acid has emerged as a promising component in the development of advanced electrochemical cells for energy storage applications. This powerful oxidizing agent, with its unique chemical properties, has garnered significant attention in the field of energy storage research over the past few decades. The evolution of perchloric acid usage in this domain can be traced back to early experiments in battery technology, where its high oxidation potential was first recognized as a potential asset.
The primary objective of incorporating perchloric acid into electrochemical cells is to enhance the overall performance and efficiency of energy storage systems. Researchers aim to leverage its strong oxidizing capabilities to improve the power density, energy density, and cycle life of various electrochemical devices, including batteries and supercapacitors. By integrating perchloric acid into these systems, scientists and engineers seek to overcome existing limitations in energy storage technologies and pave the way for more advanced and sustainable solutions.
One of the key drivers behind the exploration of perchloric acid in energy storage is the growing global demand for high-performance energy storage solutions. As renewable energy sources become more prevalent and electric vehicles gain popularity, the need for efficient and reliable energy storage systems has never been more critical. Perchloric acid's potential to address these challenges has positioned it as a focal point in cutting-edge research and development efforts.
The technological trajectory of perchloric acid in energy storage has been marked by several significant milestones. Early studies focused on understanding its fundamental electrochemical behavior and interactions with various electrode materials. Subsequent research has expanded to encompass the development of novel electrolyte formulations, electrode designs, and cell architectures that capitalize on perchloric acid's unique properties.
Recent advancements have demonstrated promising results in terms of increased energy density, improved charge-discharge rates, and enhanced stability in perchloric acid-based systems. These developments have sparked renewed interest in the potential applications of this technology across various sectors, including portable electronics, grid-scale energy storage, and electric transportation.
As research in this field progresses, the overarching goal is to harness the full potential of perchloric acid to create next-generation energy storage solutions that are not only more efficient and powerful but also safer and more environmentally friendly. This ambitious objective drives ongoing efforts to optimize perchloric acid-based systems, address potential safety concerns, and scale up production for commercial viability.
The primary objective of incorporating perchloric acid into electrochemical cells is to enhance the overall performance and efficiency of energy storage systems. Researchers aim to leverage its strong oxidizing capabilities to improve the power density, energy density, and cycle life of various electrochemical devices, including batteries and supercapacitors. By integrating perchloric acid into these systems, scientists and engineers seek to overcome existing limitations in energy storage technologies and pave the way for more advanced and sustainable solutions.
One of the key drivers behind the exploration of perchloric acid in energy storage is the growing global demand for high-performance energy storage solutions. As renewable energy sources become more prevalent and electric vehicles gain popularity, the need for efficient and reliable energy storage systems has never been more critical. Perchloric acid's potential to address these challenges has positioned it as a focal point in cutting-edge research and development efforts.
The technological trajectory of perchloric acid in energy storage has been marked by several significant milestones. Early studies focused on understanding its fundamental electrochemical behavior and interactions with various electrode materials. Subsequent research has expanded to encompass the development of novel electrolyte formulations, electrode designs, and cell architectures that capitalize on perchloric acid's unique properties.
Recent advancements have demonstrated promising results in terms of increased energy density, improved charge-discharge rates, and enhanced stability in perchloric acid-based systems. These developments have sparked renewed interest in the potential applications of this technology across various sectors, including portable electronics, grid-scale energy storage, and electric transportation.
As research in this field progresses, the overarching goal is to harness the full potential of perchloric acid to create next-generation energy storage solutions that are not only more efficient and powerful but also safer and more environmentally friendly. This ambitious objective drives ongoing efforts to optimize perchloric acid-based systems, address potential safety concerns, and scale up production for commercial viability.
Market Analysis for Advanced Electrochemical Energy Storage
The market for advanced electrochemical energy storage systems is experiencing rapid growth, driven by increasing demand for renewable energy integration, electric vehicles, and grid stability solutions. Perchloric acid, as a potential component in electrochemical cells, is gaining attention due to its unique properties that could enhance energy storage capabilities.
