Reversible Calcium Metal Anodes and SEI Formation in Calcium Ion Batteries
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Calcium Battery Technology Background and Objectives
Calcium-ion batteries (CIBs) have emerged as a promising alternative to lithium-ion batteries due to the abundance of calcium resources, which constitute approximately 3% of the Earth's crust compared to lithium's 0.006%. This natural abundance translates to significantly lower raw material costs and reduced geopolitical supply chain risks, positioning calcium as a sustainable candidate for next-generation energy storage technologies.
The evolution of battery technology has been driven by increasing demands for higher energy density, longer cycle life, and enhanced safety profiles. While lithium-ion batteries have dominated the market since their commercialization in the 1990s, their theoretical limits are being approached, necessitating exploration of alternative chemistries. Calcium, with its divalent nature, offers a theoretical volumetric capacity of 2073 mAh/cm³, surpassing lithium's 2046 mAh/cm³, while maintaining a relatively low standard reduction potential of -2.87 V vs. SHE.
Historical development of calcium battery research dates back to the 1960s, but significant progress has been hindered by fundamental challenges, particularly the formation of stable solid electrolyte interphase (SEI) layers on calcium metal anodes and the limited reversibility of calcium plating/stripping processes. The breakthrough in 2016 demonstrating reversible calcium plating in specific electrolytes has reignited interest in this field, marking a pivotal moment in calcium battery development.
Current technological objectives for calcium battery research center on addressing several critical challenges. Primary among these is understanding and controlling the SEI formation on calcium metal anodes, which directly impacts the reversibility and efficiency of calcium deposition and dissolution. Unlike lithium, calcium's divalent nature creates complex interfacial chemistry that remains poorly understood.
Additional objectives include developing electrolytes with wider electrochemical stability windows that are compatible with calcium metal, identifying suitable cathode materials capable of accommodating calcium ions' larger size and stronger electrostatic interactions, and engineering practical cell designs that mitigate calcium's tendency toward dendrite formation during cycling.
The ultimate goal of calcium battery technology development is to create energy storage systems that offer comparable or superior performance to current lithium-ion technologies while providing advantages in cost, safety, and environmental impact. This requires a multidisciplinary approach combining materials science, electrochemistry, and engineering to overcome the fundamental challenges associated with calcium's electrochemical behavior.
The evolution of battery technology has been driven by increasing demands for higher energy density, longer cycle life, and enhanced safety profiles. While lithium-ion batteries have dominated the market since their commercialization in the 1990s, their theoretical limits are being approached, necessitating exploration of alternative chemistries. Calcium, with its divalent nature, offers a theoretical volumetric capacity of 2073 mAh/cm³, surpassing lithium's 2046 mAh/cm³, while maintaining a relatively low standard reduction potential of -2.87 V vs. SHE.
Historical development of calcium battery research dates back to the 1960s, but significant progress has been hindered by fundamental challenges, particularly the formation of stable solid electrolyte interphase (SEI) layers on calcium metal anodes and the limited reversibility of calcium plating/stripping processes. The breakthrough in 2016 demonstrating reversible calcium plating in specific electrolytes has reignited interest in this field, marking a pivotal moment in calcium battery development.
Current technological objectives for calcium battery research center on addressing several critical challenges. Primary among these is understanding and controlling the SEI formation on calcium metal anodes, which directly impacts the reversibility and efficiency of calcium deposition and dissolution. Unlike lithium, calcium's divalent nature creates complex interfacial chemistry that remains poorly understood.
Additional objectives include developing electrolytes with wider electrochemical stability windows that are compatible with calcium metal, identifying suitable cathode materials capable of accommodating calcium ions' larger size and stronger electrostatic interactions, and engineering practical cell designs that mitigate calcium's tendency toward dendrite formation during cycling.
The ultimate goal of calcium battery technology development is to create energy storage systems that offer comparable or superior performance to current lithium-ion technologies while providing advantages in cost, safety, and environmental impact. This requires a multidisciplinary approach combining materials science, electrochemistry, and engineering to overcome the fundamental challenges associated with calcium's electrochemical behavior.
Market Analysis for Next-Generation Battery Technologies
The global battery market is experiencing a significant shift towards next-generation technologies, with calcium ion batteries (CIBs) emerging as a promising alternative to current lithium-ion dominance. The market for advanced battery technologies is projected to reach $240 billion by 2027, growing at a CAGR of 12.3% from 2022. Within this expanding landscape, calcium-based battery systems are attracting increasing attention due to their theoretical advantages in safety, cost, and environmental impact.
