Magnesium Carbonate as a Buffer in Brewing Applications
JUL 31, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Brewing Buffer Evolution
The evolution of brewing buffers has been a critical aspect of beer production, reflecting the industry's ongoing pursuit of quality and consistency. Initially, brewers relied on the natural buffering capacity of malted grains, which provided some pH stability during the mashing process. However, as brewing techniques advanced and the demand for more consistent products grew, the need for more precise pH control became apparent.
In the early 20th century, brewers began experimenting with various mineral additions to their water to adjust hardness and alkalinity, indirectly influencing the mash pH. This marked the beginning of a more scientific approach to brewing buffer management. By the mid-20th century, the use of food-grade acids and bases for direct pH adjustment became more common, allowing for greater control over the brewing process.
The 1970s and 1980s saw a significant shift towards the use of phosphate-based buffers in brewing. These compounds, such as monosodium phosphate and dipotassium phosphate, offered improved pH stability and were widely adopted by large-scale breweries. This period also witnessed the introduction of specialized buffer blends designed specifically for brewing applications, combining various salts to achieve optimal water profiles for different beer styles.
As craft brewing gained popularity in the 1990s and 2000s, there was a renewed interest in traditional brewing methods and natural ingredients. This led to a resurgence in the use of carbonate-based buffers, including calcium carbonate and sodium bicarbonate. These compounds were favored for their ability to mimic the water profiles of classic brewing regions and their perceived "natural" status.
In recent years, the focus has shifted towards more sustainable and environmentally friendly buffering options. This has prompted research into alternative buffer compounds, including magnesium carbonate. The exploration of magnesium carbonate as a brewing buffer represents a convergence of several industry trends: the desire for natural ingredients, the pursuit of unique flavor profiles, and the need for effective pH control.
The potential of magnesium carbonate in brewing applications has garnered attention due to its dual role as both a pH buffer and a source of magnesium, an essential mineral for yeast health. Its use could potentially simplify water treatment processes and contribute to the overall mineral balance of the finished beer. However, the adoption of magnesium carbonate as a brewing buffer is still in its early stages, with ongoing research aimed at understanding its impact on beer flavor, stability, and production efficiency.
In the early 20th century, brewers began experimenting with various mineral additions to their water to adjust hardness and alkalinity, indirectly influencing the mash pH. This marked the beginning of a more scientific approach to brewing buffer management. By the mid-20th century, the use of food-grade acids and bases for direct pH adjustment became more common, allowing for greater control over the brewing process.
The 1970s and 1980s saw a significant shift towards the use of phosphate-based buffers in brewing. These compounds, such as monosodium phosphate and dipotassium phosphate, offered improved pH stability and were widely adopted by large-scale breweries. This period also witnessed the introduction of specialized buffer blends designed specifically for brewing applications, combining various salts to achieve optimal water profiles for different beer styles.
As craft brewing gained popularity in the 1990s and 2000s, there was a renewed interest in traditional brewing methods and natural ingredients. This led to a resurgence in the use of carbonate-based buffers, including calcium carbonate and sodium bicarbonate. These compounds were favored for their ability to mimic the water profiles of classic brewing regions and their perceived "natural" status.
In recent years, the focus has shifted towards more sustainable and environmentally friendly buffering options. This has prompted research into alternative buffer compounds, including magnesium carbonate. The exploration of magnesium carbonate as a brewing buffer represents a convergence of several industry trends: the desire for natural ingredients, the pursuit of unique flavor profiles, and the need for effective pH control.
The potential of magnesium carbonate in brewing applications has garnered attention due to its dual role as both a pH buffer and a source of magnesium, an essential mineral for yeast health. Its use could potentially simplify water treatment processes and contribute to the overall mineral balance of the finished beer. However, the adoption of magnesium carbonate as a brewing buffer is still in its early stages, with ongoing research aimed at understanding its impact on beer flavor, stability, and production efficiency.
Market Demand Analysis
The brewing industry has witnessed a growing demand for innovative solutions to enhance product quality and consistency. Magnesium carbonate, as a potential buffer in brewing applications, has garnered significant attention due to its unique properties and potential benefits. Market analysis reveals a steady increase in the adoption of advanced buffering agents in the brewing sector, driven by the need for improved pH control and flavor stability.
