Automation And Robotics In Labs For DNA Data Storage
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
DNA Data Storage Evolution and Objectives
DNA data storage has evolved significantly since its conceptual introduction in the 1960s, transitioning from theoretical possibility to practical implementation. The foundational breakthrough came in 1988 when scientists first demonstrated the ability to encode and retrieve digital information using DNA molecules. This milestone established DNA's potential as a storage medium, leveraging its inherent data density capabilities that far exceed conventional electronic storage systems.
The evolution accelerated in the early 2000s with advancements in DNA synthesis and sequencing technologies, particularly through next-generation sequencing methods that dramatically reduced costs and increased throughput. By 2012, researchers at Harvard University successfully stored a 5.27-megabit book in DNA, demonstrating practical feasibility at a meaningful scale. This achievement was followed by Microsoft and University of Washington's 2016 milestone of storing and retrieving 200 megabytes of data in DNA with 100% accuracy.
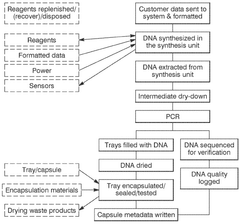
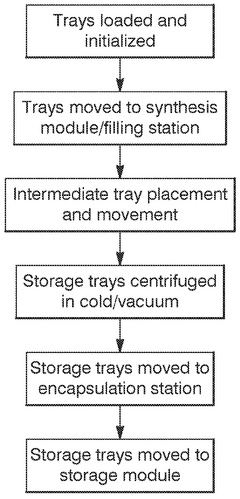
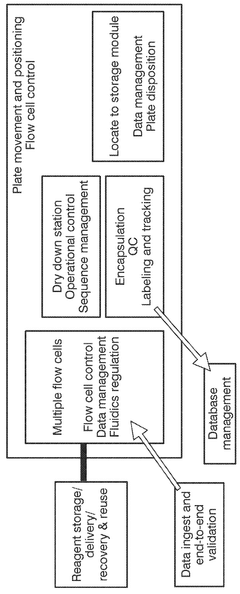
Recent developments have focused on addressing key challenges in the DNA data storage workflow, particularly through automation and robotics integration. These technologies aim to overcome manual handling limitations that restrict scalability and introduce errors. The evolution now centers on creating end-to-end automated systems that can encode digital information, synthesize corresponding DNA sequences, store them reliably, and retrieve and decode the information when needed.
The primary objective of automation and robotics in DNA data storage is to establish a commercially viable, high-throughput system capable of competing with traditional storage technologies. This includes developing robotic platforms for precise liquid handling, automated DNA synthesis reactors, and integrated storage and retrieval systems that maintain DNA integrity while minimizing human intervention.
Additional objectives include error reduction through standardized protocols and quality control mechanisms implemented via automated systems. These systems aim to achieve write speeds exceeding 1 MB per second and read speeds of multiple GB per second, targets necessary for practical application in data centers and archival storage facilities.
Long-term objectives extend to creating modular, scalable automated systems that can adapt to evolving DNA synthesis and sequencing technologies while maintaining backward compatibility with previously stored data. The ultimate goal is establishing DNA as a mainstream storage medium for archival purposes, offering unparalleled data density (potentially storing all current digital information in a space the size of a standard shipping container) with exceptional longevity (thousands of years under proper storage conditions).
The evolution accelerated in the early 2000s with advancements in DNA synthesis and sequencing technologies, particularly through next-generation sequencing methods that dramatically reduced costs and increased throughput. By 2012, researchers at Harvard University successfully stored a 5.27-megabit book in DNA, demonstrating practical feasibility at a meaningful scale. This achievement was followed by Microsoft and University of Washington's 2016 milestone of storing and retrieving 200 megabytes of data in DNA with 100% accuracy.
Recent developments have focused on addressing key challenges in the DNA data storage workflow, particularly through automation and robotics integration. These technologies aim to overcome manual handling limitations that restrict scalability and introduce errors. The evolution now centers on creating end-to-end automated systems that can encode digital information, synthesize corresponding DNA sequences, store them reliably, and retrieve and decode the information when needed.
