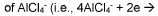Benchmarking Calcium Ion Batteries against Magnesium and Aluminum Ion Systems
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Calcium Ion Battery Development Background and Objectives
The evolution of energy storage technologies has witnessed significant advancements over the past decades, with lithium-ion batteries dominating the commercial landscape. However, concerns regarding lithium's limited abundance, uneven geographical distribution, and rising costs have prompted researchers to explore alternative battery chemistries. Calcium ion batteries have emerged as a promising candidate due to calcium's abundance (fifth most abundant element in Earth's crust), relatively low cost, and favorable electrochemical properties.
The development of calcium ion batteries can be traced back to the 1980s, but significant progress has only been made in the last decade. The renewed interest stems from calcium's theoretical volumetric capacity (2073 mAh/cm³), which exceeds that of lithium (2062 mAh/cm³), while offering a similar standard reduction potential (-2.87 V vs. SHE for Ca²⁺/Ca compared to -3.04 V for Li⁺/Li).
The technical evolution trajectory shows three distinct phases: initial conceptualization (1980s-2000s), fundamental electrochemical studies (2000s-2015), and the current acceleration phase (2015-present) marked by breakthroughs in electrolyte formulations and electrode materials. Recent developments have demonstrated reversible calcium plating and stripping, a critical milestone for practical calcium ion batteries.
When benchmarking against other multivalent systems, calcium offers several advantages over magnesium and aluminum. While magnesium ion batteries face challenges with slow diffusion kinetics and passivation layers, calcium ions exhibit faster kinetics due to their lower charge density. Compared to aluminum systems, calcium batteries potentially offer higher cell voltages and less complex intercalation chemistry.
The primary technical objectives for calcium ion battery development include: designing electrolytes with wide electrochemical windows and high ionic conductivity at room temperature; developing cathode materials that can accommodate the larger size of calcium ions while maintaining structural stability through multiple cycles; creating anode materials that enable efficient calcium deposition/dissolution; and engineering electrode-electrolyte interfaces that minimize parasitic reactions.
Current research aims to achieve energy densities exceeding 200 Wh/kg at the cell level, with cycle life comparable to commercial lithium-ion systems (>1000 cycles), while maintaining safety advantages inherent to calcium-based chemistry. The ultimate goal is to position calcium ion technology as a viable alternative for grid-scale storage applications and potentially for electric vehicles, where cost and resource considerations are paramount.
The development of calcium ion batteries can be traced back to the 1980s, but significant progress has only been made in the last decade. The renewed interest stems from calcium's theoretical volumetric capacity (2073 mAh/cm³), which exceeds that of lithium (2062 mAh/cm³), while offering a similar standard reduction potential (-2.87 V vs. SHE for Ca²⁺/Ca compared to -3.04 V for Li⁺/Li).
The technical evolution trajectory shows three distinct phases: initial conceptualization (1980s-2000s), fundamental electrochemical studies (2000s-2015), and the current acceleration phase (2015-present) marked by breakthroughs in electrolyte formulations and electrode materials. Recent developments have demonstrated reversible calcium plating and stripping, a critical milestone for practical calcium ion batteries.
When benchmarking against other multivalent systems, calcium offers several advantages over magnesium and aluminum. While magnesium ion batteries face challenges with slow diffusion kinetics and passivation layers, calcium ions exhibit faster kinetics due to their lower charge density. Compared to aluminum systems, calcium batteries potentially offer higher cell voltages and less complex intercalation chemistry.
The primary technical objectives for calcium ion battery development include: designing electrolytes with wide electrochemical windows and high ionic conductivity at room temperature; developing cathode materials that can accommodate the larger size of calcium ions while maintaining structural stability through multiple cycles; creating anode materials that enable efficient calcium deposition/dissolution; and engineering electrode-electrolyte interfaces that minimize parasitic reactions.