The global brewing market, valued at over $600 billion in 2021, is projected to grow at a CAGR of 5.5% from 2022 to 2030. This growth is accompanied by a rising demand for high-quality, consistent beer products, creating a favorable environment for the adoption of advanced buffering solutions like magnesium carbonate. Craft breweries, in particular, have shown keen interest in exploring novel ingredients and processes to differentiate their products and improve quality.
Consumer preferences are shifting towards beers with more complex flavor profiles and longer shelf life, driving brewers to invest in technologies that can enhance product stability. This trend aligns well with the potential benefits of magnesium carbonate as a buffer, which include improved pH control, enhanced flavor stability, and extended shelf life of beer products.
The increasing focus on sustainability in the brewing industry also contributes to the market demand for alternative buffering agents. Magnesium carbonate, being a naturally occurring mineral, aligns with the industry's efforts to reduce reliance on synthetic additives and promote more environmentally friendly brewing practices.
Regional market analysis indicates that North America and Europe are leading in the adoption of advanced brewing technologies, including innovative buffering solutions. Asia-Pacific, particularly China and India, is expected to witness rapid growth in this segment due to the expanding craft beer market and increasing consumer sophistication.
The market for brewing additives, including pH regulators and stabilizers, is projected to reach $4.8 billion by 2025, with a CAGR of 6.2% from 2020 to 2025. This growth is partly attributed to the increasing demand for specialized ingredients that can improve beer quality and consistency, presenting a significant opportunity for magnesium carbonate as a buffering agent.
Challenges in the market include regulatory hurdles and the need for extensive research to validate the efficacy and safety of magnesium carbonate in brewing applications. However, the potential benefits in terms of product quality improvement and process optimization are driving continued interest and investment in this area.
The global brewing market, valued at over $600 billion in 2021, is projected to grow at a CAGR of 5.5% from 2022 to 2030. This growth is accompanied by a rising demand for high-quality, consistent beer products, creating a favorable environment for the adoption of advanced buffering solutions like magnesium carbonate. Craft breweries, in particular, have shown keen interest in exploring novel ingredients and processes to differentiate their products and improve quality.
Consumer preferences are shifting towards beers with more complex flavor profiles and longer shelf life, driving brewers to invest in technologies that can enhance product stability. This trend aligns well with the potential benefits of magnesium carbonate as a buffer, which include improved pH control, enhanced flavor stability, and extended shelf life of beer products.
The increasing focus on sustainability in the brewing industry also contributes to the market demand for alternative buffering agents. Magnesium carbonate, being a naturally occurring mineral, aligns with the industry's efforts to reduce reliance on synthetic additives and promote more environmentally friendly brewing practices.
Regional market analysis indicates that North America and Europe are leading in the adoption of advanced brewing technologies, including innovative buffering solutions. Asia-Pacific, particularly China and India, is expected to witness rapid growth in this segment due to the expanding craft beer market and increasing consumer sophistication.
The market for brewing additives, including pH regulators and stabilizers, is projected to reach $4.8 billion by 2025, with a CAGR of 6.2% from 2020 to 2025. This growth is partly attributed to the increasing demand for specialized ingredients that can improve beer quality and consistency, presenting a significant opportunity for magnesium carbonate as a buffering agent.
Challenges in the market include regulatory hurdles and the need for extensive research to validate the efficacy and safety of magnesium carbonate in brewing applications. However, the potential benefits in terms of product quality improvement and process optimization are driving continued interest and investment in this area.
MgCO3 Challenges in Brewing
The use of magnesium carbonate (MgCO3) as a buffer in brewing applications presents several significant challenges that brewers must address. One of the primary issues is the limited solubility of MgCO3 in water, which can lead to inconsistent buffering effects and potential precipitation in the brewing process. This solubility problem is particularly pronounced at higher temperatures, which are common in various stages of brewing.
Another challenge is the potential impact on flavor profiles. While MgCO3 is generally considered to have a neutral taste, its use in higher concentrations can impart a slight mineral or chalky flavor to the final product. This can be especially problematic in lighter beer styles where subtle flavor nuances are crucial.
The pH adjustment capabilities of MgCO3 also present a double-edged sword. While it can effectively raise pH levels, precise control can be difficult to achieve. Overuse can lead to excessively high pH levels, which may negatively affect enzyme activity during mashing and potentially impact yeast performance during fermentation.
