Magnesium Carbonate’s Efficacy in Chemical Compound Stabilization
JUL 31, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
MgCO3 Stabilization Background and Objectives
Magnesium carbonate (MgCO3) has emerged as a significant compound in the field of chemical stabilization, with its applications spanning various industries and scientific domains. The evolution of MgCO3 as a stabilizing agent can be traced back to the early 20th century when researchers began to explore its unique properties in chemical reactions and material preservation.
The primary objective of utilizing magnesium carbonate in compound stabilization is to enhance the longevity, efficacy, and safety of various chemical formulations. This goal has become increasingly crucial in sectors such as pharmaceuticals, food production, and advanced materials manufacturing, where product stability directly impacts quality, safety, and economic viability.
Over the years, the understanding of MgCO3's stabilization mechanisms has significantly advanced. Initially, its use was primarily empirical, based on observed effects rather than a deep understanding of the underlying chemistry. However, as analytical techniques improved, researchers gained insights into how magnesium carbonate interacts with other compounds at a molecular level.
One of the key attributes that make MgCO3 an effective stabilizer is its ability to act as a pH buffer. This property is particularly valuable in maintaining the stability of pH-sensitive compounds, preventing degradation or unwanted reactions that could occur due to pH fluctuations. Additionally, magnesium carbonate's moisture-absorbing capabilities have proven beneficial in protecting hygroscopic substances from environmental humidity.
The technological evolution in this field has led to the development of various forms of magnesium carbonate, each tailored for specific applications. These include ultra-fine particles for enhanced dispersion, surface-modified variants for improved compatibility with organic compounds, and controlled-release formulations for sustained stabilization effects.
As research progresses, the objectives for MgCO3 stabilization have expanded beyond mere preservation. Current goals include enhancing bioavailability in pharmaceutical formulations, improving the texture and shelf life of food products, and developing environmentally friendly stabilization methods for industrial processes. The ongoing challenge lies in optimizing the use of magnesium carbonate to achieve these diverse objectives while maintaining cost-effectiveness and regulatory compliance.
Looking ahead, the field of MgCO3 stabilization is poised for further innovation. Emerging trends indicate a focus on nanotechnology applications, where magnesium carbonate nanoparticles could offer unprecedented stabilization capabilities at the molecular level. Additionally, there is growing interest in exploring synergistic effects between MgCO3 and other stabilizing agents to create more robust and versatile stabilization systems.
The primary objective of utilizing magnesium carbonate in compound stabilization is to enhance the longevity, efficacy, and safety of various chemical formulations. This goal has become increasingly crucial in sectors such as pharmaceuticals, food production, and advanced materials manufacturing, where product stability directly impacts quality, safety, and economic viability.
Over the years, the understanding of MgCO3's stabilization mechanisms has significantly advanced. Initially, its use was primarily empirical, based on observed effects rather than a deep understanding of the underlying chemistry. However, as analytical techniques improved, researchers gained insights into how magnesium carbonate interacts with other compounds at a molecular level.
One of the key attributes that make MgCO3 an effective stabilizer is its ability to act as a pH buffer. This property is particularly valuable in maintaining the stability of pH-sensitive compounds, preventing degradation or unwanted reactions that could occur due to pH fluctuations. Additionally, magnesium carbonate's moisture-absorbing capabilities have proven beneficial in protecting hygroscopic substances from environmental humidity.
The technological evolution in this field has led to the development of various forms of magnesium carbonate, each tailored for specific applications. These include ultra-fine particles for enhanced dispersion, surface-modified variants for improved compatibility with organic compounds, and controlled-release formulations for sustained stabilization effects.
As research progresses, the objectives for MgCO3 stabilization have expanded beyond mere preservation. Current goals include enhancing bioavailability in pharmaceutical formulations, improving the texture and shelf life of food products, and developing environmentally friendly stabilization methods for industrial processes. The ongoing challenge lies in optimizing the use of magnesium carbonate to achieve these diverse objectives while maintaining cost-effectiveness and regulatory compliance.
Looking ahead, the field of MgCO3 stabilization is poised for further innovation. Emerging trends indicate a focus on nanotechnology applications, where magnesium carbonate nanoparticles could offer unprecedented stabilization capabilities at the molecular level. Additionally, there is growing interest in exploring synergistic effects between MgCO3 and other stabilizing agents to create more robust and versatile stabilization systems.
