Assessing Magnesium Carbonate in Probiotic Delivery Systems
JUL 31, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Magnesium Carbonate in Probiotics: Background and Objectives
Magnesium carbonate has emerged as a promising component in probiotic delivery systems, marking a significant advancement in the field of nutraceuticals and functional foods. The integration of this inorganic compound with probiotics represents a convergence of materials science and microbiology, aimed at enhancing the viability and efficacy of beneficial bacteria in various applications.
The historical context of this technology dates back to the early 2000s when researchers began exploring novel methods to protect probiotics from harsh environmental conditions. Traditional encapsulation techniques, while effective to some degree, often fell short in providing adequate protection against acidity, heat, and moisture. This limitation led to the investigation of inorganic compounds, with magnesium carbonate emerging as a particularly promising candidate due to its unique properties.
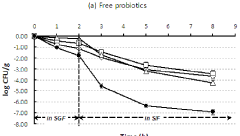
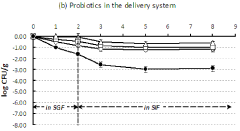
Magnesium carbonate's potential in probiotic delivery systems stems from its ability to create a favorable microenvironment for probiotic bacteria. Its alkaline nature helps neutralize acids, potentially improving probiotic survival through the gastrointestinal tract. Additionally, its porous structure offers physical protection and controlled release capabilities, addressing key challenges in probiotic stability and delivery.
The evolution of this technology has been driven by the growing consumer demand for functional foods and supplements that offer health benefits beyond basic nutrition. As awareness of the importance of gut health has increased, so too has the market for probiotic products. This trend has spurred research into more effective delivery systems, with magnesium carbonate-based solutions representing a cutting-edge approach.
The primary objectives of incorporating magnesium carbonate in probiotic delivery systems are multifaceted. Firstly, there is a focus on enhancing the survival rate of probiotics during processing, storage, and gastrointestinal transit. This improved viability is crucial for ensuring that an effective dose of live bacteria reaches the intended site of action in the gut.
Secondly, researchers aim to develop controlled release mechanisms that can target specific areas of the gastrointestinal tract. This targeted delivery is essential for maximizing the therapeutic potential of probiotics, as different strains may exert their beneficial effects in different regions of the gut.
Another key objective is to improve the stability of probiotic formulations, extending their shelf life and reducing the need for refrigeration. This aspect is particularly important for expanding the accessibility of probiotic products in regions with limited cold chain infrastructure.
Furthermore, there is ongoing research into the potential synergistic effects between magnesium carbonate and probiotics. Some studies suggest that the presence of magnesium may enhance the growth and metabolic activity of certain probiotic strains, potentially amplifying their health benefits.
As the field progresses, researchers are also exploring the scalability and cost-effectiveness of magnesium carbonate-based delivery systems. The ultimate goal is to develop commercially viable products that can be widely adopted by the food and pharmaceutical industries, thereby bringing the benefits of this technology to a broader consumer base.
The historical context of this technology dates back to the early 2000s when researchers began exploring novel methods to protect probiotics from harsh environmental conditions. Traditional encapsulation techniques, while effective to some degree, often fell short in providing adequate protection against acidity, heat, and moisture. This limitation led to the investigation of inorganic compounds, with magnesium carbonate emerging as a particularly promising candidate due to its unique properties.
Magnesium carbonate's potential in probiotic delivery systems stems from its ability to create a favorable microenvironment for probiotic bacteria. Its alkaline nature helps neutralize acids, potentially improving probiotic survival through the gastrointestinal tract. Additionally, its porous structure offers physical protection and controlled release capabilities, addressing key challenges in probiotic stability and delivery.
The evolution of this technology has been driven by the growing consumer demand for functional foods and supplements that offer health benefits beyond basic nutrition. As awareness of the importance of gut health has increased, so too has the market for probiotic products. This trend has spurred research into more effective delivery systems, with magnesium carbonate-based solutions representing a cutting-edge approach.