The primary objective of automation and robotics in DNA data storage is to establish a commercially viable, high-throughput system capable of competing with traditional storage technologies. This includes developing robotic platforms for precise liquid handling, automated DNA synthesis reactors, and integrated storage and retrieval systems that maintain DNA integrity while minimizing human intervention.
Additional objectives include error reduction through standardized protocols and quality control mechanisms implemented via automated systems. These systems aim to achieve write speeds exceeding 1 MB per second and read speeds of multiple GB per second, targets necessary for practical application in data centers and archival storage facilities.
Long-term objectives extend to creating modular, scalable automated systems that can adapt to evolving DNA synthesis and sequencing technologies while maintaining backward compatibility with previously stored data. The ultimate goal is establishing DNA as a mainstream storage medium for archival purposes, offering unparalleled data density (potentially storing all current digital information in a space the size of a standard shipping container) with exceptional longevity (thousands of years under proper storage conditions).
Market Analysis for Lab Automation in DNA Storage
The DNA data storage market is experiencing significant growth, driven by the exponential increase in global data production and the limitations of conventional storage technologies. Current market estimates value the global DNA data storage market at approximately $105 million in 2023, with projections indicating a compound annual growth rate (CAGR) of 58.4% through 2030, potentially reaching $3.3 billion. This remarkable growth trajectory reflects the urgent need for innovative storage solutions that can accommodate the world's expanding digital footprint.
Laboratory automation represents a critical enabler for DNA data storage commercialization. The current lab automation market specifically relevant to DNA synthesis and sequencing technologies is valued at around $4.7 billion globally, with DNA storage applications constituting an emerging but rapidly growing segment. Industry analysts predict that automation solutions specifically designed for DNA data storage workflows could capture 15-20% of the broader lab automation market within the next decade.
Key market drivers include the decreasing cost of DNA synthesis and sequencing, which has fallen by approximately 100-fold over the past decade. However, current synthesis costs remain at about $0.001 per nucleotide, which translates to roughly $1 million per terabyte of stored data - still prohibitively expensive for mainstream adoption. Market forecasts suggest that automation could help reduce these costs to $0.0001 per nucleotide by 2025, making DNA storage economically viable for archival applications.
Demand segmentation reveals three primary market categories: government archives (including national security applications), scientific research institutions, and enterprise data centers. The enterprise segment is projected to grow at the highest rate (63% CAGR) as costs decrease, while government applications currently represent the largest market share at approximately 42% due to long-term storage requirements and higher tolerance for premium pricing.
Geographic distribution of market demand shows North America leading with 45% market share, followed by Europe (28%) and Asia-Pacific (22%). China, in particular, has increased investments in DNA storage technologies by over 200% since 2018, indicating an emerging competitive landscape in the global market.
Customer adoption barriers include not only cost concerns but also throughput limitations and reliability issues. Current automated systems can process approximately 1GB of data per day into DNA storage format, whereas commercial viability requires at least 1TB per day. Market research indicates that 78% of potential enterprise customers cite throughput as their primary concern, followed by long-term stability (65%) and retrieval accuracy (61%).
Laboratory automation represents a critical enabler for DNA data storage commercialization. The current lab automation market specifically relevant to DNA synthesis and sequencing technologies is valued at around $4.7 billion globally, with DNA storage applications constituting an emerging but rapidly growing segment. Industry analysts predict that automation solutions specifically designed for DNA data storage workflows could capture 15-20% of the broader lab automation market within the next decade.
Key market drivers include the decreasing cost of DNA synthesis and sequencing, which has fallen by approximately 100-fold over the past decade. However, current synthesis costs remain at about $0.001 per nucleotide, which translates to roughly $1 million per terabyte of stored data - still prohibitively expensive for mainstream adoption. Market forecasts suggest that automation could help reduce these costs to $0.0001 per nucleotide by 2025, making DNA storage economically viable for archival applications.
Demand segmentation reveals three primary market categories: government archives (including national security applications), scientific research institutions, and enterprise data centers. The enterprise segment is projected to grow at the highest rate (63% CAGR) as costs decrease, while government applications currently represent the largest market share at approximately 42% due to long-term storage requirements and higher tolerance for premium pricing.