Current research aims to achieve energy densities exceeding 200 Wh/kg at the cell level, with cycle life comparable to commercial lithium-ion systems (>1000 cycles), while maintaining safety advantages inherent to calcium-based chemistry. The ultimate goal is to position calcium ion technology as a viable alternative for grid-scale storage applications and potentially for electric vehicles, where cost and resource considerations are paramount.
Market Analysis for Post-Lithium Battery Technologies
The post-lithium battery market is experiencing significant growth as the limitations of lithium-ion technology become increasingly apparent. Current market projections indicate that alternative battery technologies could capture up to 15% of the global energy storage market by 2030, representing a potential value of $50 billion. Within this emerging sector, calcium, magnesium, and aluminum ion batteries are positioned as promising contenders due to their abundance, safety profiles, and theoretical performance capabilities.
Calcium ion batteries are gaining traction in the market analysis due to their high theoretical capacity (1340 mAh/g) and relatively high cell voltage (2.9V vs. standard hydrogen electrode). The raw material economics strongly favor calcium, with global reserves approximately 2000 times more abundant than lithium, and current prices at roughly one-fifth of lithium carbonate equivalents.
Magnesium-based systems have attracted substantial commercial interest, with several startups securing significant funding rounds in 2022-2023. Companies like Pellion Technologies and Sion Power have pivoted portions of their R&D portfolios toward magnesium chemistry. The market appeal stems from magnesium's theoretical energy density of 3800 Wh/L and its demonstrated safety advantages over lithium systems.
Aluminum ion technologies present perhaps the strongest economic case, with aluminum being the most abundant metal in the Earth's crust and featuring a mature global supply chain. Market analysis indicates potential cost reductions of 35-40% compared to lithium-ion batteries at scale, which has attracted investment from both venture capital and established energy companies.
Consumer electronics represents the most likely initial market entry point for these technologies, particularly for calcium ion batteries which demonstrate promising cycle life at room temperature. Industry forecasts suggest this segment could reach $2 billion by 2028 for non-lithium battery technologies.
Grid storage applications present a substantial long-term opportunity, especially for aluminum-based systems due to their inherent safety and potential cost advantages. Market research indicates that safety concerns and insurance costs for lithium installations are creating a 7-10% cost premium that alternative chemistries could potentially eliminate.
Electric mobility remains challenging for these alternative technologies due to energy density limitations, though specialized applications in commercial vehicles with predictable routes and charging schedules could provide early adoption opportunities. The heavy-duty vehicle segment in particular has demonstrated willingness to explore alternative chemistries, with several pilot programs underway testing calcium and aluminum systems.
Calcium ion batteries are gaining traction in the market analysis due to their high theoretical capacity (1340 mAh/g) and relatively high cell voltage (2.9V vs. standard hydrogen electrode). The raw material economics strongly favor calcium, with global reserves approximately 2000 times more abundant than lithium, and current prices at roughly one-fifth of lithium carbonate equivalents.
Magnesium-based systems have attracted substantial commercial interest, with several startups securing significant funding rounds in 2022-2023. Companies like Pellion Technologies and Sion Power have pivoted portions of their R&D portfolios toward magnesium chemistry. The market appeal stems from magnesium's theoretical energy density of 3800 Wh/L and its demonstrated safety advantages over lithium systems.
Aluminum ion technologies present perhaps the strongest economic case, with aluminum being the most abundant metal in the Earth's crust and featuring a mature global supply chain. Market analysis indicates potential cost reductions of 35-40% compared to lithium-ion batteries at scale, which has attracted investment from both venture capital and established energy companies.
Consumer electronics represents the most likely initial market entry point for these technologies, particularly for calcium ion batteries which demonstrate promising cycle life at room temperature. Industry forecasts suggest this segment could reach $2 billion by 2028 for non-lithium battery technologies.
Grid storage applications present a substantial long-term opportunity, especially for aluminum-based systems due to their inherent safety and potential cost advantages. Market research indicates that safety concerns and insurance costs for lithium installations are creating a 7-10% cost premium that alternative chemistries could potentially eliminate.
