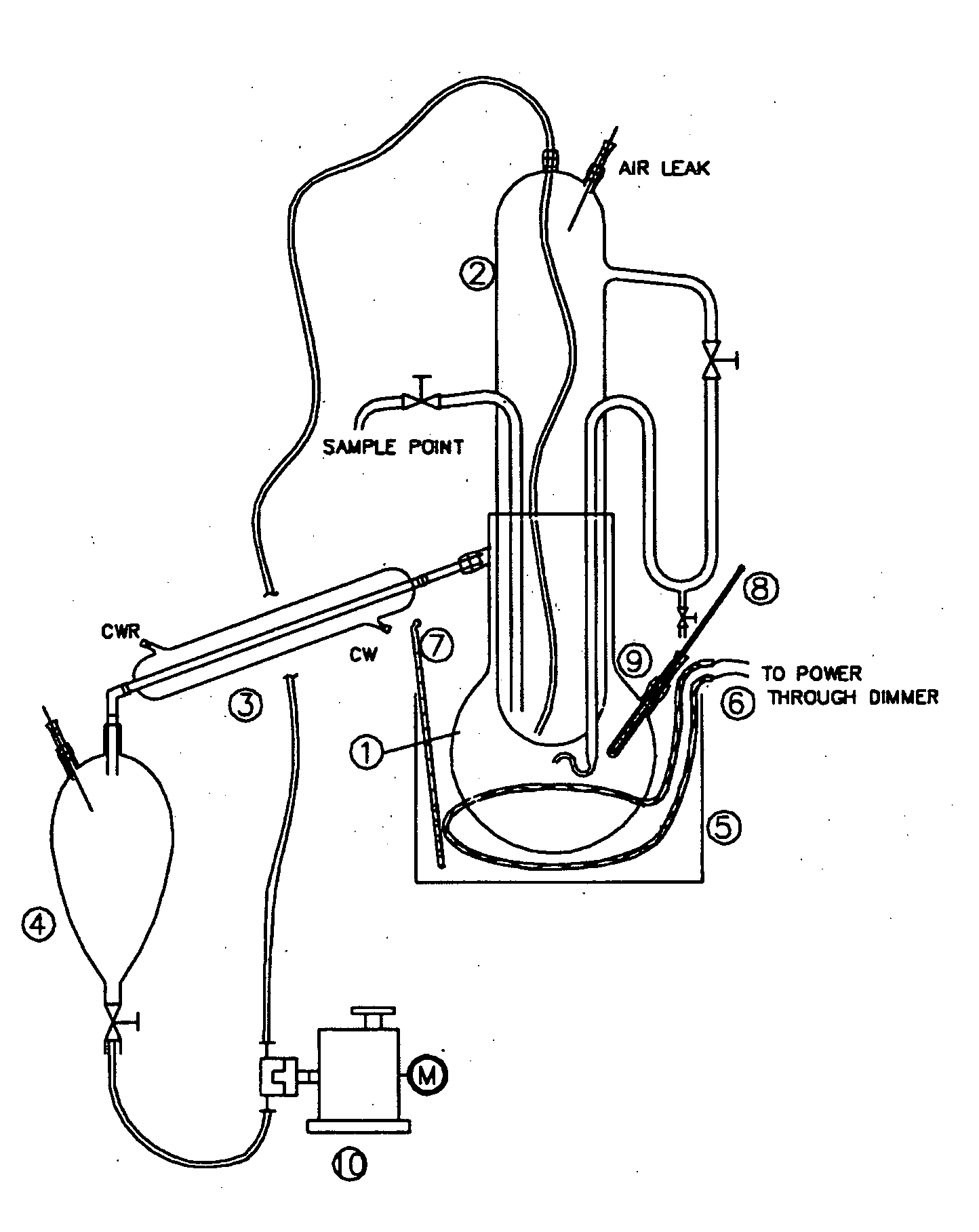
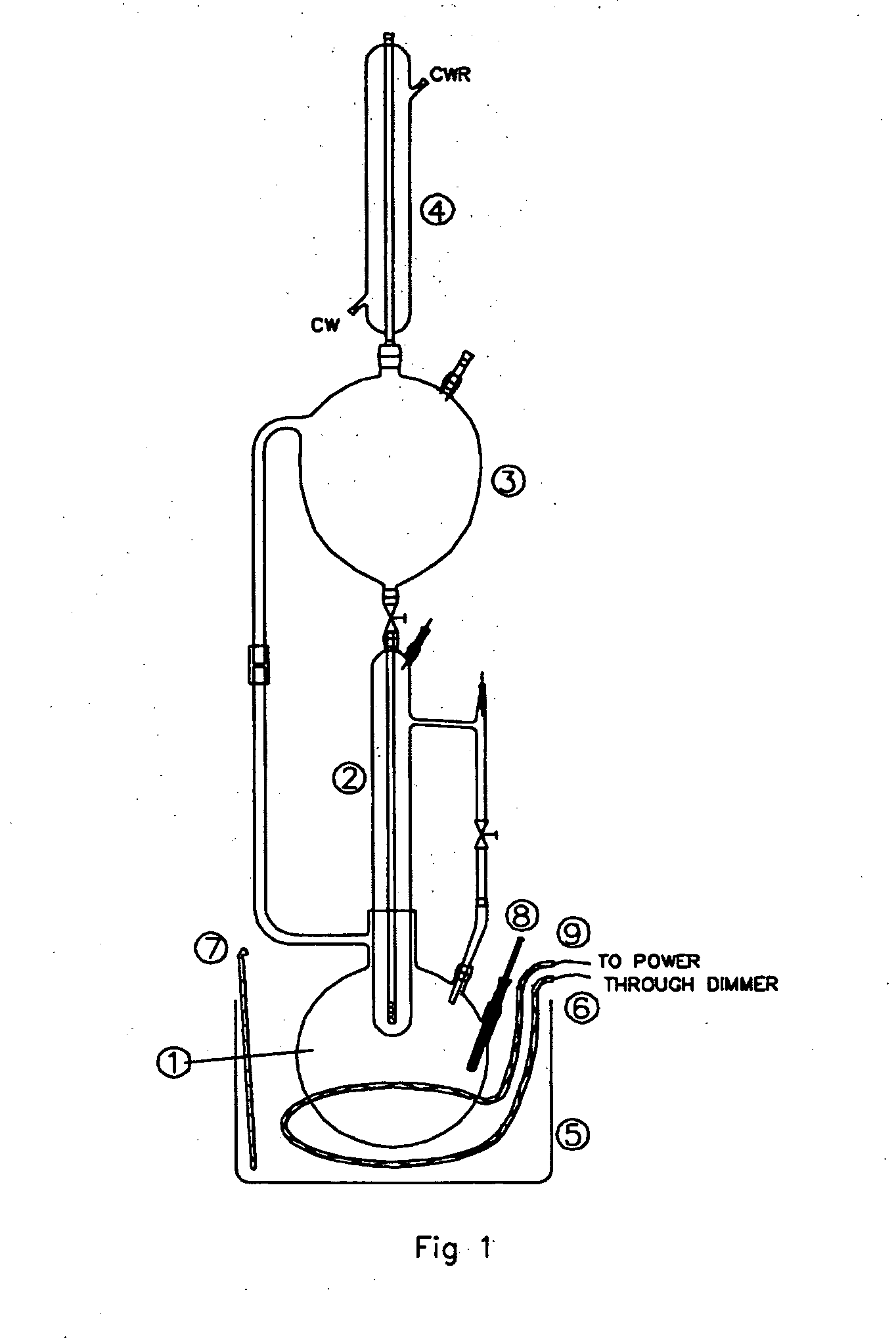
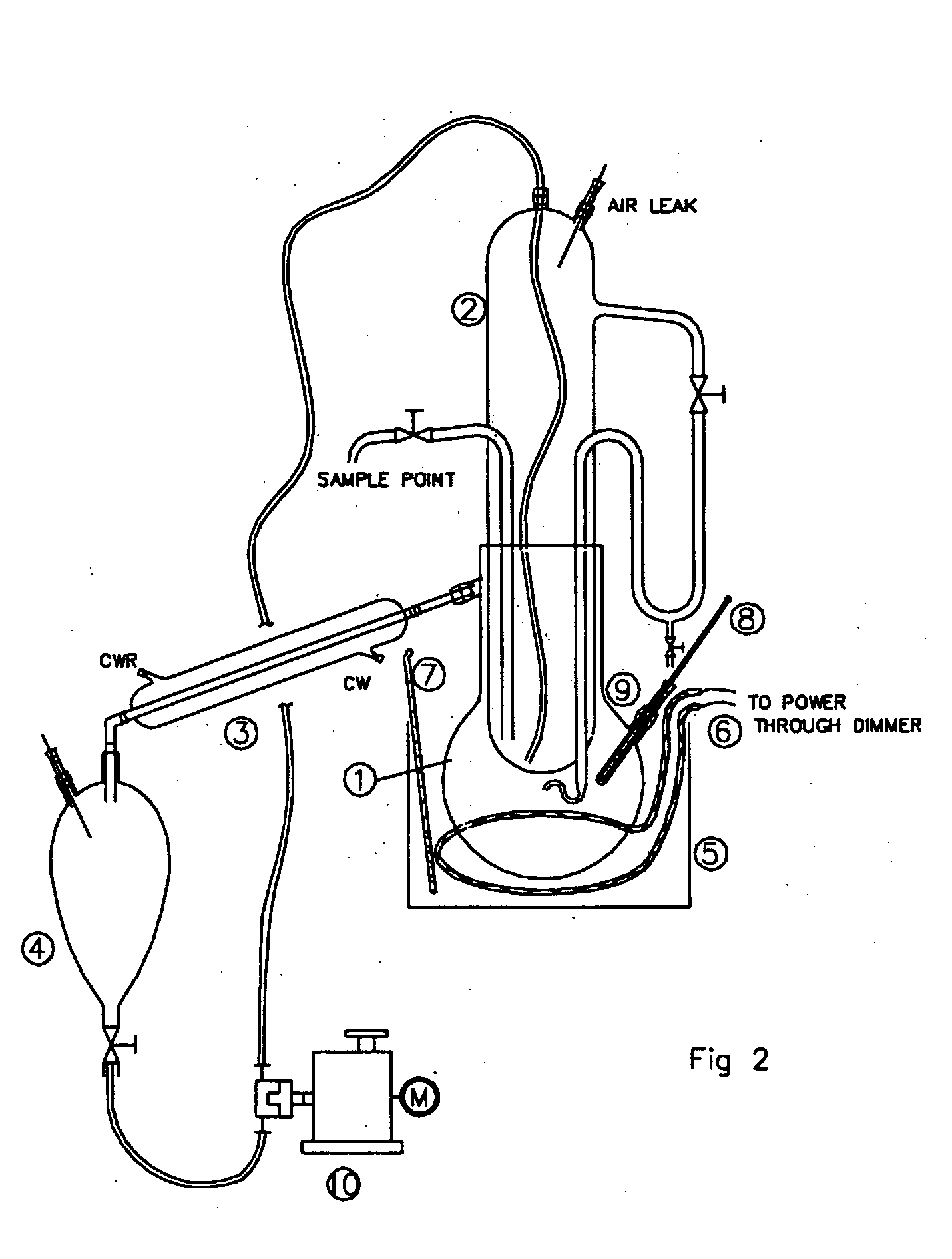
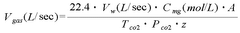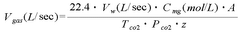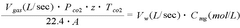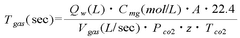The Use of Magnesium Carbonate in Biodiesel Purification Processes
JUL 31, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Biodiesel Purification Evolution and Objectives
Biodiesel purification has undergone significant evolution since its inception, driven by the growing demand for cleaner and more sustainable fuel alternatives. The journey began in the late 20th century when biodiesel emerged as a promising renewable fuel source. Initially, the purification process was rudimentary, focusing primarily on removing glycerol, the main by-product of transesterification.
As environmental concerns and fuel quality standards became more stringent, the objectives of biodiesel purification expanded. The industry recognized the need to remove not only