Calcium's abundance in the Earth's crust (fifth most abundant element) presents a compelling economic case for CIBs. Unlike lithium, which faces supply constraints and geopolitical complications, calcium resources are widely distributed globally, potentially reducing supply chain vulnerabilities that currently plague lithium-ion battery production. This accessibility could translate to a 30-40% reduction in raw material costs compared to lithium-ion batteries once scaled to commercial production.
Market research indicates that the electric vehicle sector represents the most significant potential market for calcium ion batteries, with global EV sales expected to reach 26.8 million units by 2030. If calcium ion technology can overcome current technical challenges, particularly those related to reversible calcium metal anodes and stable SEI formation, it could capture up to 15% of the EV battery market by 2035.
Grid-scale energy storage represents another substantial market opportunity, with projected growth to $31.2 billion by 2029. Calcium's higher volumetric capacity compared to sodium and its better safety profile compared to lithium make it particularly attractive for stationary storage applications where space constraints are less critical than in transportation.
Consumer electronics manufacturers are also closely monitoring calcium ion battery development, with particular interest in the technology's potential safety advantages. After several high-profile lithium-ion battery failures in consumer products, the industry is actively seeking safer alternatives that maintain competitive energy densities.
Investment in calcium ion battery research has seen a 65% increase between 2018 and 2022, though it remains significantly lower than funding for lithium-ion improvements or solid-state battery research. Major battery manufacturers and automotive companies are establishing dedicated research divisions for multivalent ion batteries, with calcium receiving particular attention alongside magnesium systems.
Market adoption timelines suggest that initial commercial applications of calcium ion batteries may emerge in niche markets by 2026-2028, with broader adoption dependent on solving the critical challenges of reversible calcium metal anodes and stable SEI formation. Industry analysts predict that if these technical hurdles are overcome, calcium ion batteries could achieve a 7-10% market share of the global battery market by 2035.
Calcium's abundance in the Earth's crust (fifth most abundant element) presents a compelling economic case for CIBs. Unlike lithium, which faces supply constraints and geopolitical complications, calcium resources are widely distributed globally, potentially reducing supply chain vulnerabilities that currently plague lithium-ion battery production. This accessibility could translate to a 30-40% reduction in raw material costs compared to lithium-ion batteries once scaled to commercial production.
Market research indicates that the electric vehicle sector represents the most significant potential market for calcium ion batteries, with global EV sales expected to reach 26.8 million units by 2030. If calcium ion technology can overcome current technical challenges, particularly those related to reversible calcium metal anodes and stable SEI formation, it could capture up to 15% of the EV battery market by 2035.
Grid-scale energy storage represents another substantial market opportunity, with projected growth to $31.2 billion by 2029. Calcium's higher volumetric capacity compared to sodium and its better safety profile compared to lithium make it particularly attractive for stationary storage applications where space constraints are less critical than in transportation.
Consumer electronics manufacturers are also closely monitoring calcium ion battery development, with particular interest in the technology's potential safety advantages. After several high-profile lithium-ion battery failures in consumer products, the industry is actively seeking safer alternatives that maintain competitive energy densities.
Investment in calcium ion battery research has seen a 65% increase between 2018 and 2022, though it remains significantly lower than funding for lithium-ion improvements or solid-state battery research. Major battery manufacturers and automotive companies are establishing dedicated research divisions for multivalent ion batteries, with calcium receiving particular attention alongside magnesium systems.
Market adoption timelines suggest that initial commercial applications of calcium ion batteries may emerge in niche markets by 2026-2028, with broader adoption dependent on solving the critical challenges of reversible calcium metal anodes and stable SEI formation. Industry analysts predict that if these technical hurdles are overcome, calcium ion batteries could achieve a 7-10% market share of the global battery market by 2035.
Current Challenges in Calcium Ion Battery Development
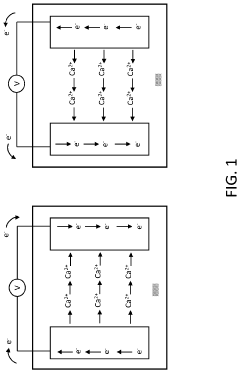
Despite the promising theoretical advantages of calcium ion batteries (CIBs), including high volumetric capacity (2073 mAh/cm³) and abundance of calcium resources, several significant challenges currently impede their practical development and commercialization. The most critical obstacle lies in the reversibility of calcium metal anodes, which suffer from poor electrochemical performance in conventional electrolytes due to the formation of passivating layers that block calcium ion transport.
