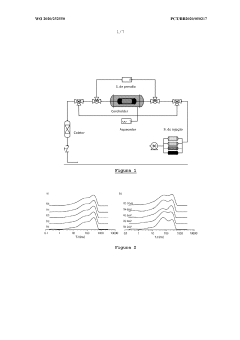
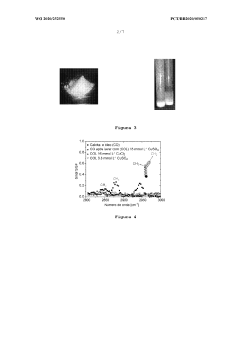
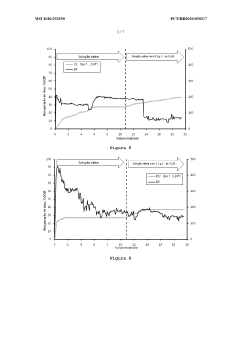
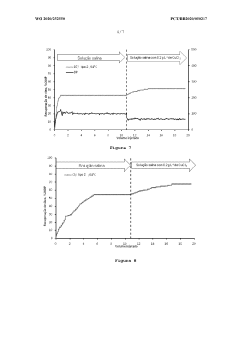
Magnesium Carbonate’s Application in Recovering Petroleum Distillates
JUL 31, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
MgCO3 in Oil Recovery: Background and Objectives
Magnesium carbonate (MgCO3) has emerged as a promising agent in the field of petroleum distillate recovery, marking a significant advancement in oil extraction technologies. This application represents a convergence of geological sciences, chemical engineering, and petroleum industry practices, aiming to enhance the efficiency and sustainability of oil recovery processes.
The journey of MgCO3 in oil recovery traces back to the ongoing quest for more effective and environmentally friendly methods in the petroleum industry. As conventional oil reserves become increasingly depleted, the focus has shifted towards enhanced oil recovery (EOR) techniques. These methods are crucial for extracting residual oil that remains trapped in reservoirs after primary and secondary recovery methods have been exhausted.
Magnesium carbonate's potential in this domain stems from its unique physicochemical properties. Its ability to interact with petroleum distillates, coupled with its relatively low environmental impact, has positioned it as a subject of intense research and development. The primary objective of utilizing MgCO3 in oil recovery is to improve the overall extraction efficiency while minimizing environmental footprint – a balance that has long been a challenge in the oil industry.
The technical goals associated with MgCO3 application in petroleum distillate recovery are multifaceted. Firstly, there is a focus on enhancing the displacement efficiency of oil from porous media. This involves studying how MgCO3 can alter the interfacial tensions between oil and rock surfaces, potentially leading to improved oil mobilization. Secondly, researchers aim to optimize the formulation and deployment methods of MgCO3-based solutions to ensure their effectiveness across various reservoir conditions.
Another critical objective is to understand and leverage the potential of MgCO3 in altering reservoir wettability. By modifying the rock surface properties, it may be possible to create more favorable conditions for oil release and flow. Additionally, there is significant interest in exploring how MgCO3 can be integrated with existing EOR techniques, such as water flooding or gas injection, to create synergistic effects.
The development trajectory of MgCO3 in oil recovery also aligns with broader industry trends towards more sustainable practices. As environmental regulations become stricter and public awareness of ecological issues grows, the petroleum industry is under increasing pressure to adopt greener technologies. In this context, the relatively benign nature of MgCO3 compared to some traditional chemical agents used in EOR presents a compelling argument for its further development and application.
The journey of MgCO3 in oil recovery traces back to the ongoing quest for more effective and environmentally friendly methods in the petroleum industry. As conventional oil reserves become increasingly depleted, the focus has shifted towards enhanced oil recovery (EOR) techniques. These methods are crucial for extracting residual oil that remains trapped in reservoirs after primary and secondary recovery methods have been exhausted.