glycerol but also other impurities such as unreacted methanol, catalyst residues, and free fatty acids. This shift in focus led to the development of more sophisticated purification techniques, including water washing, dry washing, and membrane filtration.
The use of magnesium carbonate in biodiesel purification processes represents a significant milestone in this evolutionary timeline. This innovative approach aims to address several key objectives simultaneously. Firstly, it seeks to improve the efficiency of the purification process by leveraging the adsorptive properties of magnesium carbonate to remove impurities more effectively than traditional methods.
Secondly, the incorporation of magnesium carbonate aligns with the industry's goal of developing more environmentally friendly purification techniques. Unlike some conventional methods that rely on water or synthetic adsorbents, magnesium carbonate is a naturally occurring mineral with minimal environmental impact. This aspect is particularly crucial as the biodiesel industry strives to enhance its overall sustainability profile.
Another critical objective driving the exploration of magnesium carbonate in purification processes is the reduction of production costs. By potentially simplifying the purification process and reducing the need for multiple purification steps, this approach could lead to significant economic benefits for biodiesel producers. Additionally, the reusability of magnesium carbonate as an adsorbent further contributes to cost-effectiveness and resource efficiency.
The current technological landscape surrounding magnesium carbonate in biodiesel purification is characterized by ongoing research and development efforts. Scientists and engineers are working to optimize the use of this material, focusing on aspects such as particle size, surface area, and activation methods to enhance its purification capabilities. The ultimate goal is to develop a robust, scalable, and economically viable purification process that meets or exceeds current industry standards for biodiesel quality.