Geographic distribution of market demand shows North America leading with 45% market share, followed by Europe (28%) and Asia-Pacific (22%). China, in particular, has increased investments in DNA storage technologies by over 200% since 2018, indicating an emerging competitive landscape in the global market.
Customer adoption barriers include not only cost concerns but also throughput limitations and reliability issues. Current automated systems can process approximately 1GB of data per day into DNA storage format, whereas commercial viability requires at least 1TB per day. Market research indicates that 78% of potential enterprise customers cite throughput as their primary concern, followed by long-term stability (65%) and retrieval accuracy (61%).
Current Challenges in DNA Data Storage Automation
Despite significant advancements in DNA data storage technology, the field faces substantial challenges in automation and robotics integration. Current DNA synthesis methods remain largely manual, labor-intensive processes that limit scalability. The synthesis speed typically achieves only 10-20 nucleotides per second, making the creation of large-scale DNA archives prohibitively time-consuming and expensive, with costs hovering around $3,500 per megabyte of stored data.
Laboratory workflows for DNA data storage suffer from fragmentation, with different stages requiring separate equipment and manual transfers between processes. This fragmentation introduces error risks, increases contamination possibilities, and creates workflow bottlenecks that impede throughput. The lack of standardized interfaces between different instruments further complicates integration efforts.
Miniaturization presents another significant challenge. Current DNA synthesis and sequencing equipment occupies substantial laboratory space, making compact storage solutions difficult to achieve. While microfluidic approaches show promise, they face reliability issues when scaled to production levels, particularly in maintaining consistent reaction conditions across thousands of parallel synthesis sites.
Error rates in DNA synthesis remain problematic for data storage applications. Current technologies produce error rates of approximately 1 in 100 to 1 in 200 bases, necessitating extensive error correction mechanisms that reduce effective storage density. Automated quality control systems capable of detecting and correcting these errors in real-time remain underdeveloped.
The retrieval process presents unique automation challenges. Current methods for accessing specific data within DNA archives often require PCR amplification of the entire sample, which is inefficient for random access requirements. Robotic systems for precise sample handling at nanoliter scales are still in early development stages, limiting practical implementation of selective data retrieval.
Environmental control requirements add complexity to automation systems. DNA synthesis and storage demand precise temperature, humidity, and contamination controls that must be maintained across integrated robotic platforms. Current systems struggle to maintain these conditions while allowing for the mechanical movements necessary for automation.
Cross-disciplinary expertise shortages further hinder progress. Effective automation requires specialists who understand both molecular biology and advanced robotics—a rare combination. This expertise gap slows development of truly integrated systems that can bridge the conceptual divide between information technology and biotechnology paradigms.
Laboratory workflows for DNA data storage suffer from fragmentation, with different stages requiring separate equipment and manual transfers between processes. This fragmentation introduces error risks, increases contamination possibilities, and creates workflow bottlenecks that impede throughput. The lack of standardized interfaces between different instruments further complicates integration efforts.
Miniaturization presents another significant challenge. Current DNA synthesis and sequencing equipment occupies substantial laboratory space, making compact storage solutions difficult to achieve. While microfluidic approaches show promise, they face reliability issues when scaled to production levels, particularly in maintaining consistent reaction conditions across thousands of parallel synthesis sites.
Error rates in DNA synthesis remain problematic for data storage applications. Current technologies produce error rates of approximately 1 in 100 to 1 in 200 bases, necessitating extensive error correction mechanisms that reduce effective storage density. Automated quality control systems capable of detecting and correcting these errors in real-time remain underdeveloped.
The retrieval process presents unique automation challenges. Current methods for accessing specific data within DNA archives often require PCR amplification of the entire sample, which is inefficient for random access requirements. Robotic systems for precise sample handling at nanoliter scales are still in early development stages, limiting practical implementation of selective data retrieval.
Environmental control requirements add complexity to automation systems. DNA synthesis and storage demand precise temperature, humidity, and contamination controls that must be maintained across integrated robotic platforms. Current systems struggle to maintain these conditions while allowing for the mechanical movements necessary for automation.