Electric mobility remains challenging for these alternative technologies due to energy density limitations, though specialized applications in commercial vehicles with predictable routes and charging schedules could provide early adoption opportunities. The heavy-duty vehicle segment in particular has demonstrated willingness to explore alternative chemistries, with several pilot programs underway testing calcium and aluminum systems.
Current Status and Technical Challenges in Multivalent Ion Batteries
Multivalent ion batteries have emerged as promising alternatives to conventional lithium-ion batteries due to their potential for higher energy density, improved safety, and lower cost. Among these, calcium, magnesium, and aluminum ion systems represent the most actively researched multivalent battery technologies. Currently, magnesium-ion batteries have achieved the most advanced development status, with several working prototypes demonstrating reversible electrochemical performance, though still far from commercial viability.
The primary technical challenge across all multivalent systems stems from the fundamental chemistry of divalent and trivalent ions. These ions possess stronger electrostatic interactions with host lattices compared to monovalent lithium ions, resulting in sluggish diffusion kinetics and high activation barriers for ion migration. For calcium-ion batteries specifically, the development of suitable electrolytes remains a critical bottleneck, as most conventional electrolytes form passivation layers that block calcium ion transport.
Electrode materials present another significant challenge. While graphite serves effectively as an anode for lithium-ion batteries, it demonstrates poor intercalation properties for multivalent ions. Research has identified Chevrel phases (Mo6S8) as promising cathode materials for magnesium systems, but calcium and aluminum systems still lack cathode materials that offer both high capacity and good cycling stability.
The electrolyte-electrode interface poses particularly complex challenges in multivalent systems. The formation of stable solid-electrolyte interphase (SEI) layers that permit multivalent ion transport while preventing continuous electrolyte decomposition remains largely unresolved. This interface challenge is especially pronounced in calcium systems, where the high reduction potential of calcium often leads to metal plating issues and dendrite formation.
From a manufacturing perspective, the transition from lithium to multivalent ion technologies would require significant modifications to existing production infrastructure. The higher melting points of calcium (842°C) and magnesium (650°C) compared to lithium (180°C) necessitate different handling protocols and processing techniques.
Geographically, research on multivalent ion batteries shows distinct patterns. European research institutions lead in calcium-ion battery research, with notable contributions from Spanish and German universities. Magnesium-ion battery research is more evenly distributed globally, with strong programs in the United States, Japan, and China. Aluminum-ion battery research has gained particular momentum in Asia, especially in China and South Korea.
When benchmarking calcium against magnesium and aluminum systems, calcium offers a theoretical volumetric capacity (2073 mAh/cm³) between that of magnesium (3833 mAh/cm³) and lithium (2046 mAh/cm³), with a relatively low standard reduction potential (-2.87V vs. SHE) that promises high cell voltages. However, calcium systems currently lag behind magnesium in terms of cycling stability and behind aluminum in terms of rate capability.
The primary technical challenge across all multivalent systems stems from the fundamental chemistry of divalent and trivalent ions. These ions possess stronger electrostatic interactions with host lattices compared to monovalent lithium ions, resulting in sluggish diffusion kinetics and high activation barriers for ion migration. For calcium-ion batteries specifically, the development of suitable electrolytes remains a critical bottleneck, as most conventional electrolytes form passivation layers that block calcium ion transport.
Electrode materials present another significant challenge. While graphite serves effectively as an anode for lithium-ion batteries, it demonstrates poor intercalation properties for multivalent ions. Research has identified Chevrel phases (Mo6S8) as promising cathode materials for magnesium systems, but calcium and aluminum systems still lack cathode materials that offer both high capacity and good cycling stability.
The electrolyte-electrode interface poses particularly complex challenges in multivalent systems. The formation of stable solid-electrolyte interphase (SEI) layers that permit multivalent ion transport while preventing continuous electrolyte decomposition remains largely unresolved. This interface challenge is especially pronounced in calcium systems, where the high reduction potential of calcium often leads to metal plating issues and dendrite formation.