As the biodiesel industry continues to evolve, the objectives for purification processes are likely to become even more demanding. Future developments may focus on achieving near-zero impurity levels, further reducing environmental impact, and integrating purification processes more seamlessly into overall biodiesel production systems. The use of magnesium carbonate represents a promising step towards meeting these ambitious objectives, potentially paving the way for the next generation of biodiesel purification technologies.
As environmental concerns and fuel quality standards became more stringent, the objectives of biodiesel purification expanded. The industry recognized the need to remove not only glycerol but also other impurities such as unreacted methanol, catalyst residues, and free fatty acids. This shift in focus led to the development of more sophisticated purification techniques, including water washing, dry washing, and membrane filtration.
The use of magnesium carbonate in biodiesel purification processes represents a significant milestone in this evolutionary timeline. This innovative approach aims to address several key objectives simultaneously. Firstly, it seeks to improve the efficiency of the purification process by leveraging the adsorptive properties of magnesium carbonate to remove impurities more effectively than traditional methods.
Secondly, the incorporation of magnesium carbonate aligns with the industry's goal of developing more environmentally friendly purification techniques. Unlike some conventional methods that rely on water or synthetic adsorbents, magnesium carbonate is a naturally occurring mineral with minimal environmental impact. This aspect is particularly crucial as the biodiesel industry strives to enhance its overall sustainability profile.
Another critical objective driving the exploration of magnesium carbonate in purification processes is the reduction of production costs. By potentially simplifying the purification process and reducing the need for multiple purification steps, this approach could lead to significant economic benefits for biodiesel producers. Additionally, the reusability of magnesium carbonate as an adsorbent further contributes to cost-effectiveness and resource efficiency.
The current technological landscape surrounding magnesium carbonate in biodiesel purification is characterized by ongoing research and development efforts. Scientists and engineers are working to optimize the use of this material, focusing on aspects such as particle size, surface area, and activation methods to enhance its purification capabilities. The ultimate goal is to develop a robust, scalable, and economically viable purification process that meets or exceeds current industry standards for biodiesel quality.
As the biodiesel industry continues to evolve, the objectives for purification processes are likely to become even more demanding. Future developments may focus on achieving near-zero impurity levels, further reducing environmental impact, and integrating purification processes more seamlessly into overall biodiesel production systems. The use of magnesium carbonate represents a promising step towards meeting these ambitious objectives, potentially paving the way for the next generation of biodiesel purification technologies.
Market Analysis for Eco-friendly Biodiesel Production
The global biodiesel market has been experiencing significant growth in recent years, driven by increasing environmental concerns and government mandates for renewable fuel usage. The market size for biodiesel was valued at approximately $44 billion in 2020 and is projected to reach $67 billion by 2026, growing at a CAGR of 6.9% during the forecast period. This growth is primarily attributed to the rising demand for cleaner and more sustainable fuel alternatives.
The use of magnesium carbonate in biodiesel purification processes represents a promising eco-friendly approach that aligns with the market's shift towards more sustainable production methods. This technology addresses the growing need for efficient and environmentally friendly purification techniques in biodiesel manufacturing.
Key market drivers for eco-friendly biodiesel production include stringent environmental regulations, increasing awareness of carbon emissions, and the push for energy security. Many countries have implemented policies to promote biodiesel usage, such as blending mandates and tax incentives, which are expected to further boost market growth.
The automotive sector remains the largest consumer of biodiesel, accounting for over 70% of the total market share. However, other sectors such as agriculture, construction, and power generation are also showing increased adoption of biodiesel, diversifying the market opportunities for eco-friendly production technologies.
Regionally, Europe leads the biodiesel market, followed by North America and Asia-Pacific. The European Union's Renewable Energy Directive (RED II) sets ambitious targets for renewable energy in transport, driving the demand for advanced biodiesel production technologies. The Asia-Pacific region is expected to witness the fastest growth due to increasing energy consumption and supportive government policies in countries like India and China.
The market for eco-friendly biodiesel production technologies, including purification processes using magnesium carbonate, is characterized by intense competition and rapid technological advancements. Key players are investing heavily in research and development to improve production efficiency and reduce costs. This trend is likely to continue as the industry strives to meet the growing demand for sustainable fuel solutions.
Challenges in the market include fluctuating feedstock prices, competition from other renewable energy sources, and the need for significant capital investments in production facilities. However, the long-term outlook remains positive, supported by ongoing technological innovations and the global push towards decarbonization.
The use of magnesium carbonate in biodiesel purification processes represents a promising eco-friendly approach that aligns with the market's shift towards more sustainable production methods. This technology addresses the growing need for efficient and environmentally friendly purification techniques in biodiesel manufacturing.