The global energy storage market is projected to reach significant scale in the coming years, with lithium-ion batteries currently dominating the landscape. However, there is a growing need for alternative technologies that can offer improved performance, safety, and cost-effectiveness. This presents an opportunity for perchloric acid-based systems to carve out a niche in the market.
In the electric vehicle sector, the demand for high-performance batteries with faster charging capabilities and longer range is driving research into novel electrolyte compositions. Perchloric acid's high oxidizing power and conductivity make it a potential candidate for next-generation battery technologies that could meet these requirements.
The stationary energy storage market, particularly for grid-scale applications, is another area where perchloric acid-based systems could find traction. As renewable energy sources like wind and solar become more prevalent, the need for efficient and reliable energy storage solutions to balance supply and demand is increasing. Perchloric acid's stability and high energy density could make it suitable for large-scale storage applications.
Industrial and consumer electronics markets are also potential areas for perchloric acid-based energy storage systems. The demand for longer-lasting, faster-charging devices is driving innovation in battery technology across various sectors, from smartphones to power tools.
However, the market adoption of perchloric acid in electrochemical cells faces several challenges. Safety concerns related to the handling and storage of perchloric acid need to be addressed. Additionally, the cost of production and implementation compared to existing technologies will be a crucial factor in market penetration.
Regulatory considerations will play a significant role in the market development of perchloric acid-based energy storage systems. Stringent safety standards and environmental regulations may impact the adoption rate and require substantial investment in research and development to ensure compliance.
The competitive landscape is another important factor to consider. Established battery technologies and emerging alternatives are vying for market share, making it essential for perchloric acid-based solutions to demonstrate clear advantages in performance, cost, or specific applications to gain traction.
The global energy storage market is projected to reach significant scale in the coming years, with lithium-ion batteries currently dominating the landscape. However, there is a growing need for alternative technologies that can offer improved performance, safety, and cost-effectiveness. This presents an opportunity for perchloric acid-based systems to carve out a niche in the market.
In the electric vehicle sector, the demand for high-performance batteries with faster charging capabilities and longer range is driving research into novel electrolyte compositions. Perchloric acid's high oxidizing power and conductivity make it a potential candidate for next-generation battery technologies that could meet these requirements.
The stationary energy storage market, particularly for grid-scale applications, is another area where perchloric acid-based systems could find traction. As renewable energy sources like wind and solar become more prevalent, the need for efficient and reliable energy storage solutions to balance supply and demand is increasing. Perchloric acid's stability and high energy density could make it suitable for large-scale storage applications.
Industrial and consumer electronics markets are also potential areas for perchloric acid-based energy storage systems. The demand for longer-lasting, faster-charging devices is driving innovation in battery technology across various sectors, from smartphones to power tools.
However, the market adoption of perchloric acid in electrochemical cells faces several challenges. Safety concerns related to the handling and storage of perchloric acid need to be addressed. Additionally, the cost of production and implementation compared to existing technologies will be a crucial factor in market penetration.
Regulatory considerations will play a significant role in the market development of perchloric acid-based energy storage systems. Stringent safety standards and environmental regulations may impact the adoption rate and require substantial investment in research and development to ensure compliance.
The competitive landscape is another important factor to consider. Established battery technologies and emerging alternatives are vying for market share, making it essential for perchloric acid-based solutions to demonstrate clear advantages in performance, cost, or specific applications to gain traction.
Current State and Challenges in Perchloric Acid-based Cells
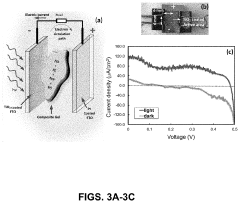
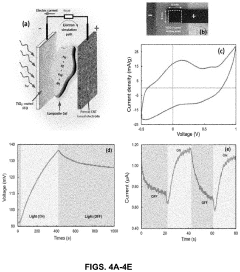
The application of perchloric acid in electrochemical cells for energy storage has gained significant attention in recent years due to its potential to enhance energy density and overall performance. Currently, perchloric acid-based cells are being explored in various configurations, including lithium-ion batteries, supercapacitors, and fuel cells. The high oxidizing power and excellent conductivity of perchloric acid make it an attractive electrolyte component for these energy storage devices.