The solid electrolyte interphase (SEI) formation on calcium anodes presents a complex challenge. Unlike lithium-ion batteries where the SEI facilitates ion transport while preventing further electrolyte decomposition, calcium-based SEI layers often become impermeable to Ca²⁺ ions. This is primarily attributed to the divalent nature of calcium ions, which creates stronger electrostatic interactions with the SEI components, resulting in sluggish ion diffusion kinetics.
Electrolyte compatibility remains another major hurdle. Traditional carbonate-based electrolytes, which work effectively in lithium-ion systems, fail to support reversible calcium plating and stripping. This limitation stems from the high reduction potential of calcium and the tendency of conventional electrolytes to decompose at the calcium metal interface, forming resistive surface films. Recent research has explored alternative electrolyte systems, including borates and certain ethereal solvents, but these often suffer from narrow electrochemical stability windows or poor anodic stability.
The high reactivity of calcium metal with atmospheric components presents additional challenges for practical battery assembly and operation. Calcium readily reacts with oxygen, nitrogen, and moisture, necessitating stringent manufacturing conditions and sophisticated cell designs to prevent performance degradation. This reactivity also complicates the study of fundamental calcium electrochemistry, as surface contamination can significantly alter experimental results.
Dendrite formation during calcium deposition represents another significant concern. While initially thought to be less problematic than in lithium systems due to calcium's higher mechanical strength, recent studies have revealed that under certain conditions, calcium can also form dendritic structures during electrodeposition. These dendrites can lead to internal short circuits and safety hazards, particularly at higher current densities.
The development of suitable cathode materials that can accommodate the larger size and divalent nature of calcium ions presents yet another challenge. Most existing cathode architectures, optimized for lithium-ion intercalation, exhibit poor calcium ion insertion kinetics and structural stability. This limitation restricts the overall energy density and cycle life of calcium-based systems, necessitating novel cathode designs specifically tailored for calcium ion storage.
The solid electrolyte interphase (SEI) formation on calcium anodes presents a complex challenge. Unlike lithium-ion batteries where the SEI facilitates ion transport while preventing further electrolyte decomposition, calcium-based SEI layers often become impermeable to Ca²⁺ ions. This is primarily attributed to the divalent nature of calcium ions, which creates stronger electrostatic interactions with the SEI components, resulting in sluggish ion diffusion kinetics.
Electrolyte compatibility remains another major hurdle. Traditional carbonate-based electrolytes, which work effectively in lithium-ion systems, fail to support reversible calcium plating and stripping. This limitation stems from the high reduction potential of calcium and the tendency of conventional electrolytes to decompose at the calcium metal interface, forming resistive surface films. Recent research has explored alternative electrolyte systems, including borates and certain ethereal solvents, but these often suffer from narrow electrochemical stability windows or poor anodic stability.
The high reactivity of calcium metal with atmospheric components presents additional challenges for practical battery assembly and operation. Calcium readily reacts with oxygen, nitrogen, and moisture, necessitating stringent manufacturing conditions and sophisticated cell designs to prevent performance degradation. This reactivity also complicates the study of fundamental calcium electrochemistry, as surface contamination can significantly alter experimental results.
Dendrite formation during calcium deposition represents another significant concern. While initially thought to be less problematic than in lithium systems due to calcium's higher mechanical strength, recent studies have revealed that under certain conditions, calcium can also form dendritic structures during electrodeposition. These dendrites can lead to internal short circuits and safety hazards, particularly at higher current densities.
The development of suitable cathode materials that can accommodate the larger size and divalent nature of calcium ions presents yet another challenge. Most existing cathode architectures, optimized for lithium-ion intercalation, exhibit poor calcium ion insertion kinetics and structural stability. This limitation restricts the overall energy density and cycle life of calcium-based systems, necessitating novel cathode designs specifically tailored for calcium ion storage.