Key market drivers for eco-friendly biodiesel production include stringent environmental regulations, increasing awareness of carbon emissions, and the push for energy security. Many countries have implemented policies to promote biodiesel usage, such as blending mandates and tax incentives, which are expected to further boost market growth.
The automotive sector remains the largest consumer of biodiesel, accounting for over 70% of the total market share. However, other sectors such as agriculture, construction, and power generation are also showing increased adoption of biodiesel, diversifying the market opportunities for eco-friendly production technologies.
Regionally, Europe leads the biodiesel market, followed by North America and Asia-Pacific. The European Union's Renewable Energy Directive (RED II) sets ambitious targets for renewable energy in transport, driving the demand for advanced biodiesel production technologies. The Asia-Pacific region is expected to witness the fastest growth due to increasing energy consumption and supportive government policies in countries like India and China.
The market for eco-friendly biodiesel production technologies, including purification processes using magnesium carbonate, is characterized by intense competition and rapid technological advancements. Key players are investing heavily in research and development to improve production efficiency and reduce costs. This trend is likely to continue as the industry strives to meet the growing demand for sustainable fuel solutions.
Challenges in the market include fluctuating feedstock prices, competition from other renewable energy sources, and the need for significant capital investments in production facilities. However, the long-term outlook remains positive, supported by ongoing technological innovations and the global push towards decarbonization.
Magnesium Carbonate in Biodiesel: Current Status and Challenges
The current status of magnesium carbonate in biodiesel purification processes reflects a growing interest in sustainable and efficient methods for refining biofuels. Magnesium carbonate has emerged as a promising adsorbent material due to its high surface area, porosity, and alkaline nature, which make it effective in removing impurities from biodiesel.
One of the primary challenges in biodiesel production is the removal of glycerol, free fatty acids, and other contaminants that can negatively impact fuel quality and engine performance. Traditional purification methods often involve water washing, which is water-intensive and generates significant wastewater. Magnesium carbonate offers a dry purification alternative that addresses these environmental concerns.
Recent studies have demonstrated the efficacy of magnesium carbonate in adsorbing glycerol and reducing the acid value of biodiesel. Its ability to neutralize acidic compounds makes it particularly useful in treating biodiesel produced from high free fatty acid feedstocks. However, the optimal conditions for using magnesium carbonate in biodiesel purification are still being investigated, including factors such as temperature, contact time, and adsorbent dosage.
A significant challenge in the widespread adoption of magnesium carbonate for biodiesel purification is the need for standardization and scalability. While laboratory-scale experiments have shown promising results, translating these findings to industrial-scale operations requires further research and development. The variability in biodiesel feedstocks also presents a challenge, as the effectiveness of magnesium carbonate may differ depending on the source and composition of the raw materials.
Another hurdle is the regeneration and reusability of magnesium carbonate adsorbents. Developing cost-effective methods for regenerating spent adsorbents is crucial for making the process economically viable on a large scale. Additionally, the potential for magnesium leaching into the purified biodiesel needs to be carefully monitored and controlled to meet fuel quality standards.
The integration of magnesium carbonate into existing biodiesel production processes also poses technical challenges. Optimizing the purification step to minimize production time and energy consumption while maintaining high-quality output is an ongoing area of research. Furthermore, the long-term stability and performance of magnesium carbonate under various operating conditions need to be thoroughly evaluated to ensure consistent purification results.
As the biodiesel industry continues to evolve, addressing these challenges will be crucial for realizing the full potential of magnesium carbonate in biodiesel purification. Collaborative efforts between researchers, industry stakeholders, and regulatory bodies will be essential in overcoming these hurdles and establishing magnesium carbonate as a viable and sustainable option for biodiesel refinement.
One of the primary challenges in biodiesel production is the removal of glycerol, free fatty acids, and other contaminants that can negatively impact fuel quality and engine performance. Traditional purification methods often involve water washing, which is water-intensive and generates significant wastewater. Magnesium carbonate offers a dry purification alternative that addresses these environmental concerns.
Recent studies have demonstrated the efficacy of magnesium carbonate in adsorbing glycerol and reducing the acid value of biodiesel. Its ability to neutralize acidic compounds makes it particularly useful in treating biodiesel produced from high free fatty acid feedstocks. However, the optimal conditions for using magnesium carbonate in biodiesel purification are still being investigated, including factors such as temperature, contact time, and adsorbent dosage.
A significant challenge in the widespread adoption of magnesium carbonate for biodiesel purification is the need for standardization and scalability. While laboratory-scale experiments have shown promising results, translating these findings to industrial-scale operations requires further research and development. The variability in biodiesel feedstocks also presents a challenge, as the effectiveness of magnesium carbonate may differ depending on the source and composition of the raw materials.