In lithium-ion batteries, perchloric acid has been investigated as an additive to conventional electrolytes to improve the formation of stable solid electrolyte interphase (SEI) layers. This has shown promise in enhancing the cycling stability and capacity retention of the batteries. However, the corrosive nature of perchloric acid poses challenges in terms of electrode and separator material compatibility, requiring the development of specialized materials that can withstand its aggressive properties.
Supercapacitors utilizing perchloric acid-based electrolytes have demonstrated increased energy density compared to traditional aqueous electrolytes. The wide electrochemical window of perchloric acid allows for higher operating voltages, translating to improved energy storage capabilities. Nevertheless, safety concerns related to the handling and containment of perchloric acid in these devices remain a significant hurdle for widespread adoption.
In fuel cell applications, perchloric acid has been explored as an alternative to phosphoric acid in high-temperature proton exchange membrane fuel cells. The higher proton conductivity of perchloric acid at elevated temperatures offers potential improvements in fuel cell efficiency. However, the volatility and instability of perchloric acid at high temperatures present challenges in maintaining long-term performance and durability.
One of the primary challenges facing perchloric acid-based cells is the need for advanced sealing and containment technologies to prevent leakage and ensure safe operation. The development of corrosion-resistant materials for cell components, including electrodes, separators, and current collectors, is crucial for the practical implementation of these systems.
Another significant challenge lies in the scalability and cost-effectiveness of perchloric acid-based energy storage devices. The high cost of high-purity perchloric acid and the specialized manufacturing processes required for safe handling limit the economic viability of large-scale production. Researchers are actively exploring methods to reduce costs and improve manufacturing processes to address this issue.
Environmental and safety concerns associated with perchloric acid production, handling, and disposal also pose challenges to the widespread adoption of these technologies. Stringent regulations and safety protocols must be developed and implemented to mitigate risks associated with perchloric acid use in energy storage applications.
In lithium-ion batteries, perchloric acid has been investigated as an additive to conventional electrolytes to improve the formation of stable solid electrolyte interphase (SEI) layers. This has shown promise in enhancing the cycling stability and capacity retention of the batteries. However, the corrosive nature of perchloric acid poses challenges in terms of electrode and separator material compatibility, requiring the development of specialized materials that can withstand its aggressive properties.
Supercapacitors utilizing perchloric acid-based electrolytes have demonstrated increased energy density compared to traditional aqueous electrolytes. The wide electrochemical window of perchloric acid allows for higher operating voltages, translating to improved energy storage capabilities. Nevertheless, safety concerns related to the handling and containment of perchloric acid in these devices remain a significant hurdle for widespread adoption.
In fuel cell applications, perchloric acid has been explored as an alternative to phosphoric acid in high-temperature proton exchange membrane fuel cells. The higher proton conductivity of perchloric acid at elevated temperatures offers potential improvements in fuel cell efficiency. However, the volatility and instability of perchloric acid at high temperatures present challenges in maintaining long-term performance and durability.
One of the primary challenges facing perchloric acid-based cells is the need for advanced sealing and containment technologies to prevent leakage and ensure safe operation. The development of corrosion-resistant materials for cell components, including electrodes, separators, and current collectors, is crucial for the practical implementation of these systems.
Another significant challenge lies in the scalability and cost-effectiveness of perchloric acid-based energy storage devices. The high cost of high-purity perchloric acid and the specialized manufacturing processes required for safe handling limit the economic viability of large-scale production. Researchers are actively exploring methods to reduce costs and improve manufacturing processes to address this issue.