Magnesium carbonate's potential in this domain stems from its unique physicochemical properties. Its ability to interact with petroleum distillates, coupled with its relatively low environmental impact, has positioned it as a subject of intense research and development. The primary objective of utilizing MgCO3 in oil recovery is to improve the overall extraction efficiency while minimizing environmental footprint – a balance that has long been a challenge in the oil industry.
The technical goals associated with MgCO3 application in petroleum distillate recovery are multifaceted. Firstly, there is a focus on enhancing the displacement efficiency of oil from porous media. This involves studying how MgCO3 can alter the interfacial tensions between oil and rock surfaces, potentially leading to improved oil mobilization. Secondly, researchers aim to optimize the formulation and deployment methods of MgCO3-based solutions to ensure their effectiveness across various reservoir conditions.
Another critical objective is to understand and leverage the potential of MgCO3 in altering reservoir wettability. By modifying the rock surface properties, it may be possible to create more favorable conditions for oil release and flow. Additionally, there is significant interest in exploring how MgCO3 can be integrated with existing EOR techniques, such as water flooding or gas injection, to create synergistic effects.
The development trajectory of MgCO3 in oil recovery also aligns with broader industry trends towards more sustainable practices. As environmental regulations become stricter and public awareness of ecological issues grows, the petroleum industry is under increasing pressure to adopt greener technologies. In this context, the relatively benign nature of MgCO3 compared to some traditional chemical agents used in EOR presents a compelling argument for its further development and application.
Market Analysis for Enhanced Oil Recovery
The enhanced oil recovery (EOR) market has been experiencing significant growth due to the increasing global demand for oil and the need to maximize production from existing oil fields. As conventional oil reserves become depleted, the industry is turning to advanced technologies to extract remaining oil from mature fields. The global EOR market was valued at approximately $26 billion in 2020 and is projected to reach $34 billion by 2025, growing at a CAGR of 5.5% during the forecast period.
The market for EOR technologies can be segmented based on the type of technique used, including thermal recovery, gas injection, and chemical injection. Among these, thermal recovery methods currently dominate the market, accounting for about 40% of the total EOR market share. However, chemical injection methods, including the use of magnesium carbonate for recovering petroleum distillates, are gaining traction due to their effectiveness in certain geological formations.
Geographically, North America leads the EOR market, with the United States being the largest consumer of EOR technologies. This is primarily due to the presence of mature oil fields and the country's focus on increasing domestic oil production. The Middle East and Asia-Pacific regions are also significant markets, with countries like China, India, and Saudi Arabia investing heavily in EOR technologies to boost their oil production capabilities.
The market drivers for EOR technologies include the rising global energy demand, declining production from conventional oil fields, and technological advancements in oil recovery techniques. The increasing focus on maximizing recovery from existing fields, rather than exploring new ones, is also contributing to market growth. Additionally, government initiatives and favorable regulations supporting EOR projects are further propelling market expansion.
However, the EOR market faces challenges such as high initial investment costs, technical complexities associated with implementation, and environmental concerns. The volatility of oil prices also impacts the adoption of EOR technologies, as low oil prices can make these advanced recovery methods less economically viable.
The application of magnesium carbonate in recovering petroleum distillates represents a niche but growing segment within the chemical EOR market. This technology is particularly promising for its potential to improve oil recovery rates while being environmentally friendly. As the industry continues to seek more efficient and sustainable EOR methods, innovations like magnesium carbonate-based solutions are likely to gain more attention and market share in the coming years.
The market for EOR technologies can be segmented based on the type of technique used, including thermal recovery, gas injection, and chemical injection. Among these, thermal recovery methods currently dominate the market, accounting for about 40% of the total EOR market share. However, chemical injection methods, including the use of magnesium carbonate for recovering petroleum distillates, are gaining traction due to their effectiveness in certain geological formations.
Geographically, North America leads the EOR market, with the United States being the largest consumer of EOR technologies. This is primarily due to the presence of mature oil fields and the country's focus on increasing domestic oil production. The Middle East and Asia-Pacific regions are also significant markets, with countries like China, India, and Saudi Arabia investing heavily in EOR technologies to boost their oil production capabilities.