Another hurdle is the regeneration and reusability of magnesium carbonate adsorbents. Developing cost-effective methods for regenerating spent adsorbents is crucial for making the process economically viable on a large scale. Additionally, the potential for magnesium leaching into the purified biodiesel needs to be carefully monitored and controlled to meet fuel quality standards.
The integration of magnesium carbonate into existing biodiesel production processes also poses technical challenges. Optimizing the purification step to minimize production time and energy consumption while maintaining high-quality output is an ongoing area of research. Furthermore, the long-term stability and performance of magnesium carbonate under various operating conditions need to be thoroughly evaluated to ensure consistent purification results.
As the biodiesel industry continues to evolve, addressing these challenges will be crucial for realizing the full potential of magnesium carbonate in biodiesel purification. Collaborative efforts between researchers, industry stakeholders, and regulatory bodies will be essential in overcoming these hurdles and establishing magnesium carbonate as a viable and sustainable option for biodiesel refinement.
Existing Magnesium Carbonate-based Purification Methods
01 Precipitation and crystallization methods
Magnesium carbonate purification can be achieved through precipitation and crystallization techniques. These methods involve controlling factors such as temperature, pH, and concentration to promote the formation of high-purity magnesium carbonate crystals. The process may include steps like seeding, cooling, and filtration to enhance crystal growth and separation.- Precipitation and crystallization methods: Magnesium carbonate purification can be achieved through precipitation and crystallization techniques. These methods involve controlling factors such as temperature, pH, and concentration to selectively precipitate magnesium carbonate from solution. The process may include steps like dissolving raw materials, adding precipitating agents, and controlling crystallization conditions to obtain high-purity magnesium carbonate.
- Carbonation process for purification: The carbonation process is an effective method for purifying magnesium carbonate. This technique involves treating magnesium-containing solutions or suspensions with carbon dioxide under controlled conditions. The process can be optimized by adjusting parameters such as pressure, temperature, and reaction time to produce high-quality magnesium carbonate with desired properties.
- Filtration and washing techniques: Filtration and washing steps play a crucial role in the purification of magnesium carbonate. These processes help remove impurities and unwanted byproducts from the magnesium carbonate slurry. Various filtration methods, such as pressure filtration or vacuum filtration, can be employed, followed by washing with purified water or other suitable solvents to enhance the purity of the final product.
- Thermal treatment for purification: Thermal treatment methods can be used to purify magnesium carbonate. These techniques involve heating the raw material under controlled conditions to decompose impurities or convert them into easily removable forms. The process may include calcination, roasting, or other high-temperature treatments, followed by subsequent processing steps to obtain purified magnesium carbonate.
- Solvent extraction and recrystallization: Solvent extraction and recrystallization techniques can be employed for the purification of magnesium carbonate. These methods involve dissolving the raw material in a suitable solvent, selectively extracting impurities, and then recrystallizing the purified magnesium carbonate. The choice of solvents and process conditions can be optimized to achieve high purity levels and desired crystal morphology.
02 Carbonation of magnesium hydroxide
One method for producing pure magnesium carbonate involves the carbonation of magnesium hydroxide. This process typically includes reacting magnesium hydroxide with carbon dioxide under controlled conditions. The reaction parameters, such as pressure, temperature, and CO2 concentration, are optimized to yield high-purity magnesium carbonate.Expand Specific Solutions03 Purification using organic solvents
Organic solvents can be employed in the purification of magnesium carbonate. This method often involves dissolving impure magnesium carbonate in a suitable organic solvent, followed by recrystallization or selective precipitation. The choice of solvent and process conditions are crucial for achieving high purity levels.Expand Specific Solutions04 Ion exchange and membrane separation
Advanced purification techniques for magnesium carbonate include ion exchange and membrane separation processes. These methods can effectively remove impurities and contaminants from magnesium carbonate solutions or suspensions. The use of specific ion exchange resins or selective membranes allows for the isolation of pure magnesium carbonate.Expand Specific Solutions05 Thermal decomposition and recarbonation
This purification method involves the thermal decomposition of impure magnesium carbonate to form magnesium oxide, followed by a controlled recarbonation process. The intermediate magnesium oxide step allows for the removal of certain impurities, and the subsequent recarbonation can be optimized to produce high-purity magnesium carbonate.Expand Specific Solutions
Innovative Approaches in MgCO3 Biodiesel Purification
Process for the Preparation of Biodiesel from Vegetable Oils Containing High FFA
PatentInactiveUS20100205853A1
Innovation
- A process involving liquid-liquid extraction with a polar organic solvent to deacidify crude vegetable oils, followed by in situ esterification and transesterification, and subsequent purification using celite adsorption or liquid-liquid extraction to produce biodiesel, minimizing the need for mineral acids and bases and reducing effluents.