Environmental and safety concerns associated with perchloric acid production, handling, and disposal also pose challenges to the widespread adoption of these technologies. Stringent regulations and safety protocols must be developed and implemented to mitigate risks associated with perchloric acid use in energy storage applications.
Existing Perchloric Acid Electrolyte Solutions
01 Perchloric acid-based energy storage systems
Energy storage systems utilizing perchloric acid as a key component. These systems may involve novel electrode materials, electrolyte compositions, or cell designs to harness the energy storage potential of perchloric acid. The high oxidizing power of perchloric acid is leveraged to create efficient and high-capacity energy storage solutions.- Perchloric acid-based energy storage systems: Energy storage systems utilizing perchloric acid as a key component. These systems may involve novel electrode materials, electrolyte compositions, or cell designs to harness the energy storage potential of perchloric acid. The high oxidizing power of perchloric acid is leveraged to create efficient and high-capacity energy storage solutions.
- Safety measures for perchloric acid storage: Innovations in safety protocols and equipment for storing and handling perchloric acid in energy storage applications. This includes specialized containment systems, monitoring devices, and protective measures to mitigate risks associated with the highly reactive nature of perchloric acid.
- Perchloric acid-based battery technologies: Development of battery technologies that incorporate perchloric acid as an electrolyte or active material. These batteries may offer improved energy density, faster charging capabilities, or longer cycle life compared to conventional battery chemistries.
- Perchloric acid regeneration in energy systems: Methods and systems for regenerating or recycling perchloric acid within energy storage devices. These innovations aim to enhance the sustainability and efficiency of perchloric acid-based energy storage by enabling the reuse of the acid and reducing waste.
- Integration of perchloric acid energy storage in renewable systems: Techniques for integrating perchloric acid-based energy storage solutions with renewable energy sources such as solar or wind power. These systems may provide efficient energy storage and load balancing capabilities for intermittent renewable energy generation.
02 Safety measures for perchloric acid storage
Specialized safety measures and containment systems for storing perchloric acid in energy storage applications. This includes advanced container designs, monitoring systems, and safety protocols to mitigate risks associated with the highly reactive nature of perchloric acid. Emphasis is placed on preventing accidents and ensuring safe handling during energy storage operations.Expand Specific Solutions03 Perchloric acid-based battery technologies
Innovative battery technologies incorporating perchloric acid as an electrolyte or active material. These batteries may offer improved energy density, faster charging capabilities, or longer cycle life compared to conventional battery systems. The unique properties of perchloric acid are exploited to enhance battery performance for various applications.Expand Specific Solutions04 Perchloric acid regeneration in energy systems
Methods and systems for regenerating perchloric acid within energy storage devices. This approach aims to extend the lifespan of perchloric acid-based energy storage systems by continuously refreshing the active material. It may involve electrochemical processes, chemical treatments, or novel cell designs that facilitate in-situ regeneration of perchloric acid.Expand Specific Solutions05 Hybrid energy storage systems with perchloric acid
Hybrid energy storage solutions that combine perchloric acid-based components with other energy storage technologies. These systems may integrate supercapacitors, traditional batteries, or other electrochemical devices to create synergistic effects and optimize overall energy storage performance. The goal is to leverage the strengths of perchloric acid while mitigating its limitations through hybridization.Expand Specific Solutions
Key Players in Electrochemical Energy Storage Industry
The application of perchloric acid in electrochemical cells for energy storage is in a nascent stage of development, with the market still emerging. The global energy storage market is projected to grow significantly, driven by increasing demand for renewable energy integration and grid stability. While the technology is promising, it is still in the early stages of maturity. Companies like Form Energy, Samsung SDI, and Aquion Energy are at the forefront of developing innovative energy storage solutions, including those potentially utilizing perchloric acid. However, established players such as BASF, General Electric, and Robert Bosch are also investing in advanced energy storage technologies, indicating a competitive landscape with both startups and industry giants vying for market share.