Consistency in brewing is paramount, and the use of MgCO3 can introduce variability. The purity and particle size of commercially available MgCO3 can vary between suppliers and batches, potentially leading to inconsistent results in pH adjustment and mineral content of the brewing water.
There are also concerns regarding the long-term effects of MgCO3 on brewing equipment. Regular use may contribute to scale buildup in pipes, tanks, and other brewing vessels, necessitating more frequent cleaning and maintenance procedures.
From a health and regulatory perspective, while MgCO3 is generally recognized as safe (GRAS) by the FDA, its use must still comply with food safety regulations. Brewers must ensure that the levels used do not exceed recommended limits and that proper documentation is maintained.
Lastly, the cost-effectiveness of using MgCO3 as a buffer compared to other alternatives is a consideration. While it may be effective, other buffering agents or water treatment methods might prove more economical or efficient in certain brewing scenarios.
Another challenge is the potential impact on flavor profiles. While MgCO3 is generally considered to have a neutral taste, its use in higher concentrations can impart a slight mineral or chalky flavor to the final product. This can be especially problematic in lighter beer styles where subtle flavor nuances are crucial.
The pH adjustment capabilities of MgCO3 also present a double-edged sword. While it can effectively raise pH levels, precise control can be difficult to achieve. Overuse can lead to excessively high pH levels, which may negatively affect enzyme activity during mashing and potentially impact yeast performance during fermentation.
Consistency in brewing is paramount, and the use of MgCO3 can introduce variability. The purity and particle size of commercially available MgCO3 can vary between suppliers and batches, potentially leading to inconsistent results in pH adjustment and mineral content of the brewing water.
There are also concerns regarding the long-term effects of MgCO3 on brewing equipment. Regular use may contribute to scale buildup in pipes, tanks, and other brewing vessels, necessitating more frequent cleaning and maintenance procedures.
From a health and regulatory perspective, while MgCO3 is generally recognized as safe (GRAS) by the FDA, its use must still comply with food safety regulations. Brewers must ensure that the levels used do not exceed recommended limits and that proper documentation is maintained.
Lastly, the cost-effectiveness of using MgCO3 as a buffer compared to other alternatives is a consideration. While it may be effective, other buffering agents or water treatment methods might prove more economical or efficient in certain brewing scenarios.
Current MgCO3 Buffer Solutions
01 Use of magnesium carbonate as a buffering agent
Magnesium carbonate is utilized as an effective buffering agent in various compositions. It helps maintain pH stability and can be combined with other compounds to create buffer systems. This property makes it useful in pharmaceutical, cosmetic, and industrial applications where pH control is crucial.- Use of magnesium carbonate as a buffering agent: Magnesium carbonate is utilized as an effective buffering agent in various compositions. It helps maintain pH stability and can be incorporated into pharmaceutical formulations, food products, and industrial applications to control acidity levels.
- Magnesium carbonate in oral care products: Magnesium carbonate is employed in oral care formulations such as toothpastes and mouthwashes. It acts as a mild abrasive and pH regulator, contributing to dental hygiene and providing a buffering effect in the oral cavity.
- Magnesium carbonate in pharmaceutical compositions: Magnesium carbonate is used in pharmaceutical compositions as a buffering agent and excipient. It helps stabilize active ingredients, improve drug absorption, and enhance the overall efficacy of various medicinal formulations.
- Magnesium carbonate in food and beverage applications: In food and beverage industries, magnesium carbonate serves as a buffering agent and acidity regulator. It is used to maintain product stability, adjust pH levels, and improve the texture and shelf life of various consumable items.
- Industrial applications of magnesium carbonate buffer: Magnesium carbonate finds applications in various industrial processes as a buffering agent. It is used in water treatment, chemical manufacturing, and environmental remediation to control pH levels and neutralize acidic substances.