Method for producing high-purity magnesium carbonate using seawater desalination brine
PatentWO2025116218A1
Innovation
- A method involving continuous reactors and solid-liquid separators to produce high-purity magnesium carbonate by reacting magnesium hydroxide with carbon dioxide, while using a polymer hydrogel to remove residual calcium and magnesium, and implementing a chlor-alkali process to reduce sodium concentration and produce sodium hydroxide and chlorine.
Environmental Impact Assessment of MgCO3 in Biodiesel Production
The environmental impact assessment of magnesium carbonate (MgCO3) in biodiesel production is a critical aspect of evaluating the sustainability and ecological footprint of this purification process. MgCO3 has gained attention as an effective adsorbent for removing impurities from biodiesel, potentially offering a more environmentally friendly alternative to traditional methods.
One of the primary environmental benefits of using MgCO3 in biodiesel purification is its potential to reduce water consumption. Traditional wet washing techniques often require significant amounts of water, which can strain local water resources and generate large volumes of wastewater. MgCO3-based dry washing methods can substantially decrease water usage, minimizing the environmental impact associated with water consumption and treatment.
The production and disposal of MgCO3 also play crucial roles in its overall environmental impact. MgCO3 is typically sourced from natural deposits or synthesized through industrial processes. The mining and processing of natural MgCO3 can lead to habitat disruption and energy consumption. However, compared to some other adsorbents, MgCO3 production generally has a lower carbon footprint and requires less energy-intensive processes.
In terms of waste management, spent MgCO3 from biodiesel purification can be recycled or repurposed for other applications, such as soil amendment or construction materials. This potential for circular economy practices can significantly reduce the environmental burden associated with waste disposal. However, proper handling and disposal protocols must be established to prevent any potential contamination from residual biodiesel or impurities.
The use of MgCO3 in biodiesel purification may also contribute to improved air quality. By effectively removing impurities from biodiesel, MgCO3 can help produce cleaner-burning fuel, potentially reducing harmful emissions when the biodiesel is used in vehicles or industrial applications. This indirect environmental benefit should be considered in the overall assessment of MgCO3's environmental impact.
It is important to note that the environmental impact of MgCO3 in biodiesel production can vary depending on factors such as the scale of production, the specific purification process employed, and local environmental regulations. A comprehensive life cycle assessment (LCA) would be necessary to fully quantify the environmental impacts and benefits of MgCO3 use in biodiesel purification compared to alternative methods.
Future research should focus on optimizing MgCO3-based purification processes to further minimize environmental impacts, such as developing more efficient regeneration techniques for spent MgCO3 and exploring the use of waste-derived or synthetic MgCO3 to reduce reliance on natural resources. Additionally, long-term studies on the potential ecological effects of MgCO3 use in large-scale biodiesel production are needed to ensure its sustainability and environmental safety.
One of the primary environmental benefits of using MgCO3 in biodiesel purification is its potential to reduce water consumption. Traditional wet washing techniques often require significant amounts of water, which can strain local water resources and generate large volumes of wastewater. MgCO3-based dry washing methods can substantially decrease water usage, minimizing the environmental impact associated with water consumption and treatment.
The production and disposal of MgCO3 also play crucial roles in its overall environmental impact. MgCO3 is typically sourced from natural deposits or synthesized through industrial processes. The mining and processing of natural MgCO3 can lead to habitat disruption and energy consumption. However, compared to some other adsorbents, MgCO3 production generally has a lower carbon footprint and requires less energy-intensive processes.
In terms of waste management, spent MgCO3 from biodiesel purification can be recycled or repurposed for other applications, such as soil amendment or construction materials. This potential for circular economy practices can significantly reduce the environmental burden associated with waste disposal. However, proper handling and disposal protocols must be established to prevent any potential contamination from residual biodiesel or impurities.
The use of MgCO3 in biodiesel purification may also contribute to improved air quality. By effectively removing impurities from biodiesel, MgCO3 can help produce cleaner-burning fuel, potentially reducing harmful emissions when the biodiesel is used in vehicles or industrial applications. This indirect environmental benefit should be considered in the overall assessment of MgCO3's environmental impact.
It is important to note that the environmental impact of MgCO3 in biodiesel production can vary depending on factors such as the scale of production, the specific purification process employed, and local environmental regulations. A comprehensive life cycle assessment (LCA) would be necessary to fully quantify the environmental impacts and benefits of MgCO3 use in biodiesel purification compared to alternative methods.