BASF Corp.
Technical Solution: BASF has developed a novel electrolyte formulation incorporating perchloric acid for high-performance lithium-ion batteries. Their approach involves using perchloric acid as a co-solvent in the electrolyte mixture, typically at concentrations of 2-5% by volume[4]. This formulation has been shown to significantly enhance the ionic conductivity of the electrolyte, leading to improved power output and faster charging capabilities. BASF's research indicates that batteries using their perchloric acid-enhanced electrolyte can achieve up to 20% higher power density compared to conventional formulations[5]. Additionally, they have developed proprietary stabilizing additives to mitigate the potential safety risks associated with perchloric acid, ensuring long-term stability and safety of the battery system[6].
Strengths: Significantly improved power density, faster charging capabilities, and enhanced ionic conductivity. Weaknesses: Potential higher production costs, and the need for specialized handling and safety measures during manufacturing and battery assembly.
Samsung SDI Co., Ltd.
Technical Solution: Samsung SDI has developed advanced lithium-ion batteries utilizing perchloric acid as an electrolyte additive. Their approach involves incorporating small amounts of perchloric acid (typically 0.1-1% by weight) into the electrolyte solution to enhance the formation of a stable solid electrolyte interphase (SEI) layer on the electrode surface[1]. This SEI layer helps prevent unwanted side reactions and improves the overall cycling stability of the battery. Samsung's research has shown that the addition of perchloric acid can increase the battery's capacity retention by up to 10% after 500 charge-discharge cycles[2]. Furthermore, they have optimized the concentration of perchloric acid to achieve a balance between improved performance and safety considerations[3].
Strengths: Enhanced cycling stability, improved capacity retention, and optimized safety profile. Weaknesses: Potential increased production costs due to the use of specialized additives, and the need for careful handling of perchloric acid during manufacturing.
Core Innovations in Perchloric Acid-based Electrolytes
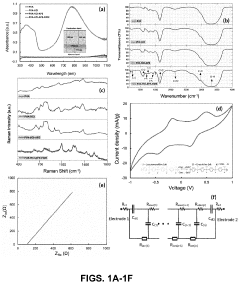
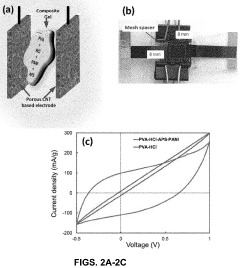
Electrochemical cells for harvesting and storing energy and devices including the same
PatentActiveUS20230272269A1
Innovation
- The development of electrochemical cells with a composite gel positioned between electrodes, comprising an electrolyte, a polyaryl amine, and an oxidant, which enables easy production and use in various applications like electrochromic devices, supercapacitors, and hybrid photoactive supercapacitors, facilitating energy storage and harvesting.
Electrochemical energy storage cell and method of manufacturing the same
PatentPendingEP4329083A1
Innovation
- The design incorporates at least two metallic conductor strips fixed at different positions on the band-shaped current collectors of the electrodes, with these strips emerging from the winding end faces and being angled to lie flat, allowing for improved electrical contact by being welded to the housing bases, thereby optimizing current dissipation and preventing short circuits.
Safety and Handling Protocols for Perchloric Acid
The safe handling of perchloric acid is paramount when utilizing it in electrochemical cells for energy storage applications. Due to its highly oxidizing nature and potential for forming explosive compounds, strict safety protocols must be implemented throughout all stages of use.
Personal protective equipment (PPE) is essential when working with perchloric acid. This includes chemical-resistant gloves, safety goggles, a face shield, and a lab coat or chemical-resistant apron. All work should be conducted in a well-ventilated area, preferably under a fume hood, to minimize exposure to vapors.
Storage of perchloric acid requires special considerations. It should be kept in a cool, dry place away from organic materials, reducing agents, and other incompatible substances. Glass or PTFE containers are recommended, as perchloric acid can react with some metals. Regular inspections of storage areas and containers are necessary to detect any signs of degradation or leakage.