The primary objectives of incorporating magnesium carbonate in probiotic delivery systems are multifaceted. Firstly, there is a focus on enhancing the survival rate of probiotics during processing, storage, and gastrointestinal transit. This improved viability is crucial for ensuring that an effective dose of live bacteria reaches the intended site of action in the gut.
Secondly, researchers aim to develop controlled release mechanisms that can target specific areas of the gastrointestinal tract. This targeted delivery is essential for maximizing the therapeutic potential of probiotics, as different strains may exert their beneficial effects in different regions of the gut.
Another key objective is to improve the stability of probiotic formulations, extending their shelf life and reducing the need for refrigeration. This aspect is particularly important for expanding the accessibility of probiotic products in regions with limited cold chain infrastructure.
Furthermore, there is ongoing research into the potential synergistic effects between magnesium carbonate and probiotics. Some studies suggest that the presence of magnesium may enhance the growth and metabolic activity of certain probiotic strains, potentially amplifying their health benefits.
As the field progresses, researchers are also exploring the scalability and cost-effectiveness of magnesium carbonate-based delivery systems. The ultimate goal is to develop commercially viable products that can be widely adopted by the food and pharmaceutical industries, thereby bringing the benefits of this technology to a broader consumer base.
Market Analysis of Probiotic Delivery Systems
The global probiotic market has been experiencing significant growth, driven by increasing consumer awareness of the health benefits associated with probiotics. Within this market, probiotic delivery systems play a crucial role in ensuring the efficacy and stability of probiotic products. The market for probiotic delivery systems is expected to continue its upward trajectory, with a particular focus on innovative technologies that enhance probiotic viability and targeted delivery.
The probiotic market encompasses various sectors, including dietary supplements, functional foods, and beverages. Among these, the dietary supplement segment holds a substantial market share, with consumers increasingly turning to probiotic supplements for digestive health, immune support, and overall well-being. The functional food and beverage sector is also witnessing rapid growth, as manufacturers incorporate probiotics into a wide range of products to meet consumer demand for health-enhancing foods.
In recent years, there has been a growing interest in advanced probiotic delivery systems that can protect probiotics from harsh environmental conditions, such as stomach acid and bile salts. This has led to the development of novel encapsulation technologies and controlled-release mechanisms. Magnesium carbonate, in particular, has gained attention as a potential component in probiotic delivery systems due to its ability to create a favorable microenvironment for probiotic survival.
The market for probiotic delivery systems is characterized by intense competition and continuous innovation. Key players in this space include major pharmaceutical companies, specialized probiotic manufacturers, and ingredient suppliers. These companies are investing heavily in research and development to create more effective and efficient delivery systems that can differentiate their products in a crowded market.
Geographically, North America and Europe currently dominate the probiotic delivery systems market, owing to high consumer awareness and well-established health and wellness industries. However, the Asia-Pacific region is emerging as a lucrative market, driven by increasing disposable incomes, changing dietary habits, and a growing focus on preventive healthcare.
Consumer preferences are shifting towards more personalized probiotic solutions, creating opportunities for targeted delivery systems that cater to specific health conditions or demographic groups. This trend is likely to drive further innovation in the field of probiotic delivery, including the exploration of novel materials and technologies.
The regulatory landscape plays a significant role in shaping the probiotic delivery systems market. Stringent regulations regarding probiotic claims and product safety have led to increased demand for delivery systems that can ensure probiotic stability and efficacy throughout the product's shelf life. This regulatory environment is expected to continue influencing market dynamics and driving technological advancements in probiotic delivery systems.
The probiotic market encompasses various sectors, including dietary supplements, functional foods, and beverages. Among these, the dietary supplement segment holds a substantial market share, with consumers increasingly turning to probiotic supplements for digestive health, immune support, and overall well-being. The functional food and beverage sector is also witnessing rapid growth, as manufacturers incorporate probiotics into a wide range of products to meet consumer demand for health-enhancing foods.