The market drivers for EOR technologies include the rising global energy demand, declining production from conventional oil fields, and technological advancements in oil recovery techniques. The increasing focus on maximizing recovery from existing fields, rather than exploring new ones, is also contributing to market growth. Additionally, government initiatives and favorable regulations supporting EOR projects are further propelling market expansion.
However, the EOR market faces challenges such as high initial investment costs, technical complexities associated with implementation, and environmental concerns. The volatility of oil prices also impacts the adoption of EOR technologies, as low oil prices can make these advanced recovery methods less economically viable.
The application of magnesium carbonate in recovering petroleum distillates represents a niche but growing segment within the chemical EOR market. This technology is particularly promising for its potential to improve oil recovery rates while being environmentally friendly. As the industry continues to seek more efficient and sustainable EOR methods, innovations like magnesium carbonate-based solutions are likely to gain more attention and market share in the coming years.
Technical Challenges in Petroleum Distillate Recovery
The recovery of petroleum distillates using magnesium carbonate faces several technical challenges that require innovative solutions. One of the primary obstacles is the efficient separation of petroleum distillates from complex mixtures, particularly in the presence of water and other contaminants. Magnesium carbonate's hydrophobic properties make it a promising adsorbent, but optimizing its selectivity for specific petroleum fractions remains a significant challenge.
Another technical hurdle is the development of a cost-effective and scalable production process for magnesium carbonate with the desired physical and chemical properties. The surface area, pore size distribution, and particle morphology of magnesium carbonate significantly influence its adsorption capacity and kinetics. Achieving consistent and tailored characteristics at an industrial scale is crucial for widespread application in petroleum distillate recovery.
The regeneration of spent magnesium carbonate adsorbents presents a further technical challenge. Efficient desorption of captured petroleum distillates and restoration of the adsorbent's original properties are essential for economic viability. Current regeneration methods often involve energy-intensive thermal treatments or chemical processes that may compromise the adsorbent's structure and performance over multiple cycles.
Environmental concerns and regulatory compliance add another layer of complexity to the technical challenges. Developing environmentally friendly processes for both the production of magnesium carbonate and the recovery of petroleum distillates is crucial. This includes minimizing waste generation, reducing energy consumption, and ensuring that any by-products or emissions meet stringent environmental standards.
The integration of magnesium carbonate-based recovery systems into existing petroleum refining and processing infrastructure poses significant engineering challenges. Designing robust and efficient systems that can handle varying feed compositions, flow rates, and operating conditions while maintaining high recovery rates is a complex task. Additionally, ensuring compatibility with existing equipment and processes without compromising safety or operational efficiency requires careful consideration and innovative engineering solutions.
Lastly, the long-term stability and performance of magnesium carbonate under diverse operating conditions remain areas of concern. Factors such as temperature fluctuations, pressure variations, and exposure to various chemical species in petroleum distillates can potentially degrade the adsorbent's performance over time. Developing strategies to enhance the durability and maintain consistent performance of magnesium carbonate-based recovery systems is crucial for their successful implementation in industrial settings.
Another technical hurdle is the development of a cost-effective and scalable production process for magnesium carbonate with the desired physical and chemical properties. The surface area, pore size distribution, and particle morphology of magnesium carbonate significantly influence its adsorption capacity and kinetics. Achieving consistent and tailored characteristics at an industrial scale is crucial for widespread application in petroleum distillate recovery.
The regeneration of spent magnesium carbonate adsorbents presents a further technical challenge. Efficient desorption of captured petroleum distillates and restoration of the adsorbent's original properties are essential for economic viability. Current regeneration methods often involve energy-intensive thermal treatments or chemical processes that may compromise the adsorbent's structure and performance over multiple cycles.
Environmental concerns and regulatory compliance add another layer of complexity to the technical challenges. Developing environmentally friendly processes for both the production of magnesium carbonate and the recovery of petroleum distillates is crucial. This includes minimizing waste generation, reducing energy consumption, and ensuring that any by-products or emissions meet stringent environmental standards.
