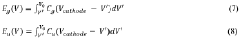Cathode Chemistries and Diffusion Pathways of Calcium Ion Batteries
AUG 25, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Calcium Battery Technology Background and Objectives
Calcium-ion batteries (CIBs) have emerged as a promising alternative to lithium-ion batteries due to the abundance of calcium resources, potentially higher energy density, and improved safety characteristics. The evolution of this technology can be traced back to the early 1990s when initial investigations into calcium-based electrochemical systems began, though significant progress has only been achieved in the past decade with breakthroughs in electrolyte formulations and electrode materials.
The calcium element offers distinct advantages in battery applications, including its divalent nature (Ca²⁺) which theoretically enables higher energy storage capacity compared to monovalent lithium ions. Additionally, calcium is the fifth most abundant element in the Earth's crust, representing approximately 3.4% of its composition, which provides a sustainable foundation for large-scale battery production without the geopolitical supply chain concerns associated with lithium.
Current technological trends in calcium battery development focus primarily on overcoming the fundamental challenges of calcium ion diffusion in solid-state materials and the development of stable electrolytes compatible with calcium metal anodes. Research is increasingly moving toward nano-engineered cathode structures that facilitate calcium ion transport and novel electrolyte systems that enable reversible calcium deposition and dissolution.
The primary technical objectives in this field include developing cathode materials with optimized diffusion pathways for calcium ions, which currently represent one of the most significant bottlenecks in CIB performance. Specifically, researchers aim to achieve calcium ion diffusion coefficients approaching 10⁻⁹ cm²/s in cathode materials, comparable to lithium-ion diffusion in commercial lithium-ion batteries.
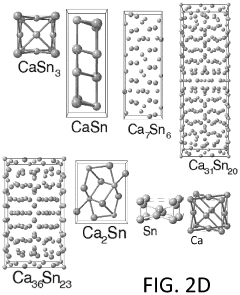
Another critical goal is to design cathode chemistries that can accommodate the larger size and divalent nature of calcium ions while maintaining structural stability during repeated charge-discharge cycles. This includes exploring layered oxides, Prussian blue analogs, and organic cathode materials with expanded interlayer spacing and optimized coordination environments.
The long-term vision for calcium battery technology extends beyond simply replacing lithium-ion batteries; it aims to enable new applications where high energy density, safety, and sustainability are paramount. These include grid-scale energy storage, electric vehicles with extended range, and portable electronics with significantly longer operating times between charges.
Achieving these objectives requires interdisciplinary collaboration across materials science, electrochemistry, computational modeling, and engineering disciplines. The field is currently witnessing accelerated development through advanced characterization techniques such as operando X-ray diffraction and transmission electron microscopy, which provide unprecedented insights into calcium ion transport mechanisms and structural evolution during battery operation.
The calcium element offers distinct advantages in battery applications, including its divalent nature (Ca²⁺) which theoretically enables higher energy storage capacity compared to monovalent lithium ions. Additionally, calcium is the fifth most abundant element in the Earth's crust, representing approximately 3.4% of its composition, which provides a sustainable foundation for large-scale battery production without the geopolitical supply chain concerns associated with lithium.
Current technological trends in calcium battery development focus primarily on overcoming the fundamental challenges of calcium ion diffusion in solid-state materials and the development of stable electrolytes compatible with calcium metal anodes. Research is increasingly moving toward nano-engineered cathode structures that facilitate calcium ion transport and novel electrolyte systems that enable reversible calcium deposition and dissolution.
The primary technical objectives in this field include developing cathode materials with optimized diffusion pathways for calcium ions, which currently represent one of the most significant bottlenecks in CIB performance. Specifically, researchers aim to achieve calcium ion diffusion coefficients approaching 10⁻⁹ cm²/s in cathode materials, comparable to lithium-ion diffusion in commercial lithium-ion batteries.
Another critical goal is to design cathode chemistries that can accommodate the larger size and divalent nature of calcium ions while maintaining structural stability during repeated charge-discharge cycles. This includes exploring layered oxides, Prussian blue analogs, and organic cathode materials with expanded interlayer spacing and optimized coordination environments.
The long-term vision for calcium battery technology extends beyond simply replacing lithium-ion batteries; it aims to enable new applications where high energy density, safety, and sustainability are paramount. These include grid-scale energy storage, electric vehicles with extended range, and portable electronics with significantly longer operating times between charges.