In recent years, there has been a growing interest in advanced probiotic delivery systems that can protect probiotics from harsh environmental conditions, such as stomach acid and bile salts. This has led to the development of novel encapsulation technologies and controlled-release mechanisms. Magnesium carbonate, in particular, has gained attention as a potential component in probiotic delivery systems due to its ability to create a favorable microenvironment for probiotic survival.
The market for probiotic delivery systems is characterized by intense competition and continuous innovation. Key players in this space include major pharmaceutical companies, specialized probiotic manufacturers, and ingredient suppliers. These companies are investing heavily in research and development to create more effective and efficient delivery systems that can differentiate their products in a crowded market.
Geographically, North America and Europe currently dominate the probiotic delivery systems market, owing to high consumer awareness and well-established health and wellness industries. However, the Asia-Pacific region is emerging as a lucrative market, driven by increasing disposable incomes, changing dietary habits, and a growing focus on preventive healthcare.
Consumer preferences are shifting towards more personalized probiotic solutions, creating opportunities for targeted delivery systems that cater to specific health conditions or demographic groups. This trend is likely to drive further innovation in the field of probiotic delivery, including the exploration of novel materials and technologies.
The regulatory landscape plays a significant role in shaping the probiotic delivery systems market. Stringent regulations regarding probiotic claims and product safety have led to increased demand for delivery systems that can ensure probiotic stability and efficacy throughout the product's shelf life. This regulatory environment is expected to continue influencing market dynamics and driving technological advancements in probiotic delivery systems.
Current Challenges in Probiotic Encapsulation
Probiotic encapsulation faces several significant challenges that hinder the effective delivery and functionality of these beneficial microorganisms. One of the primary issues is maintaining probiotic viability during processing, storage, and transit through the gastrointestinal tract. The harsh conditions of the stomach, including low pH and the presence of digestive enzymes, can significantly reduce the number of viable probiotic cells reaching the intestines.
Another challenge lies in controlling the release kinetics of probiotics. Ideally, probiotics should be released at specific sites in the gut to maximize their beneficial effects. However, achieving targeted delivery and controlled release remains difficult due to the complex and variable nature of the gastrointestinal environment.
The selection of appropriate encapsulation materials poses another hurdle. Materials must be biocompatible, food-grade, and capable of protecting probiotics while allowing for their timely release. Additionally, these materials should not negatively impact the sensory properties of the final product, which is crucial for consumer acceptance.
Scale-up and manufacturing processes present further complications. Techniques that work well in laboratory settings may not be feasible or cost-effective at industrial scales. Ensuring uniformity in particle size, shape, and probiotic distribution within the encapsulation matrix becomes increasingly challenging as production volumes increase.
Stability during product shelf life is another critical concern. Encapsulated probiotics must remain viable and effective throughout the product's intended storage period, which can be affected by factors such as temperature, humidity, and packaging materials.
The interaction between encapsulated probiotics and other food components also presents challenges. Some food ingredients may have antimicrobial properties or create unfavorable conditions for probiotic survival, necessitating careful formulation and compatibility testing.
Regulatory considerations add another layer of complexity. Different regions have varying requirements for probiotic products, and demonstrating the efficacy and safety of novel encapsulation techniques can be time-consuming and costly.
Lastly, there is a growing demand for natural, clean-label products, which limits the use of certain synthetic materials in encapsulation. This trend pushes researchers to explore and develop new, natural encapsulation materials that can provide adequate protection and functionality.
Another challenge lies in controlling the release kinetics of probiotics. Ideally, probiotics should be released at specific sites in the gut to maximize their beneficial effects. However, achieving targeted delivery and controlled release remains difficult due to the complex and variable nature of the gastrointestinal environment.
The selection of appropriate encapsulation materials poses another hurdle. Materials must be biocompatible, food-grade, and capable of protecting probiotics while allowing for their timely release. Additionally, these materials should not negatively impact the sensory properties of the final product, which is crucial for consumer acceptance.