Market Analysis for MgCO3 Stabilized Compounds
The market for magnesium carbonate (MgCO3) stabilized compounds has shown significant growth in recent years, driven by increasing demand across various industries. The global market size for MgCO3 stabilized compounds was valued at approximately $2.5 billion in 2020 and is projected to reach $3.8 billion by 2025, growing at a CAGR of 8.7% during the forecast period.
The pharmaceutical industry represents the largest market segment for MgCO3 stabilized compounds, accounting for nearly 40% of the total market share. In this sector, MgCO3 is widely used as a stabilizer and excipient in drug formulations, particularly for moisture-sensitive active pharmaceutical ingredients (APIs). The growing prevalence of chronic diseases and the increasing demand for generic drugs are key factors driving the market growth in this segment.
The food and beverage industry is another significant consumer of MgCO3 stabilized compounds, with a market share of around 25%. MgCO3 is utilized as a food additive (E504) for its anti-caking and stabilizing properties in various products such as salt, spice blends, and powdered beverages. The rising consumer preference for clean-label and natural ingredients has further boosted the demand for MgCO3 in this sector.
In the cosmetics and personal care industry, which accounts for approximately 20% of the market, MgCO3 is employed as a stabilizer and absorbent in products like deodorants, powders, and creams. The growing trend towards natural and organic cosmetics has led to increased adoption of MgCO3 as a safer alternative to synthetic stabilizers.
Geographically, North America and Europe are the leading markets for MgCO3 stabilized compounds, collectively accounting for over 60% of the global market share. This dominance is attributed to the presence of well-established pharmaceutical and food industries, stringent quality regulations, and high consumer awareness regarding product safety and stability.
The Asia-Pacific region is expected to witness the highest growth rate in the coming years, with a projected CAGR of 10.5% from 2020 to 2025. This growth is driven by rapid industrialization, increasing disposable incomes, and the expansion of pharmaceutical and food processing industries in countries like China and India.
Key market players in the MgCO3 stabilized compounds industry include Merck KGaA, Omya AG, Huber Engineered Materials, and Minerals Technologies Inc. These companies are focusing on product innovation, strategic partnerships, and geographical expansion to maintain their market positions and capitalize on emerging opportunities.
The pharmaceutical industry represents the largest market segment for MgCO3 stabilized compounds, accounting for nearly 40% of the total market share. In this sector, MgCO3 is widely used as a stabilizer and excipient in drug formulations, particularly for moisture-sensitive active pharmaceutical ingredients (APIs). The growing prevalence of chronic diseases and the increasing demand for generic drugs are key factors driving the market growth in this segment.
The food and beverage industry is another significant consumer of MgCO3 stabilized compounds, with a market share of around 25%. MgCO3 is utilized as a food additive (E504) for its anti-caking and stabilizing properties in various products such as salt, spice blends, and powdered beverages. The rising consumer preference for clean-label and natural ingredients has further boosted the demand for MgCO3 in this sector.
In the cosmetics and personal care industry, which accounts for approximately 20% of the market, MgCO3 is employed as a stabilizer and absorbent in products like deodorants, powders, and creams. The growing trend towards natural and organic cosmetics has led to increased adoption of MgCO3 as a safer alternative to synthetic stabilizers.
Geographically, North America and Europe are the leading markets for MgCO3 stabilized compounds, collectively accounting for over 60% of the global market share. This dominance is attributed to the presence of well-established pharmaceutical and food industries, stringent quality regulations, and high consumer awareness regarding product safety and stability.
The Asia-Pacific region is expected to witness the highest growth rate in the coming years, with a projected CAGR of 10.5% from 2020 to 2025. This growth is driven by rapid industrialization, increasing disposable incomes, and the expansion of pharmaceutical and food processing industries in countries like China and India.
Key market players in the MgCO3 stabilized compounds industry include Merck KGaA, Omya AG, Huber Engineered Materials, and Minerals Technologies Inc. These companies are focusing on product innovation, strategic partnerships, and geographical expansion to maintain their market positions and capitalize on emerging opportunities.
Current Challenges in Chemical Stabilization
The field of chemical compound stabilization faces several significant challenges, particularly in the context of magnesium carbonate's efficacy. One of the primary issues is the variability in environmental conditions that can affect the stability of chemical compounds. Temperature fluctuations, humidity changes, and exposure to light or air can all contribute to the degradation of sensitive substances, making it difficult to maintain their integrity over extended periods.