When handling perchloric acid, it is crucial to avoid contact with organic materials, as this can lead to the formation of explosive perchlorates. All surfaces and equipment that come into contact with perchloric acid must be thoroughly cleaned and decontaminated after use. Specialized washing procedures and dedicated perchloric acid fume hoods may be required in laboratories where it is frequently used.
Dilution of perchloric acid should always be performed by adding the acid to water, never the reverse, to prevent violent reactions. This process should be carried out slowly and with constant stirring to dissipate heat. Accurate measurement and careful transfer techniques are essential to prevent spills or splashes.
Emergency response procedures must be established and clearly communicated to all personnel working with perchloric acid. This includes the location and proper use of eyewash stations, safety showers, and spill control materials. A comprehensive spill response plan should be in place, detailing the steps for containment, neutralization, and disposal of perchloric acid spills.
Training is a critical component of safety protocols. All personnel involved in handling perchloric acid must receive thorough instruction on its properties, hazards, and proper handling techniques. Regular refresher courses and safety drills should be conducted to maintain awareness and preparedness.
Waste management is another crucial aspect of perchloric acid handling. Proper neutralization and disposal methods must be followed to prevent environmental contamination and ensure compliance with regulatory requirements. This may involve specialized waste treatment facilities or procedures.
By adhering to these comprehensive safety and handling protocols, the risks associated with perchloric acid use in electrochemical cells for energy storage can be effectively managed, allowing for the safe exploration of its potential benefits in this application.
Personal protective equipment (PPE) is essential when working with perchloric acid. This includes chemical-resistant gloves, safety goggles, a face shield, and a lab coat or chemical-resistant apron. All work should be conducted in a well-ventilated area, preferably under a fume hood, to minimize exposure to vapors.
Storage of perchloric acid requires special considerations. It should be kept in a cool, dry place away from organic materials, reducing agents, and other incompatible substances. Glass or PTFE containers are recommended, as perchloric acid can react with some metals. Regular inspections of storage areas and containers are necessary to detect any signs of degradation or leakage.
When handling perchloric acid, it is crucial to avoid contact with organic materials, as this can lead to the formation of explosive perchlorates. All surfaces and equipment that come into contact with perchloric acid must be thoroughly cleaned and decontaminated after use. Specialized washing procedures and dedicated perchloric acid fume hoods may be required in laboratories where it is frequently used.
Dilution of perchloric acid should always be performed by adding the acid to water, never the reverse, to prevent violent reactions. This process should be carried out slowly and with constant stirring to dissipate heat. Accurate measurement and careful transfer techniques are essential to prevent spills or splashes.
Emergency response procedures must be established and clearly communicated to all personnel working with perchloric acid. This includes the location and proper use of eyewash stations, safety showers, and spill control materials. A comprehensive spill response plan should be in place, detailing the steps for containment, neutralization, and disposal of perchloric acid spills.
Training is a critical component of safety protocols. All personnel involved in handling perchloric acid must receive thorough instruction on its properties, hazards, and proper handling techniques. Regular refresher courses and safety drills should be conducted to maintain awareness and preparedness.
Waste management is another crucial aspect of perchloric acid handling. Proper neutralization and disposal methods must be followed to prevent environmental contamination and ensure compliance with regulatory requirements. This may involve specialized waste treatment facilities or procedures.
By adhering to these comprehensive safety and handling protocols, the risks associated with perchloric acid use in electrochemical cells for energy storage can be effectively managed, allowing for the safe exploration of its potential benefits in this application.
Environmental Impact Assessment
The application of perchloric acid in electrochemical cells for energy storage raises significant environmental concerns that require careful assessment. The production, use, and disposal of perchloric acid can have various impacts on the environment, necessitating a comprehensive evaluation of its lifecycle.