The integration of magnesium carbonate-based recovery systems into existing petroleum refining and processing infrastructure poses significant engineering challenges. Designing robust and efficient systems that can handle varying feed compositions, flow rates, and operating conditions while maintaining high recovery rates is a complex task. Additionally, ensuring compatibility with existing equipment and processes without compromising safety or operational efficiency requires careful consideration and innovative engineering solutions.
Lastly, the long-term stability and performance of magnesium carbonate under diverse operating conditions remain areas of concern. Factors such as temperature fluctuations, pressure variations, and exposure to various chemical species in petroleum distillates can potentially degrade the adsorbent's performance over time. Developing strategies to enhance the durability and maintain consistent performance of magnesium carbonate-based recovery systems is crucial for their successful implementation in industrial settings.
Current MgCO3 Application Techniques
01 Magnesium carbonate in pharmaceutical compositions
Magnesium carbonate is used in various pharmaceutical compositions as an excipient or active ingredient. It can be utilized in antacid formulations, oral care products, and as a filler or binder in tablets and capsules. Its properties make it suitable for improving drug stability, controlling release rates, and enhancing bioavailability of certain medications.- Magnesium carbonate in pharmaceutical compositions: Magnesium carbonate is used in various pharmaceutical compositions as an excipient or active ingredient. It can be utilized in antacid formulations, oral care products, and as a filler or binder in tablets and capsules. Its properties make it suitable for improving drug stability, controlling release rates, and enhancing bioavailability of certain medications.
- Industrial applications of magnesium carbonate: Magnesium carbonate finds widespread use in industrial applications. It is employed as a filler and whitening agent in paper production, as a reinforcing agent in rubber manufacturing, and as a raw material in the production of magnesium oxide. Additionally, it is used in fireproofing materials, ceramics, and as a drying agent in various industrial processes.
- Magnesium carbonate in food and beverage industry: In the food and beverage industry, magnesium carbonate serves multiple purposes. It is used as an anticaking agent in powdered products, a pH regulator in certain food preparations, and a color retention agent. It can also be found in some dietary supplements as a source of magnesium and in some sports products for its moisture-absorbing properties.
- Environmental applications of magnesium carbonate: Magnesium carbonate has emerging applications in environmental technologies. It is being researched for its potential in carbon capture and storage processes, where it can react with carbon dioxide to form stable carbonate minerals. Additionally, it is used in water treatment processes and as a component in some air purification systems.
- Magnesium carbonate in cosmetics and personal care products: In the cosmetics and personal care industry, magnesium carbonate is utilized for its absorbent and mattifying properties. It is commonly found in powder-based makeup products, deodorants, and antiperspirants. Its ability to absorb excess oils and moisture makes it a valuable ingredient in various skincare formulations and body powders.
02 Magnesium carbonate in personal care products
Magnesium carbonate finds applications in personal care products such as deodorants, antiperspirants, and cosmetics. It can act as an absorbent, pH adjuster, and anti-caking agent. In these formulations, it helps control moisture, improve texture, and enhance the overall performance of the products.Expand Specific Solutions03 Industrial applications of magnesium carbonate
Magnesium carbonate is widely used in various industrial processes and products. It serves as a flame retardant, a filler in rubber and plastics, and a component in ceramics and refractory materials. Its properties make it valuable in applications such as paper production, wastewater treatment, and as a desiccant in certain industrial processes.Expand Specific Solutions04 Magnesium carbonate in food and beverage industry
In the food and beverage industry, magnesium carbonate is used as an additive and processing aid. It functions as an acidity regulator, anti-caking agent, and color retention agent. Applications include confectionery, dairy products, and flour treatment. It also finds use in mineral water fortification and as a processing aid in coffee production.Expand Specific Solutions05 Synthesis and production methods of magnesium carbonate
Various methods are employed for the synthesis and production of magnesium carbonate. These include precipitation reactions, carbonation processes, and extraction from natural sources. Advanced techniques focus on controlling particle size, morphology, and purity of the produced magnesium carbonate to suit specific applications. Some methods aim to improve efficiency and reduce environmental impact in the production process.Expand Specific Solutions
Key Players in EOR Technology
The application of magnesium carbonate in recovering petroleum distillates is in a developing stage, with the market showing potential for growth. The technology's maturity is still evolving, as evidenced by ongoing research and development efforts from various players. Key companies like Saudi Arabian Oil Co., China Petroleum & Chemical Corp., and ExxonMobil Technology & Engineering Co. are actively involved in advancing this technology. The competitive landscape includes a mix of oil and gas giants, research institutions, and specialized chemical companies, indicating a diverse approach to innovation. While the market size is not yet substantial, increasing focus on efficient petroleum recovery methods and environmental considerations are likely to drive future expansion in this niche sector.