Another challenge lies in the complexity of chemical interactions between magnesium carbonate and various compounds. While magnesium carbonate has shown promise as a stabilizing agent, its effectiveness can vary greatly depending on the specific chemical properties of the target compound. This variability necessitates extensive research and testing to determine optimal stabilization protocols for different substances.
The scalability of stabilization techniques using magnesium carbonate also presents a significant hurdle. Methods that prove effective in laboratory settings may not always translate seamlessly to industrial-scale applications. Factors such as mixing efficiency, uniform distribution of the stabilizing agent, and maintaining consistent quality across large batches can pose substantial technical difficulties.
Furthermore, the pharmaceutical and food industries face stringent regulatory requirements regarding the use of stabilizing agents. Ensuring that magnesium carbonate-based stabilization methods meet these regulatory standards while maintaining efficacy and cost-effectiveness is an ongoing challenge. This includes addressing potential concerns about the long-term effects of magnesium carbonate on human health and the environment.
The development of novel formulations that can enhance the stabilizing properties of magnesium carbonate without compromising the integrity or efficacy of the target compounds is another area of active research. Scientists are exploring various modifications and combinations to overcome limitations in current stabilization techniques.
Lastly, the economic viability of using magnesium carbonate for chemical stabilization on a large scale remains a concern. While it may offer superior stabilization properties in some cases, the cost-benefit analysis must justify its use over alternative methods or compounds. This economic factor can significantly influence the adoption and further development of magnesium carbonate-based stabilization technologies across different industries.
Another challenge lies in the complexity of chemical interactions between magnesium carbonate and various compounds. While magnesium carbonate has shown promise as a stabilizing agent, its effectiveness can vary greatly depending on the specific chemical properties of the target compound. This variability necessitates extensive research and testing to determine optimal stabilization protocols for different substances.
The scalability of stabilization techniques using magnesium carbonate also presents a significant hurdle. Methods that prove effective in laboratory settings may not always translate seamlessly to industrial-scale applications. Factors such as mixing efficiency, uniform distribution of the stabilizing agent, and maintaining consistent quality across large batches can pose substantial technical difficulties.
Furthermore, the pharmaceutical and food industries face stringent regulatory requirements regarding the use of stabilizing agents. Ensuring that magnesium carbonate-based stabilization methods meet these regulatory standards while maintaining efficacy and cost-effectiveness is an ongoing challenge. This includes addressing potential concerns about the long-term effects of magnesium carbonate on human health and the environment.
The development of novel formulations that can enhance the stabilizing properties of magnesium carbonate without compromising the integrity or efficacy of the target compounds is another area of active research. Scientists are exploring various modifications and combinations to overcome limitations in current stabilization techniques.
Lastly, the economic viability of using magnesium carbonate for chemical stabilization on a large scale remains a concern. While it may offer superior stabilization properties in some cases, the cost-benefit analysis must justify its use over alternative methods or compounds. This economic factor can significantly influence the adoption and further development of magnesium carbonate-based stabilization technologies across different industries.
Existing MgCO3 Stabilization Techniques
01 Pharmaceutical applications of magnesium carbonate
Magnesium carbonate is utilized in various pharmaceutical formulations due to its efficacy as an antacid, anti-caking agent, and excipient. It helps neutralize stomach acid, improve powder flowability, and enhance drug delivery in tablets and capsules.- Antacid and digestive health applications: Magnesium carbonate is widely used as an antacid to neutralize stomach acid and relieve indigestion, heartburn, and acid reflux. Its efficacy in treating digestive issues is due to its ability to rapidly neutralize excess stomach acid without causing an overproduction of acid afterward.
- Athletic performance enhancement: Magnesium carbonate is utilized in sports and fitness applications to improve grip and reduce moisture. It is particularly effective in enhancing performance in activities such as weightlifting, rock climbing, and gymnastics by absorbing sweat and providing better traction.
- Pharmaceutical formulations and drug delivery: Magnesium carbonate is employed in pharmaceutical formulations as an excipient or carrier for active ingredients. It can improve drug stability, modify release profiles, and enhance the bioavailability of certain medications, contributing to the overall efficacy of various pharmaceutical products.