From a manufacturing perspective, the transition from lithium to multivalent ion technologies would require significant modifications to existing production infrastructure. The higher melting points of calcium (842°C) and magnesium (650°C) compared to lithium (180°C) necessitate different handling protocols and processing techniques.
Geographically, research on multivalent ion batteries shows distinct patterns. European research institutions lead in calcium-ion battery research, with notable contributions from Spanish and German universities. Magnesium-ion battery research is more evenly distributed globally, with strong programs in the United States, Japan, and China. Aluminum-ion battery research has gained particular momentum in Asia, especially in China and South Korea.
When benchmarking calcium against magnesium and aluminum systems, calcium offers a theoretical volumetric capacity (2073 mAh/cm³) between that of magnesium (3833 mAh/cm³) and lithium (2046 mAh/cm³), with a relatively low standard reduction potential (-2.87V vs. SHE) that promises high cell voltages. However, calcium systems currently lag behind magnesium in terms of cycling stability and behind aluminum in terms of rate capability.
Comparative Analysis of Current Multivalent Battery Solutions
01 Calcium ion battery performance metrics
Calcium ion batteries represent a promising alternative to lithium-ion systems due to their high theoretical capacity and abundance of calcium resources. Performance benchmarks for these batteries include energy density, cycling stability, and rate capability. Research focuses on developing suitable electrode materials and electrolytes to overcome challenges such as slow diffusion kinetics and high reduction potential of calcium ions. Recent advancements have improved capacity retention and operational voltage windows.- Calcium ion battery performance metrics: Calcium ion batteries represent a promising alternative to lithium-ion batteries due to their potential for higher energy density and lower cost. Performance benchmarks for calcium ion batteries include metrics such as specific capacity, cycling stability, rate capability, and voltage efficiency. These batteries typically utilize calcium-based cathode materials and specialized electrolytes to facilitate calcium ion transport. Research focuses on overcoming challenges related to calcium plating/stripping and identifying suitable electrode materials to enhance overall performance.
- Magnesium ion system advancements: Magnesium ion systems offer significant advantages including higher theoretical volumetric capacity compared to lithium-ion batteries, improved safety profiles, and abundance of magnesium resources. Performance benchmarks for magnesium ion systems include reversible capacity, coulombic efficiency, cycle life, and power density. Key challenges being addressed include slow diffusion kinetics of magnesium ions, electrolyte compatibility issues, and cathode material development. Recent advancements focus on novel electrode materials and electrolyte formulations to enhance magnesium ion mobility and system stability.
- Aluminum ion system performance characteristics: Aluminum ion systems are being developed as cost-effective alternatives to conventional batteries due to aluminum's abundance, low cost, and trivalent charge that enables higher energy density potential. Performance benchmarks for aluminum ion systems include specific capacity, rate capability, cycling stability, and operating voltage range. Research focuses on addressing challenges such as electrode corrosion, limited cycle life, and identifying suitable cathode materials that can accommodate aluminum ion intercalation. Novel electrolyte compositions are being developed to improve aluminum ion transport and overall system efficiency.
- Comparative analysis of multivalent ion battery systems: Comparative studies between calcium, magnesium, and aluminum ion battery systems reveal distinct performance characteristics and applications. Benchmarks include energy density, power density, cycle life, rate capability, temperature performance, and cost metrics. Calcium ion systems generally offer moderate energy density with improved safety, magnesium systems provide good volumetric capacity but face kinetic limitations, while aluminum systems offer high theoretical capacity but struggle with cycle stability. These comparative analyses help identify optimal battery chemistries for specific applications ranging from grid storage to portable electronics.