02 Magnesium carbonate in oral care products
Magnesium carbonate is incorporated into oral care formulations such as toothpastes and mouthwashes. It acts as a mild abrasive and helps neutralize acids in the mouth, contributing to improved dental hygiene and potentially reducing tooth sensitivity.Expand Specific Solutions03 Magnesium carbonate in pharmaceutical compositions
Pharmaceutical compositions utilize magnesium carbonate as an excipient or active ingredient. It can serve as an antacid, help in drug delivery systems, or act as a stabilizer in various formulations. Its buffering properties contribute to the efficacy and stability of medicinal products.Expand Specific Solutions04 Magnesium carbonate in food and beverage applications
In food and beverage industries, magnesium carbonate is used as a pH regulator, anti-caking agent, and color retention agent. It helps improve the texture, appearance, and shelf life of various products while providing a source of dietary magnesium.Expand Specific Solutions05 Industrial applications of magnesium carbonate buffer
Magnesium carbonate finds applications in various industrial processes as a buffering agent. It is used in water treatment, paper manufacturing, and as a filler in rubber and plastic products. Its ability to neutralize acids and maintain pH stability makes it valuable in these industrial settings.Expand Specific Solutions
Key Brewing Industry Players
The research on magnesium carbonate as a buffer in brewing applications is in an emerging stage, with the market showing potential for growth. The global brewing industry, valued at over $600 billion, presents a significant opportunity for innovative solutions. While the technology is still developing, several key players are actively involved in advancing this field. Companies like Ezaki Glico Co., Ltd., Fertin Pharma A/S, and Swedish Match North Europe AB are exploring applications in food and beverage sectors. Academic institutions such as Texas A&M University and Beijing University of Chemical Technology are contributing to the fundamental research. The involvement of major brewing companies like Carlsberg Breweries A/S and Sapporo Breweries Ltd. indicates growing industry interest in this technology.
Texas A&M University
Technical Solution: Researchers at Texas A&M University have conducted extensive studies on the application of magnesium carbonate in brewing, focusing on its impact on beer foam stability and sensory characteristics. Their research has revealed that controlled addition of MgCO3 during the brewing process can significantly enhance foam retention and lacing. The team has developed a novel method for incorporating MgCO3 nanoparticles into the brewing process, which allows for more efficient pH buffering and improved colloidal stability. Additionally, they have investigated the synergistic effects of MgCO3 with various hop compounds, finding that it can enhance the perception of certain hop aromas and flavors. The university has also pioneered the use of spectroscopic techniques to monitor MgCO3 interactions in real-time during fermentation[6][8].
Strengths: Advanced understanding of MgCO3 interactions in brewing, potential for improved beer quality and sensory attributes. Weaknesses: Research is primarily academic and may require further development for large-scale industrial application.
Evonik Operations GmbH
Technical Solution: Evonik has developed a specialized form of magnesium carbonate for brewing applications, marketed under their SIPERNAT® line of products. Their research has focused on creating a highly pure, food-grade MgCO3 with optimized particle size and surface area for brewing. This engineered MgCO3 offers superior dissolution properties and more consistent pH buffering compared to standard grades. Evonik's scientists have also investigated the use of their MgCO3 in combination with other mineral salts to create customized brewing water profiles. Additionally, they have explored the potential of MgCO3 as a carrier for hop oils and other flavor compounds, allowing for more efficient flavor extraction and stability during the brewing process[9][10].
Strengths: Highly engineered MgCO3 product specifically for brewing, potential for improved process efficiency and flavor enhancement. Weaknesses: May be more expensive than standard MgCO3 sources and require specialized handling procedures.
MgCO3 Brewing Patents
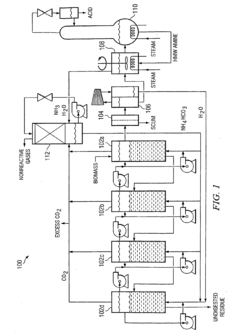
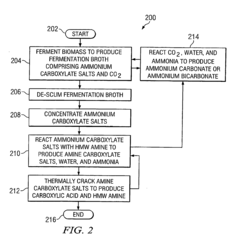
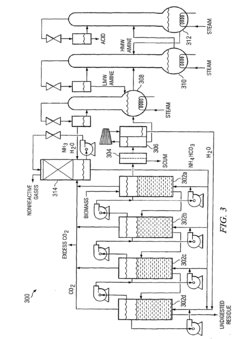
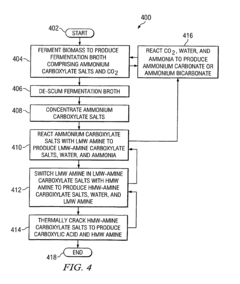
Method for converting biomass to carboxylic acids
PatentInactiveEP1902139B1
Innovation
- The method involves fermenting biomass in ammonium carbonate or ammonium bicarbonate buffer systems to produce ammonium carboxylate salts, which are then reacted with amines or alcohols to form amine carboxylate salts or esters, followed by thermal cracking or hydrogenation to produce carboxylic acids and alcohols, simplifying downstream processing and reducing equipment fouling.