Scale-up and manufacturing processes present further complications. Techniques that work well in laboratory settings may not be feasible or cost-effective at industrial scales. Ensuring uniformity in particle size, shape, and probiotic distribution within the encapsulation matrix becomes increasingly challenging as production volumes increase.
Stability during product shelf life is another critical concern. Encapsulated probiotics must remain viable and effective throughout the product's intended storage period, which can be affected by factors such as temperature, humidity, and packaging materials.
The interaction between encapsulated probiotics and other food components also presents challenges. Some food ingredients may have antimicrobial properties or create unfavorable conditions for probiotic survival, necessitating careful formulation and compatibility testing.
Regulatory considerations add another layer of complexity. Different regions have varying requirements for probiotic products, and demonstrating the efficacy and safety of novel encapsulation techniques can be time-consuming and costly.
Lastly, there is a growing demand for natural, clean-label products, which limits the use of certain synthetic materials in encapsulation. This trend pushes researchers to explore and develop new, natural encapsulation materials that can provide adequate protection and functionality.
Existing MgCO3-Based Probiotic Delivery Solutions
01 Formulation techniques for improved magnesium carbonate delivery
Various formulation techniques can be employed to enhance the delivery efficiency of magnesium carbonate. These may include the use of specific carriers, particle size reduction, or the incorporation of other compounds to improve solubility and absorption. Such techniques aim to increase the bioavailability and effectiveness of magnesium carbonate in different applications.- Formulation techniques for improved magnesium carbonate delivery: Various formulation techniques can be employed to enhance the delivery efficiency of magnesium carbonate. These may include the use of specific carriers, particle size optimization, and the incorporation of additives to improve solubility and absorption. Such formulations can help increase bioavailability and ensure more effective delivery of magnesium carbonate to the target sites.
- Controlled release systems for magnesium carbonate: Controlled release systems can be developed to optimize the delivery of magnesium carbonate over time. These systems may involve encapsulation techniques, matrix systems, or other methods to regulate the release rate of magnesium carbonate. This approach can help maintain consistent levels of the compound and improve its overall efficacy.
- Nanotechnology applications in magnesium carbonate delivery: Nanotechnology offers promising solutions for enhancing magnesium carbonate delivery efficiency. Nanoparticles or nanocarriers can be designed to improve the solubility, stability, and targeted delivery of magnesium carbonate. These advanced delivery systems may lead to increased bioavailability and reduced dosage requirements.
- Combination with other compounds for synergistic effects: Combining magnesium carbonate with other compounds can potentially enhance its delivery efficiency and overall effectiveness. Certain additives or complementary substances may improve absorption, reduce degradation, or provide synergistic effects. This approach can lead to more efficient utilization of magnesium carbonate in various applications.
- Novel delivery routes for magnesium carbonate: Exploring alternative delivery routes for magnesium carbonate can improve its efficiency and expand its applications. This may include transdermal delivery systems, inhalation methods, or other novel approaches that bypass traditional oral administration. These innovative delivery methods can potentially enhance absorption and bioavailability while minimizing side effects.