Cross-disciplinary expertise shortages further hinder progress. Effective automation requires specialists who understand both molecular biology and advanced robotics—a rare combination. This expertise gap slows development of truly integrated systems that can bridge the conceptual divide between information technology and biotechnology paradigms.
Existing Automation Solutions for DNA Data Storage
01 Industrial Automation Systems
Industrial automation systems integrate robotics, sensors, and control systems to streamline manufacturing processes. These systems enhance production efficiency, reduce human error, and enable consistent quality control in factory environments. Advanced automation solutions incorporate machine learning algorithms for predictive maintenance and adaptive manufacturing capabilities, allowing for flexible production lines that can quickly adjust to changing requirements.- Industrial Automation Systems: Industrial automation systems integrate robotics and automated machinery to streamline manufacturing processes. These systems include automated assembly lines, material handling equipment, and quality control mechanisms that improve production efficiency and reduce human intervention. Advanced sensors and control systems enable precise operations, while integration with digital platforms allows for real-time monitoring and adjustment of manufacturing parameters.
- Robotic Vision and Sensing Technologies: Vision systems and advanced sensing technologies enable robots to perceive and interact with their environment. These technologies include cameras, LiDAR, infrared sensors, and machine vision algorithms that allow robots to identify objects, navigate spaces, and perform complex tasks. The integration of multiple sensing modalities enhances robotic perception capabilities, enabling applications in various fields from manufacturing to healthcare.
- Autonomous Mobile Robots: Autonomous mobile robots are designed to navigate and operate independently in various environments without continuous human guidance. These robots utilize advanced navigation algorithms, obstacle detection systems, and path planning capabilities to move safely and efficiently. Applications include warehouse logistics, delivery services, security patrols, and exploration of hazardous environments, where they can perform tasks that would be dangerous or impractical for humans.
- Human-Robot Collaboration Systems: Collaborative robots (cobots) are designed to work alongside humans in shared workspaces. These systems incorporate safety features such as force sensing, speed monitoring, and collision detection to ensure safe operation near human workers. Human-robot collaboration enhances productivity by combining human cognitive abilities with robotic precision and strength, enabling applications in assembly, packaging, and quality inspection tasks that benefit from both human judgment and robotic consistency.
- AI and Machine Learning in Robotics: Artificial intelligence and machine learning technologies are increasingly integrated into robotic systems to enhance their adaptability and decision-making capabilities. These technologies enable robots to learn from experience, recognize patterns, and make autonomous decisions based on complex data inputs. Applications include predictive maintenance, adaptive control systems, and robots that can learn new tasks through demonstration or reinforcement learning, significantly reducing programming requirements and increasing flexibility.
02 Robotic Vision and Sensing Technologies
Vision systems and advanced sensors enable robots to perceive and interact with their environment. These technologies include cameras, LiDAR, infrared sensors, and tactile feedback mechanisms that provide robots with spatial awareness and object recognition capabilities. Machine vision algorithms process visual data to identify objects, detect defects, and guide robotic movements with precision, making them suitable for complex tasks requiring environmental adaptation.Expand Specific Solutions03 Autonomous Mobile Robots
Autonomous mobile robots can navigate environments without human intervention using sophisticated navigation systems. These robots employ simultaneous localization and mapping (SLAM) algorithms, path planning, and obstacle avoidance technologies to move efficiently through dynamic spaces. Applications include warehouse logistics, delivery services, security patrols, and agricultural operations where independent movement and decision-making capabilities are essential.Expand Specific Solutions04 Collaborative Robotics Systems
Collaborative robots (cobots) are designed to work safely alongside humans in shared workspaces. These systems feature force-limiting technology, rounded edges, and advanced sensors to detect human presence and prevent accidents. Cobots can be easily programmed through intuitive interfaces or demonstration learning, making them accessible for small and medium enterprises. They excel in tasks requiring both human judgment and robotic precision.Expand Specific Solutions05 Automation in Specialized Industries
Specialized automation solutions address unique challenges in industries such as healthcare, agriculture, construction, and logistics. These applications include surgical robots, automated farming equipment, construction robotics, and warehouse automation systems. Industry-specific robots incorporate specialized end effectors, sensors, and programming to perform tasks like precision surgery, crop harvesting, building construction, or package sorting with high efficiency and accuracy.Expand Specific Solutions
Leading Companies in DNA Storage and Lab Robotics
The DNA data storage market is in an early growth phase, characterized by significant R&D investments but limited commercial deployment. The global market size is projected to expand rapidly as storage demands increase exponentially, with estimates suggesting a multi-billion dollar opportunity by 2030. Technologically, automation and robotics integration is advancing unevenly across key players. Microsoft Technology Licensing and Catalog Technologies are pioneering automated DNA synthesis and storage workflows, while established life sciences companies like Tecan Trading and BD Kiestra are leveraging their laboratory automation expertise. Research institutions including Tsinghua University and Helmholtz-Zentrum are developing foundational technologies, while BGI Research and WuXi AppTec are building integrated platforms combining DNA synthesis, storage, and retrieval capabilities with robotic systems.