Current Approaches to Reversible Calcium Metal Anodes
01 SEI formation mechanisms in calcium ion batteries
The solid electrolyte interphase (SEI) formation in calcium ion batteries involves complex mechanisms that affect battery performance. The composition and structure of the SEI layer significantly impact the reversibility of calcium ion insertion and extraction. Various electrolyte formulations can lead to different SEI characteristics, with some promoting more stable interfaces that allow for improved calcium ion transport while preventing continuous electrolyte decomposition.- SEI formation mechanisms in calcium ion batteries: The solid electrolyte interphase (SEI) formation in calcium ion batteries involves complex mechanisms that affect battery performance. The composition and structure of the SEI layer significantly impact the reversibility of calcium ion insertion and extraction. Various electrolyte compositions can lead to different SEI characteristics, with some forming more stable and calcium-ion permeable interfaces that enhance cycling stability and coulombic efficiency.
- Electrolyte additives for improved SEI stability: Specific additives in the electrolyte can modify the SEI layer formation to improve calcium ion battery reversibility. These additives help create a more uniform and stable SEI layer that allows efficient calcium ion transport while preventing continuous electrolyte decomposition. Fluorinated compounds, boron-based additives, and certain organic molecules have shown promise in forming protective SEI layers that enhance cycling performance and reduce capacity fade.
- Electrode surface modifications for enhanced reversibility: Surface modifications of calcium ion battery electrodes can significantly improve the reversibility by controlling SEI formation. Techniques such as atomic layer deposition, surface coating with conductive polymers, and nanostructuring can create favorable interfaces that facilitate calcium ion diffusion while minimizing parasitic reactions. These modifications help maintain electrode integrity during repeated calcium insertion/extraction cycles and reduce impedance growth.
- Novel electrode materials for calcium ion transport: Advanced electrode materials designed specifically for calcium ion batteries can address reversibility challenges related to the SEI layer. Materials with optimized crystal structures, expanded interlayer spacing, or engineered defects can accommodate the large calcium ions while minimizing structural degradation. These materials often exhibit better compatibility with electrolytes, forming more favorable SEI compositions that enable sustained calcium ion insertion and extraction over multiple cycles.
- Temperature and pressure effects on SEI formation: Operating conditions such as temperature and pressure significantly influence SEI formation and calcium ion battery reversibility. Controlled formation protocols at specific temperatures can lead to more stable SEI layers with improved calcium ion conductivity. Pressure effects can alter the kinetics of interfacial reactions and the physical properties of the resulting SEI layer. Understanding these parameters enables the development of optimized formation cycles that enhance long-term battery performance and reversibility.
02 Electrolyte additives for improved reversibility
Specific electrolyte additives can be incorporated to enhance the reversibility of calcium ion batteries by forming more favorable SEI layers. These additives can modify the composition and properties of the SEI, making it more permeable to calcium ions while maintaining structural stability. Some additives help reduce the desolvation energy of calcium ions, facilitating their transport through the SEI layer and improving cycling performance.Expand Specific Solutions03 Electrode materials design for calcium ion reversibility
The design and selection of electrode materials play a crucial role in the reversibility of calcium ion batteries. Materials with appropriate crystal structures and sufficient interlayer spacing can accommodate the large size of calcium ions and facilitate their insertion/extraction. Surface modifications of electrode materials can also help control SEI formation and improve the kinetics of calcium ion transport, leading to enhanced cycling stability.Expand Specific Solutions04 Temperature effects on SEI formation and stability
Temperature significantly influences SEI formation and stability in calcium ion batteries. Higher temperatures can accelerate SEI formation but may lead to less stable interfaces, while lower temperatures can result in more stable but resistive SEI layers. Controlling the operating temperature during initial cycling can help form more favorable SEI structures that enhance the reversibility of calcium ion insertion and extraction processes.Expand Specific Solutions05 Novel electrolyte systems for calcium ion batteries
Development of novel electrolyte systems is critical for addressing the challenges of calcium ion battery reversibility. These include non-aqueous electrolytes with reduced calcium ion solvation energy, ionic liquid-based electrolytes with wider electrochemical windows, and solid-state electrolytes that can prevent continuous SEI growth. These advanced electrolyte systems aim to form more favorable interfaces that allow efficient calcium ion transport while maintaining long-term stability.Expand Specific Solutions