02 Controlled release systems for magnesium carbonate
Controlled release systems can be developed to optimize the delivery of magnesium carbonate over time. These systems may involve encapsulation techniques, matrix formulations, or other methods to regulate the release rate of magnesium carbonate. This approach can improve the overall efficiency of delivery and maintain consistent levels of the compound.Expand Specific Solutions03 Combination with other compounds for synergistic effects
Combining magnesium carbonate with other compounds can potentially enhance its delivery efficiency. Certain substances may act as absorption enhancers, improve solubility, or provide complementary effects. This strategy can lead to improved overall performance and efficacy of magnesium carbonate-based formulations.Expand Specific Solutions04 Novel delivery routes for magnesium carbonate
Exploring alternative delivery routes for magnesium carbonate can improve its efficiency. This may include transdermal, inhalation, or other non-traditional methods of administration. These novel approaches can potentially overcome limitations associated with conventional delivery methods and enhance the overall effectiveness of magnesium carbonate.Expand Specific Solutions05 Nanotechnology-based approaches for magnesium carbonate delivery
Nanotechnology offers promising avenues for improving the delivery efficiency of magnesium carbonate. Nanoparticle formulations, nanocarriers, or other nanoscale systems can be developed to enhance the solubility, stability, and targeted delivery of magnesium carbonate. These approaches may lead to significant improvements in bioavailability and overall performance.Expand Specific Solutions
Key Players in Probiotic Formulation Industry
The competitive landscape for assessing magnesium carbonate in probiotic delivery systems is in an early growth stage, with increasing market potential as the importance of probiotics in health and nutrition gains recognition. The global probiotic market size is expanding rapidly, expected to reach $69.3 billion by 2023. Technologically, the field is still evolving, with companies like Chr. Hansen A/S, Omya International AG, and Advanced BioNutrition Corp. leading research and development efforts. These firms are exploring innovative delivery systems to enhance probiotic stability and efficacy, with magnesium carbonate emerging as a promising excipient. Academic institutions such as North Carolina State University and Zhejiang University are also contributing to advancements in this area, fostering industry-academia collaborations.
Omya International AG
Technical Solution: Omya International AG has developed an innovative probiotic delivery system utilizing magnesium carbonate as a key component. Their approach involves encapsulating probiotic strains within a magnesium carbonate matrix, which serves as both a protective barrier and a pH-responsive release mechanism. The magnesium carbonate shell shields the probiotics from harsh stomach acids, allowing for targeted release in the intestines where the pH is more alkaline[1]. This controlled release system enhances the survival rate of probiotics during transit through the gastrointestinal tract, potentially increasing their efficacy. Additionally, Omya's technology incorporates a proprietary process to optimize the particle size distribution of the magnesium carbonate, which further improves the stability and dispersibility of the probiotic formulation[2].
Strengths: Enhanced probiotic survival through gastric passage, controlled release in the intestines, improved stability and shelf-life. Weaknesses: Potential alteration of product texture, possible impact on taste in certain applications.
Advanced BioNutrition Corp.
Technical Solution: Advanced BioNutrition Corp. has pioneered a novel approach to probiotic delivery using a magnesium carbonate-based microencapsulation technology. Their system, known as Bio-Tract, utilizes a patented layering process that combines magnesium carbonate with other protective compounds to create a multi-layer shield around probiotic organisms[3]. This technology not only protects probiotics from stomach acid but also provides a time-released delivery mechanism. The outer layer of magnesium carbonate acts as a pH-sensitive barrier, dissolving gradually as it moves through the digestive system. This controlled erosion allows for a sustained release of probiotics throughout the intestinal tract, potentially improving colonization and efficacy[4]. The company has also developed a method to incorporate prebiotics within the same delivery system, creating a synbiotic product that supports probiotic growth and activity.
Strengths: Time-released delivery, protection against stomach acid, potential for improved probiotic colonization. Weaknesses: Complex manufacturing process, potentially higher production costs.
Innovations in Magnesium Carbonate Probiotic Encapsulation
Probiotic delivery system based on microcapsules and protective compositions and its preparation
PatentActiveNZ591393A
Innovation
- Development of sub-100 pm probiotic microcapsules using a polysaccharide gel matrix with protective additives and coatings, including chitosan for bio- and muco-adhesion, and lecithin vesicles to withstand gastric and intestinal fluids, combined with an enteric coating for gastric resistance and a non-enteric coating for enhanced adhesion.
Improved process for the production of purified magnesium carbonate
PatentInactiveGB582023A
Innovation
- A process involving calcination, slurry formation, carbonation, and mechanical separation using froth flotation to produce and separate crystalline magnesium carbonate, followed by recrystallization to achieve a cleaner and more separable form, utilizing controlled temperature and CO2 partial pressure to favor the formation of lansfordite or nesquehonite for improved purification.