Tecan Trading AG
Technical Solution: Tecan Trading AG has developed a comprehensive laboratory automation solution specifically tailored for DNA data storage applications. Their platform centers around the Fluent® and Freedom EVO® liquid handling workstations, which have been adapted with specialized modules for DNA synthesis, amplification, and preparation for sequencing. Tecan's system employs a distributed automation architecture where multiple specialized instruments are integrated through robotic sample transport systems and centralized scheduling software. Their platform features advanced liquid handling capabilities with precision down to nanoliter volumes, critical for minimizing reagent costs in DNA synthesis operations. Tecan's automation extends to quality control processes, incorporating real-time monitoring of synthesis efficiency and sequence accuracy through integrated analytical instruments. The system utilizes a flexible scheduling algorithm that optimizes workflow based on current priorities and resource availability, allowing for dynamic adjustment of operations[5]. Their platform achieves throughput of approximately 10,000 unique DNA sequences per day while maintaining error rates below industry standards through iterative quality control processes.
Strengths: Extensive experience in laboratory automation across multiple life science applications; modular design allows customization for specific workflows; established global support infrastructure. Weaknesses: Not primarily focused on DNA data storage as core business; relies on third-party technologies for key components of the workflow; less vertical integration compared to dedicated DNA storage companies.
Microsoft Technology Licensing LLC
Technical Solution: Microsoft has developed an end-to-end automated system for DNA data storage that integrates molecular biology with semiconductor technology. Their platform employs a microfluidic device-based approach that automates DNA synthesis, storage, and retrieval processes. The system utilizes custom-designed robotic handlers to manipulate DNA libraries stored in specialized containers with minimal human intervention. Microsoft's technology incorporates machine learning algorithms to optimize encoding schemes that maximize data density while ensuring error correction capabilities. Their automated DNA storage system can achieve writing speeds of approximately 1MB per second with retrieval rates approaching real-time access for smaller datasets[1]. The platform integrates with conventional digital storage systems through specialized middleware that transparently handles the conversion between digital and molecular formats, allowing seamless integration with existing data infrastructure.
Strengths: Industry-leading integration of semiconductor technology with molecular biology; robust error correction algorithms; significant financial resources for R&D. Weaknesses: Still requires specialized laboratory conditions; higher cost per byte compared to conventional storage; limited commercial deployment experience outside research environments.
Key Patents in DNA Storage Robotics
Apparatus for constructing immobilized DNA library, gene amplification apparatus, apparatus for analyzing gene amplification product, gene diagnosis apparatus and method for controlling these apparatuses
PatentWO1999063072A1
Innovation
- An automated system comprising a reactor with a chemically modified substrate, heating/cooling means, and a control unit for temperature management, along with a sample solution delivery system, which enables simultaneous and automated preparation of an immobilized DNA library, gene amplification, and analysis of gene amplification products, including continuous PCR and data analysis.
Automated data storage system
PatentWO2024206562A2
Innovation
- A modular, automated data storage system using nucleic acids that integrates a computing system, modular rack-mount synthesis units, and hermetic sealing methods to encode and store digital information in polynucleotides, enhancing storage density and longevity while reducing complexity and cost.