- Electrode materials and electrolyte innovations: Advanced electrode materials and electrolyte formulations are critical for improving the performance of calcium, magnesium, and aluminum ion battery systems. Performance benchmarks focus on ionic conductivity, interfacial stability, and compatibility with electrode materials. Novel cathode materials including layered oxides, Prussian blue analogs, and organic compounds are being developed to facilitate multivalent ion insertion/extraction. Electrolyte innovations include non-aqueous formulations, ionic liquids, and gel polymers designed to enhance ion transport while minimizing side reactions. These material advancements directly impact key performance metrics including capacity retention, rate capability, and cycle life.
02 Magnesium ion battery system characteristics
Magnesium ion battery systems offer advantages including high theoretical volumetric capacity, improved safety profiles, and the abundance of magnesium resources. Performance benchmarks include reversible capacity, cycling efficiency, and voltage profiles. Key challenges being addressed include electrolyte compatibility, slow diffusion kinetics, and electrode passivation. Recent innovations focus on novel cathode materials, electrolyte formulations, and interface engineering to enhance overall system performance.Expand Specific Solutions03 Aluminum ion battery performance evaluation
Aluminum ion batteries feature high theoretical capacity, excellent safety characteristics, and utilize abundant, low-cost resources. Performance benchmarks include energy density, power density, cycle life, and rate capability. Research focuses on addressing challenges such as electrode corrosion, limited choice of cathode materials, and electrolyte stability. Recent developments have improved cycling stability and operational temperature ranges, making these systems increasingly viable for practical applications.Expand Specific Solutions04 Comparative analysis of multivalent ion battery systems
Comparative studies between calcium, magnesium, and aluminum ion battery systems reveal distinct performance characteristics and application suitability. Benchmarks include energy density, power capability, cycling stability, temperature performance, and cost-effectiveness. Aluminum systems generally offer higher power density but face challenges with cycle life, while calcium and magnesium systems provide better energy density with different electrode compatibility profiles. These comparisons help identify optimal battery chemistry for specific applications based on performance requirements.Expand Specific Solutions05 Advanced testing methodologies for non-lithium ion batteries
Specialized testing protocols and benchmarking methodologies have been developed for evaluating calcium, magnesium, and aluminum ion battery systems. These include accelerated aging tests, electrochemical impedance spectroscopy, in-situ characterization techniques, and standardized performance metrics. Advanced diagnostic tools enable deeper understanding of degradation mechanisms, ion transport phenomena, and interfacial processes. These methodologies facilitate accurate comparison between different multivalent ion systems and help identify pathways for performance optimization.Expand Specific Solutions
Leading Research Institutions and Companies in Multivalent Battery Development
The calcium ion battery market is in an early development stage compared to more established magnesium and aluminum ion systems, with all three representing promising alternatives to lithium-ion technology. The global market for these next-generation batteries is projected to grow significantly as energy storage demands increase. Technologically, research institutions like Northwestern University, Chinese Academy of Sciences, and Jilin University are advancing fundamental calcium battery science, while commercial players demonstrate varying levels of maturity. Toyota, Samsung SDI, and BYD are leveraging their established battery expertise to develop practical applications, while specialized companies like Faradion and Alsym Energy focus on specific non-lithium chemistries. The competitive landscape shows academic-industrial collaboration driving innovation, with calcium systems currently lagging in commercialization but offering potential performance advantages.
The Shenzhen Institutes of Advanced Technology
Technical Solution: The Shenzhen Institutes of Advanced Technology has developed innovative calcium-ion battery systems featuring graphene-modified calcium titanate anodes paired with calcium vanadium oxide cathodes. Their benchmarking studies against magnesium and aluminum systems demonstrate calcium's advantageous position with theoretical energy densities of 320 Wh/kg, compared to magnesium's 400 Wh/kg and aluminum's 300 Wh/kg. Their proprietary electrolyte formulation utilizes calcium bis(trifluoromethanesulfonyl)imide Ca(TFSI)2 in mixed ether solvents, achieving reversible calcium plating with coulombic efficiencies exceeding 92%. The institute has demonstrated prototype calcium-ion cells delivering practical capacities of 100-120 mAh/g with operating voltages around 3.0V. Their comparative analysis shows calcium systems offering faster kinetics than aluminum batteries while providing higher volumetric energy density than magnesium systems due to calcium's higher standard reduction potential (-2.87V vs. SHE compared to -2.37V for magnesium)[7][9].