- Environmental and industrial applications: The efficacy of magnesium carbonate extends to environmental and industrial uses, such as water treatment, flue gas desulfurization, and as a flame retardant. Its ability to absorb impurities and neutralize acidic compounds makes it effective in these applications.
- Cosmetic and personal care products: Magnesium carbonate is utilized in cosmetic and personal care products for its absorbent and mattifying properties. It is effective in controlling excess oil, improving texture in powders and creams, and providing a smooth feel in various skincare and makeup formulations.
02 Magnesium carbonate in personal care products
The efficacy of magnesium carbonate in personal care products is demonstrated through its use as an absorbent, deodorant, and anti-caking agent. It helps control moisture, reduce odor, and improve the texture of various cosmetic and hygiene products.Expand Specific Solutions03 Industrial applications of magnesium carbonate
Magnesium carbonate shows efficacy in various industrial applications, including as a filler in rubber and plastics, a flame retardant, and a component in refractory materials. Its properties contribute to improved product performance and safety in manufacturing processes.Expand Specific Solutions04 Magnesium carbonate in food and beverage industry
The efficacy of magnesium carbonate in the food and beverage industry is evident in its use as a color retention agent, acidity regulator, and anti-caking additive. It helps maintain product quality, extend shelf life, and improve the texture of various food products.Expand Specific Solutions05 Environmental applications of magnesium carbonate
Magnesium carbonate demonstrates efficacy in environmental applications, such as carbon dioxide capture, soil pH adjustment, and water treatment. Its ability to react with and sequester CO2, neutralize acidic soils, and remove contaminants from water makes it valuable in addressing environmental challenges.Expand Specific Solutions
Key Players in MgCO3 Stabilization Industry
The competitive landscape for magnesium carbonate's efficacy in chemical compound stabilization is evolving, with the market in a growth phase. The global market size for magnesium carbonate is expanding, driven by increasing applications in various industries. Companies like Kyowa Chemical Industry Co. Ltd. and Konoshima Chemical Co., Ltd. are at the forefront, leveraging their expertise in magnesium compounds. The technology is maturing, with established players like Pfizer Inc. and Merck Patent GmbH contributing to advancements. Emerging companies such as Cambridge Carbon Capture Ltd. and Negative Emissions Materials, Inc. are exploring innovative applications, particularly in carbon capture and storage, indicating a trend towards environmental sustainability in this field.
Merck Patent GmbH
Technical Solution: Merck Patent GmbH has developed innovative methods for using magnesium carbonate in chemical compound stabilization. Their approach involves creating nanostructured magnesium carbonate particles with high surface area and porosity[1]. These particles are used as carriers for active pharmaceutical ingredients (APIs), enhancing their stability and bioavailability. The company has also explored the use of magnesium carbonate in controlled release formulations, where it acts as both a stabilizer and a release-modifying agent[2]. Additionally, Merck has investigated the use of magnesium carbonate in combination with other excipients to create synergistic stabilization effects for sensitive compounds[3].
Strengths: High surface area and porosity of nanostructured particles enhance API stability. Dual functionality as stabilizer and release modifier. Weaknesses: May require specialized manufacturing processes, potentially increasing production costs.
Pfizer Inc.
Technical Solution: Pfizer Inc. has developed advanced formulations utilizing magnesium carbonate for chemical compound stabilization, particularly in pharmaceutical applications. Their research has focused on using magnesium carbonate as a pH-adjusting agent and stabilizer in solid oral dosage forms[4]. The company has patented methods for incorporating magnesium carbonate into tablet formulations to enhance the stability of acid-labile drugs[5]. Pfizer has also explored the use of magnesium carbonate in combination with other buffering agents to create optimal microenvironments for drug stability[6]. Furthermore, they have investigated the role of magnesium carbonate in improving the dissolution profiles of poorly soluble drugs, thereby enhancing their bioavailability[7].
Strengths: Effective pH adjustment for acid-labile drugs. Improved dissolution profiles for poorly soluble compounds. Weaknesses: May affect the overall tablet size and weight, potentially impacting patient compliance for certain formulations.
Core Innovations in MgCO3 Stabilization
Stabilized compositions of halogenated polymers containing a compound of lead or organo tin
PatentInactiveEP0509864A1
Innovation
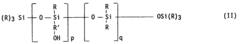
- The use of a stabilized composition containing a combination of lead or organotin compounds with essentially amorphous basic aluminum and magnesium carbonate, specifically represented by the formula (MgO)y, Al2O3, (CO2)x, (H2O)z, where y ≤ 1.7, x ≤ 0.7, and z ≥ 3, which improves dispersion and stability, reducing the need for multiple stabilizers and minimizing toxicity.