Regulatory Framework for Probiotic Supplements
The regulatory framework for probiotic supplements is a complex and evolving landscape that varies significantly across different regions and countries. In the United States, the Food and Drug Administration (FDA) regulates probiotics primarily as dietary supplements under the Dietary Supplement Health and Education Act (DSHEA) of 1994. This classification allows for a relatively streamlined path to market, but also places limitations on the health claims that can be made without substantial clinical evidence.
The European Union (EU) has a more stringent approach, with probiotics falling under the Novel Food Regulation unless they have a history of safe use prior to May 1997. The European Food Safety Authority (EFSA) is responsible for evaluating the safety and efficacy of probiotic products. Health claims for probiotics in the EU are tightly regulated and require robust scientific evidence for approval.
In Japan, probiotics are regulated under the Foods for Specified Health Uses (FOSHU) system, which allows for specific health claims if supported by scientific evidence. This system has been influential in shaping probiotic regulations in other Asian countries.
The regulatory requirements for probiotic supplements typically include safety assessments, strain identification, and quality control measures. Manufacturers must demonstrate the stability and viability of probiotic strains throughout the product's shelf life. Good Manufacturing Practices (GMP) are essential for ensuring consistent quality and safety.
Labeling requirements for probiotic supplements vary by region but generally include the identification of specific strains, colony-forming unit (CFU) count, and storage conditions. In some jurisdictions, probiotic products must also carry disclaimers or warnings about potential risks for certain populations.
The use of magnesium carbonate in probiotic delivery systems adds another layer of regulatory consideration. As an excipient, magnesium carbonate must be assessed for its impact on probiotic viability and overall product safety. Manufacturers must ensure that the inclusion of magnesium carbonate does not alter the regulatory status of the probiotic supplement or introduce any new safety concerns.
Regulatory bodies are increasingly focusing on the importance of strain-specific evidence for probiotic efficacy. This trend is likely to lead to more stringent requirements for clinical studies and documentation of health benefits. As the probiotic market continues to grow, regulatory frameworks are expected to evolve to address emerging scientific knowledge and consumer safety concerns.
The European Union (EU) has a more stringent approach, with probiotics falling under the Novel Food Regulation unless they have a history of safe use prior to May 1997. The European Food Safety Authority (EFSA) is responsible for evaluating the safety and efficacy of probiotic products. Health claims for probiotics in the EU are tightly regulated and require robust scientific evidence for approval.
In Japan, probiotics are regulated under the Foods for Specified Health Uses (FOSHU) system, which allows for specific health claims if supported by scientific evidence. This system has been influential in shaping probiotic regulations in other Asian countries.
The regulatory requirements for probiotic supplements typically include safety assessments, strain identification, and quality control measures. Manufacturers must demonstrate the stability and viability of probiotic strains throughout the product's shelf life. Good Manufacturing Practices (GMP) are essential for ensuring consistent quality and safety.
Labeling requirements for probiotic supplements vary by region but generally include the identification of specific strains, colony-forming unit (CFU) count, and storage conditions. In some jurisdictions, probiotic products must also carry disclaimers or warnings about potential risks for certain populations.
The use of magnesium carbonate in probiotic delivery systems adds another layer of regulatory consideration. As an excipient, magnesium carbonate must be assessed for its impact on probiotic viability and overall product safety. Manufacturers must ensure that the inclusion of magnesium carbonate does not alter the regulatory status of the probiotic supplement or introduce any new safety concerns.
Regulatory bodies are increasingly focusing on the importance of strain-specific evidence for probiotic efficacy. This trend is likely to lead to more stringent requirements for clinical studies and documentation of health benefits. As the probiotic market continues to grow, regulatory frameworks are expected to evolve to address emerging scientific knowledge and consumer safety concerns.
Environmental Impact of Probiotic Packaging Materials
The environmental impact of probiotic packaging materials is a crucial consideration in the development of sustainable delivery systems for probiotics, including those utilizing magnesium carbonate. Traditional packaging materials for probiotics often include plastics, which contribute to environmental pollution and waste accumulation. As the demand for probiotic products continues to grow, there is an increasing need for eco-friendly packaging solutions that maintain product efficacy while minimizing environmental harm.