Leading Research Groups and Industrial Players
The calcium-ion battery market is in an early development stage, characterized by significant research activity but limited commercial deployment. Current market size remains modest compared to lithium-ion technologies, though projections indicate substantial growth potential as calcium offers theoretical advantages in cost and abundance. Technical maturity remains low, with key players focusing on overcoming fundamental challenges in reversible calcium metal anodes and SEI formation. Research institutions like Cornell University and Deakin University are driving fundamental breakthroughs, while established battery manufacturers including LG Energy Solution, Samsung SDI, and TDK are investing in early-stage development. Automotive companies (GM, BMW, Renault, Toyota) are exploring calcium-ion technology as part of diversification strategies beyond lithium-ion, while specialized firms like Nanoscale Components and 24M Technologies are developing enabling technologies that could accelerate commercialization.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed a comprehensive approach to calcium ion battery technology with a focus on reversible calcium metal anodes. Their proprietary technology incorporates specially formulated electrolytes containing calcium salts (Ca(TFSI)2 and Ca(ClO4)2) in carefully selected solvent mixtures that demonstrate enhanced calcium ion transport properties. The company has engineered novel electrolyte additives that promote the formation of calcium-ion conductive SEI layers while suppressing dendrite formation during repeated plating/stripping cycles. LG's approach includes surface modification of current collectors with calcium-philic materials that improve nucleation behavior and adhesion of calcium deposits. Their technology also features advanced electrode architectures with 3D structured current collectors that accommodate volume changes during calcium plating/stripping, enhancing cycling stability. LG Energy Solution has integrated these innovations with their established battery manufacturing capabilities to develop prototype calcium ion cells with improved energy density compared to conventional lithium-ion batteries.
Strengths: Strong industrial manufacturing capabilities for potential commercialization; comprehensive approach addressing multiple aspects of calcium battery technology; significant R&D resources and battery engineering expertise. Weaknesses: Calcium technology still faces significant hurdles for commercial viability compared to established lithium-ion technology; challenges in achieving competitive cycle life; higher production costs compared to conventional battery technologies.
Samsung SDI Co., Ltd.
Technical Solution: Samsung SDI has developed an advanced calcium ion battery platform focusing on reversible calcium metal anodes and optimized SEI formation. Their technology employs a multi-component electrolyte system containing calcium bis(hexafluoroisopropyloxy)borate (Ca[B(hfip)4]2) salt in ether-based solvents, which has demonstrated superior calcium ion transport properties. Samsung's approach includes proprietary electrolyte additives that promote the formation of stable and calcium-ion conductive SEI layers while minimizing parasitic reactions. The company has engineered specialized current collectors with nanoscale surface modifications that improve calcium nucleation behavior and adhesion, reducing the formation of isolated calcium deposits during cycling. Their technology also incorporates pre-lithiation techniques adapted for calcium systems to compensate for initial capacity loss due to SEI formation. Samsung SDI has integrated these innovations with their established battery manufacturing infrastructure to develop prototype calcium ion cells that demonstrate improved energy density compared to conventional lithium-ion batteries.
Strengths: Extensive battery manufacturing expertise and established production infrastructure; comprehensive approach to calcium battery technology development; strong intellectual property portfolio in advanced battery materials. Weaknesses: Calcium technology still faces significant commercialization challenges compared to established battery chemistries; higher production costs for specialized materials; cycle life limitations compared to commercial lithium-ion systems.
SEI Formation Mechanisms and Critical Patents
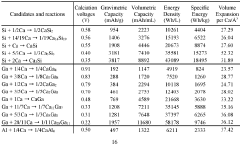
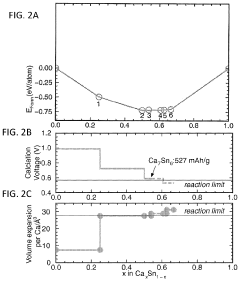
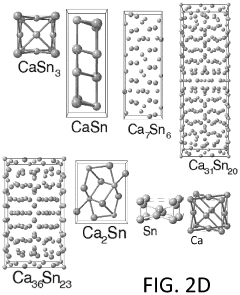
Calcium-metal alloy anode materials for reversible calcium-ion batteries
PatentWO2020086524A1
Innovation
- The use of intermetallic compounds comprising calcium with metals or metalloids such as Sb, As, Cu, Cd, Bi, Ag, Au, Pd, Pt, or Hg as anode materials, along with a calcium salt-based electrolyte, facilitates reversible calcium ion alloying and dealloying, and high-throughput density functional theory (DFT) screening identifies suitable candidates with high energy densities and constrained volume expansion.