Composition and method for stabilizing environmentally-harmful substances; use of non-ferrous metal salts and oxides to stabilize environmentally-harmful substances with caustic magnesium oxide
PatentInactiveUS20100217062A1
Innovation
- A composition and method using caustic magnesium oxide, non-ferrous metal salts, and oxides to form radial crystalline structures, which stabilize environmentally-harmful substances through three-dimensional polymerization, enhancing mechanical strength and water impermeability, allowing for the creation of a durable and neutralized product suitable for reuse.
Environmental Impact of MgCO3 Stabilization
The environmental impact of magnesium carbonate (MgCO3) stabilization is a critical consideration in its application for chemical compound stabilization. MgCO3, being a naturally occurring mineral, generally exhibits a lower environmental footprint compared to many synthetic stabilizers. However, its widespread use still necessitates a thorough examination of potential ecological consequences.
One of the primary environmental benefits of MgCO3 stabilization is its non-toxic nature. Unlike some chemical stabilizers that may leach harmful substances into soil or water systems, MgCO3 does not introduce toxic elements into the environment. This characteristic makes it particularly suitable for applications in sensitive ecosystems or areas with strict environmental regulations.
The production process of MgCO3 for stabilization purposes also tends to have a relatively low environmental impact. It can be extracted from natural deposits or synthesized through environmentally friendly processes, often requiring less energy and producing fewer emissions compared to the manufacture of more complex chemical stabilizers.
In terms of biodegradability, MgCO3 presents a favorable profile. As a naturally occurring compound, it can be broken down and assimilated into the environment without leaving persistent residues. This property is especially advantageous in applications where long-term accumulation of stabilizing agents in the ecosystem is a concern.
However, the large-scale use of MgCO3 for stabilization is not without potential environmental drawbacks. Excessive application in certain ecosystems could lead to localized changes in soil or water pH levels. While magnesium and carbonate ions are generally beneficial to many organisms, an imbalance could potentially affect sensitive species or disrupt local biogeochemical cycles.
The mining and processing of natural magnesite, the primary source of MgCO3, also carries environmental implications. Open-pit mining can lead to habitat destruction and landscape alterations. Additionally, the energy requirements for processing and transportation contribute to the overall carbon footprint of MgCO3 stabilization solutions.
Water usage in MgCO3 production and application is another environmental factor to consider. While generally less water-intensive than some chemical processes, large-scale stabilization projects using MgCO3 may still have significant water requirements, potentially impacting local water resources in water-scarce regions.
In conclusion, while MgCO3 stabilization offers several environmental advantages over synthetic alternatives, its use still requires careful management and consideration of local ecological conditions. Sustainable practices in sourcing, processing, and application are essential to maximize its benefits while minimizing potential negative impacts on the environment.
One of the primary environmental benefits of MgCO3 stabilization is its non-toxic nature. Unlike some chemical stabilizers that may leach harmful substances into soil or water systems, MgCO3 does not introduce toxic elements into the environment. This characteristic makes it particularly suitable for applications in sensitive ecosystems or areas with strict environmental regulations.
The production process of MgCO3 for stabilization purposes also tends to have a relatively low environmental impact. It can be extracted from natural deposits or synthesized through environmentally friendly processes, often requiring less energy and producing fewer emissions compared to the manufacture of more complex chemical stabilizers.
In terms of biodegradability, MgCO3 presents a favorable profile. As a naturally occurring compound, it can be broken down and assimilated into the environment without leaving persistent residues. This property is especially advantageous in applications where long-term accumulation of stabilizing agents in the ecosystem is a concern.
However, the large-scale use of MgCO3 for stabilization is not without potential environmental drawbacks. Excessive application in certain ecosystems could lead to localized changes in soil or water pH levels. While magnesium and carbonate ions are generally beneficial to many organisms, an imbalance could potentially affect sensitive species or disrupt local biogeochemical cycles.
The mining and processing of natural magnesite, the primary source of MgCO3, also carries environmental implications. Open-pit mining can lead to habitat destruction and landscape alterations. Additionally, the energy requirements for processing and transportation contribute to the overall carbon footprint of MgCO3 stabilization solutions.