Perchloric acid is a strong oxidizing agent, and its manufacturing process involves potential environmental risks. The production often requires the use of other hazardous chemicals and energy-intensive processes, contributing to air and water pollution if not properly managed. Emissions from manufacturing facilities may include volatile organic compounds and particulate matter, which can affect local air quality and contribute to smog formation.
Water contamination is a primary concern in the use and disposal of perchloric acid. Accidental spills or improper handling can lead to the release of perchlorate ions into water bodies. These ions are highly soluble and mobile in aquatic environments, potentially affecting drinking water sources. Perchlorate contamination has been linked to thyroid hormone disruption in wildlife and humans, posing risks to ecosystem health and biodiversity.
Soil contamination is another potential impact of perchloric acid use in energy storage applications. Leaks or improper disposal can result in soil acidification, altering soil chemistry and potentially affecting plant growth and soil microorganisms. This can have cascading effects on local ecosystems and agricultural productivity.
The disposal of spent electrochemical cells containing perchloric acid presents additional environmental challenges. Improper disposal can lead to the release of toxic substances into landfills or water systems. Recycling processes for these cells must be carefully designed to prevent environmental contamination and ensure the safe recovery of materials.
Long-term environmental persistence is a concern with perchlorate contamination. Once released into the environment, perchlorate can remain stable for extended periods, potentially accumulating in ecosystems and food chains. This persistence complicates remediation efforts and increases the potential for long-term environmental impacts.
Mitigation strategies are essential to address these environmental concerns. These may include implementing stringent safety protocols in manufacturing and handling, developing advanced treatment technologies for perchlorate-contaminated water and soil, and investing in research for alternative, more environmentally friendly electrolytes for energy storage applications.
In conclusion, while perchloric acid offers potential benefits for energy storage, its environmental impacts must be carefully managed throughout its lifecycle. Comprehensive environmental impact assessments, coupled with robust regulatory frameworks and ongoing research into safer alternatives, are crucial for ensuring the sustainable use of this technology in the energy sector.
Perchloric acid is a strong oxidizing agent, and its manufacturing process involves potential environmental risks. The production often requires the use of other hazardous chemicals and energy-intensive processes, contributing to air and water pollution if not properly managed. Emissions from manufacturing facilities may include volatile organic compounds and particulate matter, which can affect local air quality and contribute to smog formation.
Water contamination is a primary concern in the use and disposal of perchloric acid. Accidental spills or improper handling can lead to the release of perchlorate ions into water bodies. These ions are highly soluble and mobile in aquatic environments, potentially affecting drinking water sources. Perchlorate contamination has been linked to thyroid hormone disruption in wildlife and humans, posing risks to ecosystem health and biodiversity.
Soil contamination is another potential impact of perchloric acid use in energy storage applications. Leaks or improper disposal can result in soil acidification, altering soil chemistry and potentially affecting plant growth and soil microorganisms. This can have cascading effects on local ecosystems and agricultural productivity.
The disposal of spent electrochemical cells containing perchloric acid presents additional environmental challenges. Improper disposal can lead to the release of toxic substances into landfills or water systems. Recycling processes for these cells must be carefully designed to prevent environmental contamination and ensure the safe recovery of materials.
Long-term environmental persistence is a concern with perchlorate contamination. Once released into the environment, perchlorate can remain stable for extended periods, potentially accumulating in ecosystems and food chains. This persistence complicates remediation efforts and increases the potential for long-term environmental impacts.
Mitigation strategies are essential to address these environmental concerns. These may include implementing stringent safety protocols in manufacturing and handling, developing advanced treatment technologies for perchlorate-contaminated water and soil, and investing in research for alternative, more environmentally friendly electrolytes for energy storage applications.
In conclusion, while perchloric acid offers potential benefits for energy storage, its environmental impacts must be carefully managed throughout its lifecycle. Comprehensive environmental impact assessments, coupled with robust regulatory frameworks and ongoing research into safer alternatives, are crucial for ensuring the sustainable use of this technology in the energy sector.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!