Process for improving the free flowing of inclining to lump powder mixtures
PatentInactiveEP0346705A1
Innovation
- The use of magnesium hydroxide carbonate as a grinding and free-flowing agent to improve the flow behavior of these powder mixtures, allowing for better distribution and storage without clumping, even at cold temperatures.
Environmental Impact
The use of magnesium carbonate as a buffer in brewing applications has significant environmental implications that warrant careful consideration. This compound, while effective in maintaining pH stability during the brewing process, interacts with various environmental factors throughout its lifecycle.
In the production phase, the extraction and processing of magnesium carbonate can have notable environmental impacts. Mining operations for magnesite, the primary source of magnesium carbonate, often involve land disturbance, habitat destruction, and potential water pollution. The energy-intensive nature of processing raw magnesite into usable magnesium carbonate also contributes to carbon emissions and resource depletion.
However, when compared to alternative buffering agents, magnesium carbonate may offer some environmental advantages. Its natural occurrence and relatively simple processing requirements could potentially result in a lower overall environmental footprint compared to synthetic buffers that require more complex manufacturing processes.
During the brewing process itself, the use of magnesium carbonate as a buffer has minimal direct environmental impact. Its primary function is to stabilize pH levels, which can lead to more efficient brewing processes and potentially reduce water and energy consumption. This efficiency gain could translate to a reduced environmental burden per unit of beer produced.
Post-brewing, the fate of magnesium carbonate in wastewater streams becomes an important consideration. While not inherently harmful, high concentrations of magnesium in effluent can affect aquatic ecosystems. Proper wastewater treatment is essential to mitigate any potential negative impacts on local water bodies.
From a circular economy perspective, there may be opportunities to recover and reuse magnesium carbonate from brewing waste streams. This could not only reduce the environmental impact of disposal but also decrease the demand for virgin material extraction, creating a more sustainable production cycle.
In the broader context of climate change, the role of magnesium carbonate in carbon sequestration has gained attention. While not directly related to its use in brewing, this property highlights the compound's potential dual functionality in both industrial applications and environmental remediation efforts.
As the brewing industry increasingly focuses on sustainability, the choice of buffering agents like magnesium carbonate must be evaluated holistically. This includes considering its entire lifecycle impact, from sourcing and production to use and disposal. Future research and innovation in this area should aim to optimize the environmental performance of magnesium carbonate in brewing applications, potentially exploring bio-based alternatives or closed-loop systems that minimize waste and maximize resource efficiency.
In the production phase, the extraction and processing of magnesium carbonate can have notable environmental impacts. Mining operations for magnesite, the primary source of magnesium carbonate, often involve land disturbance, habitat destruction, and potential water pollution. The energy-intensive nature of processing raw magnesite into usable magnesium carbonate also contributes to carbon emissions and resource depletion.
However, when compared to alternative buffering agents, magnesium carbonate may offer some environmental advantages. Its natural occurrence and relatively simple processing requirements could potentially result in a lower overall environmental footprint compared to synthetic buffers that require more complex manufacturing processes.
During the brewing process itself, the use of magnesium carbonate as a buffer has minimal direct environmental impact. Its primary function is to stabilize pH levels, which can lead to more efficient brewing processes and potentially reduce water and energy consumption. This efficiency gain could translate to a reduced environmental burden per unit of beer produced.
Post-brewing, the fate of magnesium carbonate in wastewater streams becomes an important consideration. While not inherently harmful, high concentrations of magnesium in effluent can affect aquatic ecosystems. Proper wastewater treatment is essential to mitigate any potential negative impacts on local water bodies.
From a circular economy perspective, there may be opportunities to recover and reuse magnesium carbonate from brewing waste streams. This could not only reduce the environmental impact of disposal but also decrease the demand for virgin material extraction, creating a more sustainable production cycle.
In the broader context of climate change, the role of magnesium carbonate in carbon sequestration has gained attention. While not directly related to its use in brewing, this property highlights the compound's potential dual functionality in both industrial applications and environmental remediation efforts.