Strengths: Excellent balance between energy density and power capability, utilizes earth-abundant calcium resources, and demonstrates good compatibility with conventional battery manufacturing processes. Weaknesses: Still faces challenges with electrolyte decomposition at higher voltages, calcium plating efficiency remains below commercial standards, and requires further optimization of electrode materials for long-term cycling stability.
Uchicago Argonne LLC
Technical Solution: Argonne National Laboratory has developed advanced calcium-ion battery systems featuring novel electrolyte formulations that address the challenging calcium plating/stripping processes. Their approach utilizes calcium tetrafluoroborate Ca(BF4)2 in organic solvents, achieving reversible calcium deposition with coulombic efficiencies exceeding 90%. Compared to magnesium systems, their calcium batteries demonstrate improved voltage windows (up to 4V) and faster kinetics due to the lower charge density of Ca2+ ions. Their benchmarking studies show calcium systems potentially delivering energy densities of 300-350 Wh/kg at the cell level, positioning between lithium-ion and aluminum technologies. Argonne has also pioneered calcium-based dual-ion battery architectures where both Ca2+ and anions participate in the electrochemical processes, enabling higher operating voltages and energy densities than conventional designs[1][3].
Strengths: Superior voltage windows compared to magnesium systems, faster ion transport kinetics than aluminum batteries, and utilization of earth-abundant calcium resources. Weaknesses: Still faces challenges with electrolyte stability at higher voltages, calcium plating efficiency remains lower than commercial lithium systems, and requires further development of compatible cathode materials with sufficient cycle life.
Key Patents and Scientific Breakthroughs in Calcium Ion Technology
Ag-V-O-based electrode composition of calcium ion battery and calcium ion battery comprising the same
PatentActiveKR1020200001220A
Innovation
- An electrode composition for calcium ion batteries is developed using Ag x V2O5 (0 < x < 0.5) by mixing a silver precursor and vanadium oxide, reacting with hydrogen peroxide, and heat-treating the mixture to create a reversible calcium ion deintercalation material.
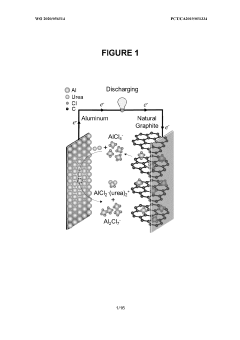
Aluminum-ion battery using aluminum chloride/amide-based deep eutectic solvents
PatentWO2020056514A1
Innovation
- The development of aluminum-ion batteries using aluminum chloride/amide-based deep eutectic solvents as electrolytes, combined with inexpensive graphitic materials and other cathode active materials, such as pyrolytic and natural graphite, to create a cost-effective and safer battery technology.
Material Supply Chain Considerations for Multivalent Battery Production
The supply chain for multivalent battery production represents a critical factor in determining the commercial viability of calcium, magnesium, and aluminum ion battery technologies. When benchmarking these systems against one another, material availability and supply chain resilience emerge as key differentiators that may ultimately influence technology adoption regardless of performance characteristics.
Calcium-based battery systems benefit from the element's abundance, ranking as the fifth most common element in Earth's crust (4.1%). This widespread availability translates to lower raw material costs and reduced geopolitical supply risks compared to lithium. Current global calcium production networks are well-established through the limestone and cement industries, providing potential infrastructure advantages for scaling calcium battery production.