Scalability and Cost Efficiency Considerations
The scalability of DNA data storage systems represents a critical challenge for widespread adoption. Current laboratory processes for DNA synthesis and sequencing remain highly manual, requiring significant human intervention and specialized expertise. Implementing automation and robotics can dramatically improve throughput capabilities, potentially scaling from gigabytes to petabytes of data storage capacity. Liquid handling robots, when integrated with DNA synthesis platforms, can operate continuously with minimal human supervision, significantly reducing labor costs which currently account for approximately 30-40% of operational expenses in DNA data storage labs.
Cost efficiency analysis reveals that DNA synthesis currently costs approximately $0.001 per nucleotide, translating to roughly $1 million per gigabyte of stored data. This represents a major barrier to commercial viability. Robotic automation offers pathways to reduce these costs through economies of scale, minimized reagent waste, and optimized reaction conditions. Industry projections suggest that fully automated systems could potentially reduce synthesis costs by 60-70% over the next five years, bringing costs closer to the $10,000 per gigabyte threshold where certain archival applications become economically feasible.
Energy consumption presents another critical consideration for scalability. Traditional data centers consume approximately 200-400 watts per terabyte of storage. Preliminary studies indicate that automated DNA storage systems could potentially operate at 5-10 watts per terabyte equivalent, representing a 40-fold improvement in energy efficiency. However, these calculations must account for the substantial energy requirements of synthesis and sequencing equipment, which currently offset much of this theoretical advantage.
Physical space utilization demonstrates one of DNA storage's most compelling advantages. Robotic systems for DNA storage can be designed with exceptional spatial efficiency, potentially storing exabytes of data in laboratory spaces measuring less than 500 square feet. This represents a storage density improvement of approximately 1,000,000× compared to conventional magnetic storage. Automated sample management systems further enhance this advantage by optimizing storage conditions and retrieval processes.
Infrastructure requirements for scaled DNA data storage operations present significant challenges. Automated DNA synthesis and sequencing platforms require specialized laboratory environments with controlled temperature, humidity, and air quality. The capital expenditure for establishing such facilities ranges from $2-5 million for mid-scale operations, excluding the robotics systems themselves. However, once established, these automated facilities can operate with minimal staffing, potentially reducing operational expenses by 50-60% compared to conventional data centers over a 10-year operational period.
Cost efficiency analysis reveals that DNA synthesis currently costs approximately $0.001 per nucleotide, translating to roughly $1 million per gigabyte of stored data. This represents a major barrier to commercial viability. Robotic automation offers pathways to reduce these costs through economies of scale, minimized reagent waste, and optimized reaction conditions. Industry projections suggest that fully automated systems could potentially reduce synthesis costs by 60-70% over the next five years, bringing costs closer to the $10,000 per gigabyte threshold where certain archival applications become economically feasible.
Energy consumption presents another critical consideration for scalability. Traditional data centers consume approximately 200-400 watts per terabyte of storage. Preliminary studies indicate that automated DNA storage systems could potentially operate at 5-10 watts per terabyte equivalent, representing a 40-fold improvement in energy efficiency. However, these calculations must account for the substantial energy requirements of synthesis and sequencing equipment, which currently offset much of this theoretical advantage.
Physical space utilization demonstrates one of DNA storage's most compelling advantages. Robotic systems for DNA storage can be designed with exceptional spatial efficiency, potentially storing exabytes of data in laboratory spaces measuring less than 500 square feet. This represents a storage density improvement of approximately 1,000,000× compared to conventional magnetic storage. Automated sample management systems further enhance this advantage by optimizing storage conditions and retrieval processes.
Infrastructure requirements for scaled DNA data storage operations present significant challenges. Automated DNA synthesis and sequencing platforms require specialized laboratory environments with controlled temperature, humidity, and air quality. The capital expenditure for establishing such facilities ranges from $2-5 million for mid-scale operations, excluding the robotics systems themselves. However, once established, these automated facilities can operate with minimal staffing, potentially reducing operational expenses by 50-60% compared to conventional data centers over a 10-year operational period.