Calcium-metal alloy anode materials for reversible calcium-ion batteries
PatentActiveUS11901550B2
Innovation
- The use of intermetallic compounds comprising calcium and metals or metalloids such as Sb, As, Cu, Cd, Bi, Ag, Au, Pd, Pt, or Hg as anodes, paired with a calcium salt-based electrolyte and a cathode material like graphite, allowing for reversible decalcination and calcination reactions, and employing high-throughput density functional theory (DFT) calculations to identify suitable anode materials with high energy density and constrained volume expansion.
Electrolyte Compatibility and Optimization Strategies
The compatibility between calcium metal anodes and electrolytes represents a critical challenge in calcium ion battery development. Conventional electrolytes often lead to passivation layers that block calcium ion transport, severely limiting reversible plating and stripping processes. Current research focuses on identifying electrolyte formulations that enable efficient calcium ion transport while maintaining electrochemical stability at the anode interface.
Ethereal solvents, particularly tetrahydrofuran (THF), have demonstrated promising compatibility with calcium metal anodes when paired with calcium tetrafluoroborate Ca(BF4)2 or calcium bis(trifluoromethanesulfonyl)imide Ca(TFSI)2 salts. These combinations facilitate relatively efficient calcium deposition and dissolution. However, the volatile nature of THF presents safety concerns and limits practical application in commercial batteries.
Carbonate-based electrolytes, while stable and commonly used in lithium-ion batteries, typically form resistive interfaces with calcium metal. Recent breakthroughs involve the addition of specific additives such as fluoroethylene carbonate (FEC) and vinylene carbonate (VC) that modify the solid electrolyte interphase (SEI) composition, potentially enabling improved calcium ion transport through the interface layer.
Optimization strategies increasingly focus on tailoring the solvation structure of calcium ions in the electrolyte. Research indicates that reducing the coordination number of solvent molecules around calcium ions can significantly enhance their mobility and reduce desolvation energy at the electrode interface. This approach has led to exploration of weakly coordinating anions and mixed solvent systems that disrupt the strong calcium-solvent interactions.
Temperature effects represent another critical optimization parameter, as elevated temperatures (40-60°C) have been shown to enhance calcium ion conductivity through otherwise passivating interfaces. This suggests potential pathways for room-temperature operation through interface engineering rather than solely focusing on electrolyte composition.
Concentrated electrolyte strategies, successful in lithium and sodium systems, are being adapted for calcium batteries. These high-salt-concentration formulations alter the solvation structure and interfacial chemistry, potentially enabling more efficient calcium ion transport. However, challenges remain regarding the limited solubility of calcium salts in most organic solvents.
Future optimization directions include the development of ionic liquid-based electrolytes with wide electrochemical windows and the incorporation of calcium-conducting solid electrolytes as protective layers on the anode surface. These approaches aim to circumvent the fundamental challenges of direct calcium metal-liquid electrolyte interfaces while maintaining the high energy density potential of calcium metal anodes.
Ethereal solvents, particularly tetrahydrofuran (THF), have demonstrated promising compatibility with calcium metal anodes when paired with calcium tetrafluoroborate Ca(BF4)2 or calcium bis(trifluoromethanesulfonyl)imide Ca(TFSI)2 salts. These combinations facilitate relatively efficient calcium deposition and dissolution. However, the volatile nature of THF presents safety concerns and limits practical application in commercial batteries.
Carbonate-based electrolytes, while stable and commonly used in lithium-ion batteries, typically form resistive interfaces with calcium metal. Recent breakthroughs involve the addition of specific additives such as fluoroethylene carbonate (FEC) and vinylene carbonate (VC) that modify the solid electrolyte interphase (SEI) composition, potentially enabling improved calcium ion transport through the interface layer.
Optimization strategies increasingly focus on tailoring the solvation structure of calcium ions in the electrolyte. Research indicates that reducing the coordination number of solvent molecules around calcium ions can significantly enhance their mobility and reduce desolvation energy at the electrode interface. This approach has led to exploration of weakly coordinating anions and mixed solvent systems that disrupt the strong calcium-solvent interactions.
Temperature effects represent another critical optimization parameter, as elevated temperatures (40-60°C) have been shown to enhance calcium ion conductivity through otherwise passivating interfaces. This suggests potential pathways for room-temperature operation through interface engineering rather than solely focusing on electrolyte composition.