Magnesium presents a similarly favorable supply profile, being the eighth most abundant element (2.3%) in the Earth's crust. The metal is commercially extracted from seawater, dolomite, and magnesite through well-established industrial processes. However, magnesium processing is energy-intensive, with approximately 85% of global magnesium production concentrated in China, creating potential supply vulnerabilities that calcium systems avoid.
Aluminum demonstrates the most robust supply chain position among the three multivalent candidates, as the most abundant metal in Earth's crust (8.1%). The global aluminum industry features diversified production across multiple continents with sophisticated recycling infrastructure already in place. This established circular economy for aluminum represents a significant advantage for aluminum ion battery systems from a sustainability perspective.
Beyond the electrode materials, electrolyte components for multivalent batteries present unique supply chain challenges. The specialized salts required for calcium and magnesium electrolytes currently have limited commercial production volumes, potentially creating bottlenecks during early commercialization phases. Aluminum electrolytes, particularly those using ionic liquids, face similar constraints with high costs and limited production capacity.
Manufacturing infrastructure requirements also differ significantly across these battery chemistries. Calcium systems may leverage some existing lithium-ion manufacturing equipment, while magnesium and aluminum systems often require specialized handling due to their higher reactivity with atmospheric moisture. This distinction impacts capital expenditure requirements for production scaling.
The environmental footprint across these supply chains varies considerably. Preliminary life cycle assessments suggest aluminum systems benefit from established recycling pathways, while calcium extraction typically generates lower carbon emissions than magnesium production. These sustainability factors increasingly influence investment decisions and regulatory frameworks governing battery technologies.
Calcium-based battery systems benefit from the element's abundance, ranking as the fifth most common element in Earth's crust (4.1%). This widespread availability translates to lower raw material costs and reduced geopolitical supply risks compared to lithium. Current global calcium production networks are well-established through the limestone and cement industries, providing potential infrastructure advantages for scaling calcium battery production.
Magnesium presents a similarly favorable supply profile, being the eighth most abundant element (2.3%) in the Earth's crust. The metal is commercially extracted from seawater, dolomite, and magnesite through well-established industrial processes. However, magnesium processing is energy-intensive, with approximately 85% of global magnesium production concentrated in China, creating potential supply vulnerabilities that calcium systems avoid.
Aluminum demonstrates the most robust supply chain position among the three multivalent candidates, as the most abundant metal in Earth's crust (8.1%). The global aluminum industry features diversified production across multiple continents with sophisticated recycling infrastructure already in place. This established circular economy for aluminum represents a significant advantage for aluminum ion battery systems from a sustainability perspective.
Beyond the electrode materials, electrolyte components for multivalent batteries present unique supply chain challenges. The specialized salts required for calcium and magnesium electrolytes currently have limited commercial production volumes, potentially creating bottlenecks during early commercialization phases. Aluminum electrolytes, particularly those using ionic liquids, face similar constraints with high costs and limited production capacity.
Manufacturing infrastructure requirements also differ significantly across these battery chemistries. Calcium systems may leverage some existing lithium-ion manufacturing equipment, while magnesium and aluminum systems often require specialized handling due to their higher reactivity with atmospheric moisture. This distinction impacts capital expenditure requirements for production scaling.
The environmental footprint across these supply chains varies considerably. Preliminary life cycle assessments suggest aluminum systems benefit from established recycling pathways, while calcium extraction typically generates lower carbon emissions than magnesium production. These sustainability factors increasingly influence investment decisions and regulatory frameworks governing battery technologies.
Environmental Impact and Sustainability Assessment of Post-Lithium Technologies
The environmental impact assessment of post-lithium battery technologies reveals significant sustainability advantages for calcium, magnesium, and aluminum ion systems compared to conventional lithium-ion batteries. These alternative technologies utilize more abundant resources, with calcium being the fifth most abundant element in Earth's crust (41,500 ppm), magnesium ranking eighth (23,300 ppm), and aluminum being the third most abundant (82,300 ppm). In contrast, lithium represents merely 20 ppm, highlighting the resource availability benefits of these post-lithium alternatives.