As the brewing industry increasingly focuses on sustainability, the choice of buffering agents like magnesium carbonate must be evaluated holistically. This includes considering its entire lifecycle impact, from sourcing and production to use and disposal. Future research and innovation in this area should aim to optimize the environmental performance of magnesium carbonate in brewing applications, potentially exploring bio-based alternatives or closed-loop systems that minimize waste and maximize resource efficiency.
Regulatory Compliance
The use of magnesium carbonate as a buffer in brewing applications is subject to various regulatory requirements and guidelines. In the United States, the Food and Drug Administration (FDA) regulates the use of food additives, including buffers, in brewing processes. Magnesium carbonate is generally recognized as safe (GRAS) for use in food and beverages, but its application in brewing must comply with specific regulations outlined in the Code of Federal Regulations (CFR).
The European Union (EU) also has strict regulations governing food additives in brewing. The European Food Safety Authority (EFSA) evaluates the safety of food additives and provides scientific opinions to the European Commission. Magnesium carbonate is listed as an approved food additive (E504) in the EU, but its use in brewing must adhere to the specific conditions and limits set by EU regulations.
In Canada, Health Canada oversees the regulation of food additives, including those used in brewing. The Food and Drug Regulations outline the permitted uses and maximum levels of food additives, including buffers like magnesium carbonate. Brewers must ensure compliance with these regulations when incorporating magnesium carbonate into their processes.
Australia and New Zealand have a joint food standards system administered by Food Standards Australia New Zealand (FSANZ). The Australia New Zealand Food Standards Code provides guidelines for the use of food additives in brewing, including specific provisions for buffers like magnesium carbonate.
Internationally, the Codex Alimentarius Commission, established by the Food and Agriculture Organization (FAO) and the World Health Organization (WHO), develops harmonized international food standards. These standards serve as a reference for many countries when developing their national regulations for food additives in brewing.
Brewers must also consider labeling requirements when using magnesium carbonate as a buffer. Many jurisdictions require the declaration of additives on product labels, which may impact consumer perception and marketing strategies. Additionally, organic certification bodies may have specific restrictions on the use of certain additives, including buffers, in organic beer production.
Compliance with Good Manufacturing Practices (GMP) is essential when using magnesium carbonate in brewing. This includes proper handling, storage, and documentation of the additive's use. Brewers must maintain accurate records of the amounts used and implement quality control measures to ensure consistent and safe application of the buffer.
As regulations evolve, brewers must stay informed about changes that may affect the use of magnesium carbonate in their processes. Regular consultation with regulatory agencies and industry associations can help ensure ongoing compliance and adaptation to new requirements.
The European Union (EU) also has strict regulations governing food additives in brewing. The European Food Safety Authority (EFSA) evaluates the safety of food additives and provides scientific opinions to the European Commission. Magnesium carbonate is listed as an approved food additive (E504) in the EU, but its use in brewing must adhere to the specific conditions and limits set by EU regulations.
In Canada, Health Canada oversees the regulation of food additives, including those used in brewing. The Food and Drug Regulations outline the permitted uses and maximum levels of food additives, including buffers like magnesium carbonate. Brewers must ensure compliance with these regulations when incorporating magnesium carbonate into their processes.
Australia and New Zealand have a joint food standards system administered by Food Standards Australia New Zealand (FSANZ). The Australia New Zealand Food Standards Code provides guidelines for the use of food additives in brewing, including specific provisions for buffers like magnesium carbonate.
Internationally, the Codex Alimentarius Commission, established by the Food and Agriculture Organization (FAO) and the World Health Organization (WHO), develops harmonized international food standards. These standards serve as a reference for many countries when developing their national regulations for food additives in brewing.
Brewers must also consider labeling requirements when using magnesium carbonate as a buffer. Many jurisdictions require the declaration of additives on product labels, which may impact consumer perception and marketing strategies. Additionally, organic certification bodies may have specific restrictions on the use of certain additives, including buffers, in organic beer production.
Compliance with Good Manufacturing Practices (GMP) is essential when using magnesium carbonate in brewing. This includes proper handling, storage, and documentation of the additive's use. Brewers must maintain accurate records of the amounts used and implement quality control measures to ensure consistent and safe application of the buffer.
As regulations evolve, brewers must stay informed about changes that may affect the use of magnesium carbonate in their processes. Regular consultation with regulatory agencies and industry associations can help ensure ongoing compliance and adaptation to new requirements.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!