Future research should focus on optimizing MgCO3-based purification processes to further minimize environmental impacts, such as developing more efficient regeneration techniques for spent MgCO3 and exploring the use of waste-derived or synthetic MgCO3 to reduce reliance on natural resources. Additionally, long-term studies on the potential ecological effects of MgCO3 use in large-scale biodiesel production are needed to ensure its sustainability and environmental safety.
Regulatory Framework for Biodiesel Purification Processes
The regulatory framework for biodiesel purification processes, including the use of magnesium carbonate, is a complex and evolving landscape. At the international level, organizations such as the International Organization for Standardization (ISO) have established guidelines for biodiesel quality, which indirectly influence purification processes. ISO 14214, for instance, sets specifications for fatty acid methyl esters (FAME) used in diesel engines, necessitating stringent purification methods to meet these standards.
In the United States, the Environmental Protection Agency (EPA) plays a crucial role in regulating biodiesel production and purification under the Renewable Fuel Standard (RFS) program. The EPA's regulations focus on ensuring that biodiesel meets specific environmental and quality criteria, including limits on contaminants and impurities. These regulations indirectly impact the choice of purification methods, potentially influencing the adoption of magnesium carbonate-based processes.
The European Union has implemented the Renewable Energy Directive (RED), which sets targets for renewable energy use in transportation. This directive includes sustainability criteria for biofuels, indirectly affecting purification processes by requiring producers to demonstrate reduced greenhouse gas emissions throughout the production chain, including the purification stage.
Specific to purification processes, many countries have regulations governing the use of chemicals in food and fuel production. For magnesium carbonate, its use in biodiesel purification may be subject to regulations typically applied to food-grade additives or industrial chemicals. In the United States, the Food and Drug Administration (FDA) classifies magnesium carbonate as Generally Recognized as Safe (GRAS) for certain applications, which may facilitate its use in biodiesel purification.
Environmental regulations also play a significant role in shaping biodiesel purification processes. Wastewater discharge from biodiesel plants, including effluents from purification stages, is subject to strict environmental regulations in many jurisdictions. The use of magnesium carbonate in purification may need to be evaluated for its environmental impact and compliance with local water quality standards.
Occupational health and safety regulations are another important aspect of the regulatory framework. The handling and storage of chemicals used in biodiesel purification, including magnesium carbonate, must comply with workplace safety standards set by agencies such as the Occupational Safety and Health Administration (OSHA) in the United States.
As the biodiesel industry continues to evolve, regulatory frameworks are likely to adapt. Emerging trends include a greater focus on lifecycle assessments of biodiesel production processes and increased scrutiny of the environmental footprint of purification methods. This may lead to new regulations that specifically address novel purification techniques, potentially including those utilizing magnesium carbonate.
In the United States, the Environmental Protection Agency (EPA) plays a crucial role in regulating biodiesel production and purification under the Renewable Fuel Standard (RFS) program. The EPA's regulations focus on ensuring that biodiesel meets specific environmental and quality criteria, including limits on contaminants and impurities. These regulations indirectly impact the choice of purification methods, potentially influencing the adoption of magnesium carbonate-based processes.
The European Union has implemented the Renewable Energy Directive (RED), which sets targets for renewable energy use in transportation. This directive includes sustainability criteria for biofuels, indirectly affecting purification processes by requiring producers to demonstrate reduced greenhouse gas emissions throughout the production chain, including the purification stage.
Specific to purification processes, many countries have regulations governing the use of chemicals in food and fuel production. For magnesium carbonate, its use in biodiesel purification may be subject to regulations typically applied to food-grade additives or industrial chemicals. In the United States, the Food and Drug Administration (FDA) classifies magnesium carbonate as Generally Recognized as Safe (GRAS) for certain applications, which may facilitate its use in biodiesel purification.
Environmental regulations also play a significant role in shaping biodiesel purification processes. Wastewater discharge from biodiesel plants, including effluents from purification stages, is subject to strict environmental regulations in many jurisdictions. The use of magnesium carbonate in purification may need to be evaluated for its environmental impact and compliance with local water quality standards.
Occupational health and safety regulations are another important aspect of the regulatory framework. The handling and storage of chemicals used in biodiesel purification, including magnesium carbonate, must comply with workplace safety standards set by agencies such as the Occupational Safety and Health Administration (OSHA) in the United States.
As the biodiesel industry continues to evolve, regulatory frameworks are likely to adapt. Emerging trends include a greater focus on lifecycle assessments of biodiesel production processes and increased scrutiny of the environmental footprint of purification methods. This may lead to new regulations that specifically address novel purification techniques, potentially including those utilizing magnesium carbonate.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!