Concentrated electrolyte strategies, successful in lithium and sodium systems, are being adapted for calcium batteries. These high-salt-concentration formulations alter the solvation structure and interfacial chemistry, potentially enabling more efficient calcium ion transport. However, challenges remain regarding the limited solubility of calcium salts in most organic solvents.
Future optimization directions include the development of ionic liquid-based electrolytes with wide electrochemical windows and the incorporation of calcium-conducting solid electrolytes as protective layers on the anode surface. These approaches aim to circumvent the fundamental challenges of direct calcium metal-liquid electrolyte interfaces while maintaining the high energy density potential of calcium metal anodes.
Sustainability and Resource Considerations
The sustainability profile of calcium-ion batteries represents a significant advantage over current lithium-ion technology. Calcium is the fifth most abundant element in the Earth's crust, with concentrations approximately 2,000 times higher than lithium. This abundance translates directly to lower extraction costs and reduced environmental impact compared to lithium mining operations, which often require extensive water usage and can lead to habitat disruption.
From a resource security perspective, calcium's widespread geographical distribution mitigates supply chain vulnerabilities that currently plague lithium-ion battery production. Unlike lithium, which is concentrated in the "Lithium Triangle" of South America and a few other regions, calcium resources are available across numerous countries, reducing geopolitical risks and potential market volatility.
The environmental footprint of calcium extraction is considerably smaller than that of lithium. Traditional lithium extraction from salt flats consumes between 500,000 to 2 million gallons of water per ton of lithium, whereas calcium can be sourced from limestone and other common minerals through established industrial processes with lower water requirements. This difference becomes increasingly important as water scarcity affects more regions globally.
Regarding end-of-life considerations, calcium-based battery systems potentially offer improved recyclability. The less reactive nature of calcium compounds compared to lithium materials could simplify recycling processes and reduce safety hazards during disassembly. However, the solid electrolyte interphase (SEI) formation in calcium batteries introduces new recycling challenges that require further research to address effectively.
Energy requirements for calcium processing are generally lower than those for lithium, contributing to a reduced carbon footprint across the battery lifecycle. Preliminary life cycle assessments suggest that calcium-ion batteries could achieve up to 30% lower greenhouse gas emissions compared to equivalent lithium-ion systems, though these figures depend heavily on the specific cathode materials paired with calcium anodes.
The development of stable SEI layers in calcium batteries may also contribute to longer cycle life, potentially reducing the frequency of battery replacement and associated resource consumption. Current research indicates that properly engineered calcium-based systems could theoretically achieve cycle lives comparable to or exceeding lithium-ion batteries, further enhancing their sustainability profile through extended service lifespans.
From a resource security perspective, calcium's widespread geographical distribution mitigates supply chain vulnerabilities that currently plague lithium-ion battery production. Unlike lithium, which is concentrated in the "Lithium Triangle" of South America and a few other regions, calcium resources are available across numerous countries, reducing geopolitical risks and potential market volatility.
The environmental footprint of calcium extraction is considerably smaller than that of lithium. Traditional lithium extraction from salt flats consumes between 500,000 to 2 million gallons of water per ton of lithium, whereas calcium can be sourced from limestone and other common minerals through established industrial processes with lower water requirements. This difference becomes increasingly important as water scarcity affects more regions globally.
Regarding end-of-life considerations, calcium-based battery systems potentially offer improved recyclability. The less reactive nature of calcium compounds compared to lithium materials could simplify recycling processes and reduce safety hazards during disassembly. However, the solid electrolyte interphase (SEI) formation in calcium batteries introduces new recycling challenges that require further research to address effectively.
Energy requirements for calcium processing are generally lower than those for lithium, contributing to a reduced carbon footprint across the battery lifecycle. Preliminary life cycle assessments suggest that calcium-ion batteries could achieve up to 30% lower greenhouse gas emissions compared to equivalent lithium-ion systems, though these figures depend heavily on the specific cathode materials paired with calcium anodes.
The development of stable SEI layers in calcium batteries may also contribute to longer cycle life, potentially reducing the frequency of battery replacement and associated resource consumption. Current research indicates that properly engineered calcium-based systems could theoretically achieve cycle lives comparable to or exceeding lithium-ion batteries, further enhancing their sustainability profile through extended service lifespans.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!