Saudi Arabian Oil Co.
Technical Solution: Saudi Arabian Oil Co. (Saudi Aramco) has developed an innovative process for recovering petroleum distillates using magnesium carbonate. Their method involves injecting a slurry of magnesium carbonate into oil reservoirs, which reacts with residual oil and enhances its recovery. The process utilizes the chemical properties of magnesium carbonate to alter the wettability of rock surfaces, improving oil displacement efficiency[1]. Additionally, the company has implemented a carbon capture and utilization system that converts CO2 into magnesium carbonate, which is then used in the oil recovery process, creating a circular economy approach[2][3].
Strengths: Dual benefit of enhanced oil recovery and carbon capture. Weaknesses: Potential scalability issues and high initial implementation costs.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed a novel application of magnesium carbonate in petroleum distillate recovery. Their approach involves using magnesium carbonate as a catalyst support in the hydrodesulfurization process of petroleum distillates. The magnesium carbonate-supported catalysts have shown improved activity and stability compared to traditional alumina-supported catalysts[4]. Sinopec has also integrated magnesium carbonate into their enhanced oil recovery (EOR) techniques, where it acts as a pH buffer and helps maintain the stability of polymer solutions used in chemical flooding[5]. This dual application has led to increased efficiency in both refining and extraction processes.
Strengths: Improved catalyst performance and EOR efficiency. Weaknesses: Potential increased costs due to specialized catalyst production.
Innovative MgCO3 Formulations for EOR
Catalyst for demercaptanization of petroleum distillates
PatentInactiveUS5849656A
Innovation
- A method using a heterogeneous catalyst with a water-soluble salt of copper, iron, or cobalt deposited on a fibrous carbonaceous material containing oxides of metals of variable valence, eliminating the need for alkali and achieving higher mercaptan oxidation at temperatures between 80°C to 220°C.
Composition for a method of optimized recovery of oil from an underground reservoir and method of optimized recovery of oil from an underground reservoir
PatentWO2020252550A1
Innovation
- The use of aqueous compositions containing controlled salinity and copper (II) ions, simulating seawater concentrations, is introduced to enhance oil recovery by injecting these solutions into carbonate reservoirs, which react with calcium carbonate to release petroleum fractions.
Environmental Impact Assessment
The application of magnesium carbonate in recovering petroleum distillates presents both potential benefits and environmental concerns that require careful assessment. The process primarily involves using magnesium carbonate as an adsorbent to separate and recover valuable petroleum components from waste streams or contaminated sites. While this technique offers promising advantages in resource recovery and waste reduction, its environmental impact must be thoroughly evaluated.
One of the primary environmental benefits of using magnesium carbonate in petroleum distillate recovery is the potential reduction of hydrocarbon pollution. By effectively capturing and separating petroleum compounds, this method can help minimize the release of harmful substances into soil and water systems. This is particularly significant in areas affected by oil spills or industrial contamination, where the recovery process can contribute to ecosystem restoration and environmental remediation efforts.