Water usage in MgCO3 production and application is another environmental factor to consider. While generally less water-intensive than some chemical processes, large-scale stabilization projects using MgCO3 may still have significant water requirements, potentially impacting local water resources in water-scarce regions.
In conclusion, while MgCO3 stabilization offers several environmental advantages over synthetic alternatives, its use still requires careful management and consideration of local ecological conditions. Sustainable practices in sourcing, processing, and application are essential to maximize its benefits while minimizing potential negative impacts on the environment.
Regulatory Framework for Stabilized Compounds
The regulatory framework for stabilized compounds using magnesium carbonate as a stabilizing agent is a complex and evolving landscape. Governments and regulatory bodies worldwide have established guidelines and standards to ensure the safety, efficacy, and quality of stabilized chemical compounds. These regulations typically cover various aspects, including manufacturing processes, quality control, labeling requirements, and safety assessments.
In the United States, the Food and Drug Administration (FDA) plays a crucial role in regulating stabilized compounds, particularly those used in pharmaceuticals and food additives. The FDA's Current Good Manufacturing Practice (cGMP) regulations provide guidelines for the production and testing of stabilized compounds. These regulations emphasize the importance of consistent quality, purity, and stability throughout the manufacturing process.
The European Medicines Agency (EMA) oversees similar regulations in the European Union. The EMA's guidelines on stability testing of existing active substances and related finished products provide specific requirements for demonstrating the long-term stability of compounds, including those stabilized with magnesium carbonate.
Internationally, the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) has developed harmonized guidelines for stability testing. These guidelines, such as ICH Q1A(R2) on stability testing of new drug substances and products, provide a framework for evaluating the stability of compounds under various environmental conditions.
Regulatory bodies also focus on the environmental impact of stabilized compounds. The Environmental Protection Agency (EPA) in the United States and the European Chemicals Agency (ECHA) in the EU have established regulations to assess and manage the potential environmental risks associated with the use and disposal of stabilized chemical compounds.
For industrial applications, occupational safety regulations come into play. Organizations such as the Occupational Safety and Health Administration (OSHA) in the US and the European Agency for Safety and Health at Work (EU-OSHA) in Europe have set standards for handling and working with stabilized compounds in industrial settings.
As research continues to advance the understanding of magnesium carbonate's efficacy in chemical compound stabilization, regulatory frameworks are likely to evolve. Regulatory bodies are increasingly focusing on the long-term effects of stabilized compounds and their potential interactions with other substances. This ongoing development in regulations underscores the need for companies and researchers to stay informed about the latest regulatory requirements and to engage in proactive compliance strategies.
In the United States, the Food and Drug Administration (FDA) plays a crucial role in regulating stabilized compounds, particularly those used in pharmaceuticals and food additives. The FDA's Current Good Manufacturing Practice (cGMP) regulations provide guidelines for the production and testing of stabilized compounds. These regulations emphasize the importance of consistent quality, purity, and stability throughout the manufacturing process.
The European Medicines Agency (EMA) oversees similar regulations in the European Union. The EMA's guidelines on stability testing of existing active substances and related finished products provide specific requirements for demonstrating the long-term stability of compounds, including those stabilized with magnesium carbonate.
Internationally, the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) has developed harmonized guidelines for stability testing. These guidelines, such as ICH Q1A(R2) on stability testing of new drug substances and products, provide a framework for evaluating the stability of compounds under various environmental conditions.
Regulatory bodies also focus on the environmental impact of stabilized compounds. The Environmental Protection Agency (EPA) in the United States and the European Chemicals Agency (ECHA) in the EU have established regulations to assess and manage the potential environmental risks associated with the use and disposal of stabilized chemical compounds.
For industrial applications, occupational safety regulations come into play. Organizations such as the Occupational Safety and Health Administration (OSHA) in the US and the European Agency for Safety and Health at Work (EU-OSHA) in Europe have set standards for handling and working with stabilized compounds in industrial settings.
As research continues to advance the understanding of magnesium carbonate's efficacy in chemical compound stabilization, regulatory frameworks are likely to evolve. Regulatory bodies are increasingly focusing on the long-term effects of stabilized compounds and their potential interactions with other substances. This ongoing development in regulations underscores the need for companies and researchers to stay informed about the latest regulatory requirements and to engage in proactive compliance strategies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!