Biosafety and Regulatory Framework
The integration of automation and robotics in DNA data storage laboratories necessitates comprehensive biosafety protocols and regulatory compliance frameworks. Current regulatory landscapes across major jurisdictions exhibit significant variations in their approach to automated DNA synthesis and storage technologies. In the United States, oversight is primarily managed through the NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules, with additional layers of regulation from the FDA for clinical applications and the EPA for environmental considerations. The European Union employs a more precautionary approach through its Directive 2009/41/EC on contained use of genetically modified microorganisms, requiring rigorous risk assessments before implementation.
Biosafety considerations for automated DNA data storage systems must address multiple dimensions of risk. Physical containment strategies, including negative pressure environments and HEPA filtration systems, form the foundation of laboratory safety infrastructure. These measures are particularly critical when robotics systems handle potentially hazardous biological materials or synthesize DNA sequences with unknown functional properties. Automated monitoring systems that continuously evaluate air quality, surface contamination, and equipment integrity provide an additional layer of protection against accidental release events.
Cyberbiosecurity has emerged as a novel regulatory concern specific to automated DNA data storage systems. The convergence of digital information systems with biological processing creates unique vulnerabilities where digital security breaches could potentially translate into biosafety risks. Regulatory frameworks are evolving to require encryption standards for DNA sequence data, access controls for synthesis equipment, and audit trails for all DNA synthesis activities.
International harmonization efforts are underway through organizations like the International Gene Synthesis Consortium (IGSC) and the WHO, which are developing standardized screening protocols for DNA synthesis orders and establishing best practices for laboratory automation. These initiatives aim to prevent the synthesis of regulated pathogen sequences while facilitating legitimate research and commercial applications of DNA data storage technologies.
Risk assessment methodologies for automated DNA storage facilities typically employ a tiered approach based on the nature of sequences being processed. Sequences with homology to known pathogens or toxins trigger enhanced containment requirements and additional oversight. The automation itself introduces novel risk factors, including the potential for higher-throughput processing of potentially hazardous sequences and reduced human supervision of critical biosafety checkpoints.
Regulatory compliance strategies for organizations implementing automated DNA data storage systems must include comprehensive staff training programs, regular third-party audits, and proactive engagement with regulatory authorities. As the technology continues to evolve, adaptive regulatory frameworks that balance innovation with appropriate safeguards will be essential to realizing the full potential of DNA data storage while maintaining public trust and safety.
Biosafety considerations for automated DNA data storage systems must address multiple dimensions of risk. Physical containment strategies, including negative pressure environments and HEPA filtration systems, form the foundation of laboratory safety infrastructure. These measures are particularly critical when robotics systems handle potentially hazardous biological materials or synthesize DNA sequences with unknown functional properties. Automated monitoring systems that continuously evaluate air quality, surface contamination, and equipment integrity provide an additional layer of protection against accidental release events.
Cyberbiosecurity has emerged as a novel regulatory concern specific to automated DNA data storage systems. The convergence of digital information systems with biological processing creates unique vulnerabilities where digital security breaches could potentially translate into biosafety risks. Regulatory frameworks are evolving to require encryption standards for DNA sequence data, access controls for synthesis equipment, and audit trails for all DNA synthesis activities.
International harmonization efforts are underway through organizations like the International Gene Synthesis Consortium (IGSC) and the WHO, which are developing standardized screening protocols for DNA synthesis orders and establishing best practices for laboratory automation. These initiatives aim to prevent the synthesis of regulated pathogen sequences while facilitating legitimate research and commercial applications of DNA data storage technologies.
Risk assessment methodologies for automated DNA storage facilities typically employ a tiered approach based on the nature of sequences being processed. Sequences with homology to known pathogens or toxins trigger enhanced containment requirements and additional oversight. The automation itself introduces novel risk factors, including the potential for higher-throughput processing of potentially hazardous sequences and reduced human supervision of critical biosafety checkpoints.
Regulatory compliance strategies for organizations implementing automated DNA data storage systems must include comprehensive staff training programs, regular third-party audits, and proactive engagement with regulatory authorities. As the technology continues to evolve, adaptive regulatory frameworks that balance innovation with appropriate safeguards will be essential to realizing the full potential of DNA data storage while maintaining public trust and safety.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!