However, the production and use of magnesium carbonate itself may have environmental implications. The mining and processing of magnesite, the primary source of magnesium carbonate, can lead to habitat disruption, soil erosion, and potential water pollution if not managed properly. Additionally, the energy-intensive nature of magnesium carbonate production contributes to carbon emissions, which must be factored into the overall environmental impact assessment.
The recovery process using magnesium carbonate may also generate secondary waste streams that require proper handling and disposal. These could include spent adsorbent materials contaminated with petroleum residues, which may pose challenges for safe disposal or regeneration. The environmental impact of managing these byproducts must be carefully considered and mitigated to ensure the overall sustainability of the recovery process.
Water usage is another critical factor to evaluate in the environmental impact assessment. The petroleum distillate recovery process may require significant amounts of water for washing and regeneration of the magnesium carbonate adsorbent. In water-stressed regions, this could potentially strain local water resources and ecosystems. Implementing water recycling and conservation measures within the recovery system would be essential to minimize this impact.
Air quality considerations are also relevant, as the recovery process may involve the release of volatile organic compounds (VOCs) during the separation and regeneration stages. Proper emission control systems and monitoring protocols would need to be implemented to ensure compliance with air quality regulations and to protect surrounding communities from potential health risks associated with VOC exposure.
The long-term effects of using magnesium carbonate for petroleum distillate recovery on soil and groundwater quality should be thoroughly investigated. While the process aims to remove contaminants, there is a need to assess any potential leaching or accumulation of magnesium or other compounds in the treated areas over time. This evaluation would help ensure that the recovery method does not inadvertently introduce new environmental challenges while addressing existing contamination issues.
One of the primary environmental benefits of using magnesium carbonate in petroleum distillate recovery is the potential reduction of hydrocarbon pollution. By effectively capturing and separating petroleum compounds, this method can help minimize the release of harmful substances into soil and water systems. This is particularly significant in areas affected by oil spills or industrial contamination, where the recovery process can contribute to ecosystem restoration and environmental remediation efforts.
However, the production and use of magnesium carbonate itself may have environmental implications. The mining and processing of magnesite, the primary source of magnesium carbonate, can lead to habitat disruption, soil erosion, and potential water pollution if not managed properly. Additionally, the energy-intensive nature of magnesium carbonate production contributes to carbon emissions, which must be factored into the overall environmental impact assessment.
The recovery process using magnesium carbonate may also generate secondary waste streams that require proper handling and disposal. These could include spent adsorbent materials contaminated with petroleum residues, which may pose challenges for safe disposal or regeneration. The environmental impact of managing these byproducts must be carefully considered and mitigated to ensure the overall sustainability of the recovery process.
Water usage is another critical factor to evaluate in the environmental impact assessment. The petroleum distillate recovery process may require significant amounts of water for washing and regeneration of the magnesium carbonate adsorbent. In water-stressed regions, this could potentially strain local water resources and ecosystems. Implementing water recycling and conservation measures within the recovery system would be essential to minimize this impact.
Air quality considerations are also relevant, as the recovery process may involve the release of volatile organic compounds (VOCs) during the separation and regeneration stages. Proper emission control systems and monitoring protocols would need to be implemented to ensure compliance with air quality regulations and to protect surrounding communities from potential health risks associated with VOC exposure.
The long-term effects of using magnesium carbonate for petroleum distillate recovery on soil and groundwater quality should be thoroughly investigated. While the process aims to remove contaminants, there is a need to assess any potential leaching or accumulation of magnesium or other compounds in the treated areas over time. This evaluation would help ensure that the recovery method does not inadvertently introduce new environmental challenges while addressing existing contamination issues.
Economic Feasibility Analysis
The economic feasibility of using magnesium carbonate in recovering petroleum distillates is a critical factor in determining its potential for widespread adoption in the oil and gas industry. Initial cost-benefit analyses indicate that the implementation of magnesium carbonate-based recovery methods could lead to significant improvements in efficiency and yield, potentially offsetting the initial investment costs.