Biodegradable materials have emerged as a promising alternative to conventional plastic packaging. These materials, such as polylactic acid (PLA) and polyhydroxyalkanoates (PHAs), are derived from renewable resources and can decompose naturally in the environment. When used in probiotic packaging, they offer the potential to reduce plastic waste and carbon footprint associated with product distribution and disposal.
Another innovative approach is the use of edible packaging materials for probiotic delivery systems. These materials, often made from natural polymers like alginate, chitosan, or cellulose derivatives, can be consumed along with the probiotic product, eliminating the need for separate packaging disposal. This approach not only reduces waste but also provides an additional matrix for probiotic encapsulation, potentially enhancing product stability and efficacy.
The incorporation of active packaging technologies in probiotic delivery systems presents another avenue for environmental improvement. These materials can interact with the product or its environment to extend shelf life, maintain probiotic viability, or indicate product quality. By prolonging product stability, active packaging can reduce food waste and the need for frequent product replacement, thereby decreasing overall environmental impact.
However, the environmental benefits of these alternative packaging materials must be weighed against their production costs and potential limitations in preserving probiotic viability. Life cycle assessments (LCAs) are essential in evaluating the true environmental impact of different packaging options, considering factors such as raw material sourcing, manufacturing processes, transportation, and end-of-life disposal.
The use of recycled materials in probiotic packaging is another strategy to reduce environmental impact. By incorporating post-consumer recycled content into packaging materials, manufacturers can decrease the demand for virgin resources and divert waste from landfills. However, careful consideration must be given to ensure that recycled materials meet the necessary standards for food-grade packaging and do not compromise product safety or efficacy.
As research in this field progresses, there is a growing focus on developing packaging materials that not only minimize environmental impact but also actively contribute to probiotic stability and delivery. This includes the exploration of novel materials with inherent prebiotic properties or those capable of creating a microenvironment conducive to probiotic survival during storage and transit.
Biodegradable materials have emerged as a promising alternative to conventional plastic packaging. These materials, such as polylactic acid (PLA) and polyhydroxyalkanoates (PHAs), are derived from renewable resources and can decompose naturally in the environment. When used in probiotic packaging, they offer the potential to reduce plastic waste and carbon footprint associated with product distribution and disposal.
Another innovative approach is the use of edible packaging materials for probiotic delivery systems. These materials, often made from natural polymers like alginate, chitosan, or cellulose derivatives, can be consumed along with the probiotic product, eliminating the need for separate packaging disposal. This approach not only reduces waste but also provides an additional matrix for probiotic encapsulation, potentially enhancing product stability and efficacy.
The incorporation of active packaging technologies in probiotic delivery systems presents another avenue for environmental improvement. These materials can interact with the product or its environment to extend shelf life, maintain probiotic viability, or indicate product quality. By prolonging product stability, active packaging can reduce food waste and the need for frequent product replacement, thereby decreasing overall environmental impact.
However, the environmental benefits of these alternative packaging materials must be weighed against their production costs and potential limitations in preserving probiotic viability. Life cycle assessments (LCAs) are essential in evaluating the true environmental impact of different packaging options, considering factors such as raw material sourcing, manufacturing processes, transportation, and end-of-life disposal.
The use of recycled materials in probiotic packaging is another strategy to reduce environmental impact. By incorporating post-consumer recycled content into packaging materials, manufacturers can decrease the demand for virgin resources and divert waste from landfills. However, careful consideration must be given to ensure that recycled materials meet the necessary standards for food-grade packaging and do not compromise product safety or efficacy.
As research in this field progresses, there is a growing focus on developing packaging materials that not only minimize environmental impact but also actively contribute to probiotic stability and delivery. This includes the exploration of novel materials with inherent prebiotic properties or those capable of creating a microenvironment conducive to probiotic survival during storage and transit.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!