Life cycle analyses demonstrate that calcium ion batteries potentially offer a 25-30% reduction in carbon footprint compared to lithium-ion equivalents, primarily due to less energy-intensive extraction processes. Similarly, aluminum ion systems show promise with up to 35% lower greenhouse gas emissions during production phases, while magnesium-based technologies present a moderate 15-20% improvement in overall environmental impact scores.
Water consumption metrics further differentiate these technologies, with calcium and aluminum extraction requiring significantly less water than lithium brine operations. Traditional lithium extraction can consume 500,000 gallons of water per ton of lithium, whereas calcium processing typically requires 40-60% less water. This advantage becomes increasingly critical in water-stressed regions where battery material extraction often occurs.
End-of-life considerations also favor these post-lithium technologies. Recycling efficiency rates for aluminum ion batteries currently reach 85-90%, substantially higher than the 50-60% typically achieved with lithium-ion systems. Calcium and magnesium components demonstrate promising recyclability, though commercial-scale recycling infrastructure remains underdeveloped compared to established lithium recycling chains.
Toxicity profiles present another sustainability dimension worth noting. Calcium and magnesium compounds generally exhibit lower environmental toxicity than lithium salts, particularly in aquatic ecosystems. Aluminum compounds require careful management but benefit from well-established industrial handling protocols developed through decades of aluminum industry experience.
Land use impact assessments indicate that calcium and magnesium mining operations typically disturb 30-40% less land area than equivalent lithium extraction operations, particularly when compared to open-pit lithium mining. This reduced footprint contributes to better preservation of biodiversity and ecosystem services in mining regions.
Policy frameworks increasingly recognize these environmental advantages, with the European Union's Battery Directive revisions and the United States' Critical Materials Initiative both highlighting the potential sustainability benefits of diversifying beyond lithium-based energy storage. These regulatory developments may accelerate the commercial adoption of these alternative battery chemistries through incentive structures and sustainability certification programs.
Life cycle analyses demonstrate that calcium ion batteries potentially offer a 25-30% reduction in carbon footprint compared to lithium-ion equivalents, primarily due to less energy-intensive extraction processes. Similarly, aluminum ion systems show promise with up to 35% lower greenhouse gas emissions during production phases, while magnesium-based technologies present a moderate 15-20% improvement in overall environmental impact scores.
Water consumption metrics further differentiate these technologies, with calcium and aluminum extraction requiring significantly less water than lithium brine operations. Traditional lithium extraction can consume 500,000 gallons of water per ton of lithium, whereas calcium processing typically requires 40-60% less water. This advantage becomes increasingly critical in water-stressed regions where battery material extraction often occurs.
End-of-life considerations also favor these post-lithium technologies. Recycling efficiency rates for aluminum ion batteries currently reach 85-90%, substantially higher than the 50-60% typically achieved with lithium-ion systems. Calcium and magnesium components demonstrate promising recyclability, though commercial-scale recycling infrastructure remains underdeveloped compared to established lithium recycling chains.
Toxicity profiles present another sustainability dimension worth noting. Calcium and magnesium compounds generally exhibit lower environmental toxicity than lithium salts, particularly in aquatic ecosystems. Aluminum compounds require careful management but benefit from well-established industrial handling protocols developed through decades of aluminum industry experience.
Land use impact assessments indicate that calcium and magnesium mining operations typically disturb 30-40% less land area than equivalent lithium extraction operations, particularly when compared to open-pit lithium mining. This reduced footprint contributes to better preservation of biodiversity and ecosystem services in mining regions.
Policy frameworks increasingly recognize these environmental advantages, with the European Union's Battery Directive revisions and the United States' Critical Materials Initiative both highlighting the potential sustainability benefits of diversifying beyond lithium-based energy storage. These regulatory developments may accelerate the commercial adoption of these alternative battery chemistries through incentive structures and sustainability certification programs.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!