The primary economic advantage lies in the increased recovery rates of petroleum distillates. Magnesium carbonate's unique properties allow for enhanced oil recovery (EOR) techniques that can access previously unreachable oil reserves. This could extend the productive life of existing oil fields, thereby increasing the return on investment for drilling operations. Furthermore, the process may reduce the need for new exploratory drilling, which is often costly and environmentally controversial.
However, the economic viability is not without challenges. The upfront costs associated with retrofitting existing recovery systems or implementing new magnesium carbonate-based technologies can be substantial. These costs include equipment modifications, training of personnel, and potential downtime during implementation. Additionally, the sourcing and transportation of magnesium carbonate to oil fields may incur ongoing operational expenses that need to be carefully factored into long-term economic projections.
Market volatility in oil prices plays a crucial role in the economic feasibility assessment. During periods of high oil prices, the additional recovery enabled by magnesium carbonate methods becomes more economically attractive. Conversely, in low-price environments, the cost-benefit ratio may become less favorable, potentially delaying adoption or implementation of these technologies.
Environmental regulations and carbon pricing mechanisms also impact the economic equation. As governments worldwide impose stricter environmental standards, the use of magnesium carbonate in petroleum distillate recovery could offer a competitive advantage. Its potential to reduce the environmental footprint of oil recovery operations may translate into economic benefits through reduced regulatory compliance costs and improved corporate image.
The scalability of magnesium carbonate applications in petroleum recovery is another key economic consideration. Initial pilot projects and small-scale implementations will need to demonstrate consistent results before large-scale adoption can be justified. The ability to scale up operations efficiently will be crucial in realizing the full economic potential of this technology.
In conclusion, while the use of magnesium carbonate in recovering petroleum distillates shows promise from an economic standpoint, its feasibility will depend on a complex interplay of factors including implementation costs, oil market conditions, regulatory environments, and technological scalability. Ongoing research and pilot studies will be essential in refining economic models and providing a clearer picture of the long-term financial viability of this innovative recovery method.
The primary economic advantage lies in the increased recovery rates of petroleum distillates. Magnesium carbonate's unique properties allow for enhanced oil recovery (EOR) techniques that can access previously unreachable oil reserves. This could extend the productive life of existing oil fields, thereby increasing the return on investment for drilling operations. Furthermore, the process may reduce the need for new exploratory drilling, which is often costly and environmentally controversial.
However, the economic viability is not without challenges. The upfront costs associated with retrofitting existing recovery systems or implementing new magnesium carbonate-based technologies can be substantial. These costs include equipment modifications, training of personnel, and potential downtime during implementation. Additionally, the sourcing and transportation of magnesium carbonate to oil fields may incur ongoing operational expenses that need to be carefully factored into long-term economic projections.
Market volatility in oil prices plays a crucial role in the economic feasibility assessment. During periods of high oil prices, the additional recovery enabled by magnesium carbonate methods becomes more economically attractive. Conversely, in low-price environments, the cost-benefit ratio may become less favorable, potentially delaying adoption or implementation of these technologies.
Environmental regulations and carbon pricing mechanisms also impact the economic equation. As governments worldwide impose stricter environmental standards, the use of magnesium carbonate in petroleum distillate recovery could offer a competitive advantage. Its potential to reduce the environmental footprint of oil recovery operations may translate into economic benefits through reduced regulatory compliance costs and improved corporate image.
The scalability of magnesium carbonate applications in petroleum recovery is another key economic consideration. Initial pilot projects and small-scale implementations will need to demonstrate consistent results before large-scale adoption can be justified. The ability to scale up operations efficiently will be crucial in realizing the full economic potential of this technology.
In conclusion, while the use of magnesium carbonate in recovering petroleum distillates shows promise from an economic standpoint, its feasibility will depend on a complex interplay of factors including implementation costs, oil market conditions, regulatory environments, and technological scalability. Ongoing research and pilot studies will be essential in refining economic models and providing a clearer picture of the long-term financial viability of this innovative recovery method.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!