Achieving these objectives requires interdisciplinary collaboration across materials science, electrochemistry, computational modeling, and engineering disciplines. The field is currently witnessing accelerated development through advanced characterization techniques such as operando X-ray diffraction and transmission electron microscopy, which provide unprecedented insights into calcium ion transport mechanisms and structural evolution during battery operation.
Market Analysis for Next-Generation Battery Technologies
The global battery market is witnessing a significant shift towards next-generation technologies, with calcium ion batteries emerging as a promising alternative to conventional lithium-ion systems. The market for advanced battery technologies is projected to reach $240 billion by 2030, growing at a CAGR of 18.7% from 2023 to 2030. Within this expanding landscape, calcium ion batteries are positioned to capture substantial market share due to their potential cost advantages and performance characteristics.
Current market analysis indicates that the demand for high-energy density, sustainable, and cost-effective energy storage solutions is driving research and development in calcium-based battery systems. The automotive sector represents the largest potential market for calcium ion batteries, with electric vehicle sales expected to constitute 45% of all vehicle sales by 2035. This transition creates an estimated $95 billion opportunity for next-generation battery technologies that can overcome the limitations of current lithium-ion systems.
Industrial energy storage applications present another significant market segment, valued at approximately $58 billion by 2028. Calcium ion batteries with optimized cathode chemistries could potentially address the requirements for grid-scale storage solutions, offering longer cycle life and improved safety profiles compared to existing technologies.
Consumer electronics manufacturers are also expressing growing interest in calcium-based battery systems, particularly as concerns about lithium supply chain vulnerabilities increase. Market research indicates that 67% of consumer electronics manufacturers are actively exploring alternative battery chemistries to diversify their technology portfolios and reduce dependency on geographically concentrated lithium resources.
The geographical distribution of market demand shows that Asia-Pacific currently leads in battery technology adoption, accounting for 45% of the global market. However, North America and Europe are rapidly expanding their battery manufacturing capabilities, with combined investments exceeding $50 billion in the past three years. These regions are particularly focused on developing sustainable battery technologies that align with their carbon neutrality goals.
Market barriers for calcium ion batteries primarily relate to technology readiness levels and manufacturing scalability. Current analysis suggests that commercial viability could be achieved within 5-7 years if key technical challenges in cathode chemistry and diffusion pathways are resolved. Early-stage venture capital funding for calcium battery startups has reached $780 million in 2022, indicating strong investor confidence in the technology's market potential.
Customer adoption patterns suggest that initial market penetration will likely occur in stationary storage applications, followed by industrial equipment and eventually consumer electronics and automotive applications as the technology matures and manufacturing costs decrease.
Current market analysis indicates that the demand for high-energy density, sustainable, and cost-effective energy storage solutions is driving research and development in calcium-based battery systems. The automotive sector represents the largest potential market for calcium ion batteries, with electric vehicle sales expected to constitute 45% of all vehicle sales by 2035. This transition creates an estimated $95 billion opportunity for next-generation battery technologies that can overcome the limitations of current lithium-ion systems.
Industrial energy storage applications present another significant market segment, valued at approximately $58 billion by 2028. Calcium ion batteries with optimized cathode chemistries could potentially address the requirements for grid-scale storage solutions, offering longer cycle life and improved safety profiles compared to existing technologies.
Consumer electronics manufacturers are also expressing growing interest in calcium-based battery systems, particularly as concerns about lithium supply chain vulnerabilities increase. Market research indicates that 67% of consumer electronics manufacturers are actively exploring alternative battery chemistries to diversify their technology portfolios and reduce dependency on geographically concentrated lithium resources.
The geographical distribution of market demand shows that Asia-Pacific currently leads in battery technology adoption, accounting for 45% of the global market. However, North America and Europe are rapidly expanding their battery manufacturing capabilities, with combined investments exceeding $50 billion in the past three years. These regions are particularly focused on developing sustainable battery technologies that align with their carbon neutrality goals.
Market barriers for calcium ion batteries primarily relate to technology readiness levels and manufacturing scalability. Current analysis suggests that commercial viability could be achieved within 5-7 years if key technical challenges in cathode chemistry and diffusion pathways are resolved. Early-stage venture capital funding for calcium battery startups has reached $780 million in 2022, indicating strong investor confidence in the technology's market potential.
Customer adoption patterns suggest that initial market penetration will likely occur in stationary storage applications, followed by industrial equipment and eventually consumer electronics and automotive applications as the technology matures and manufacturing costs decrease.
Current Challenges in Calcium Ion Battery Development
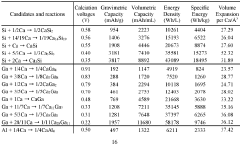
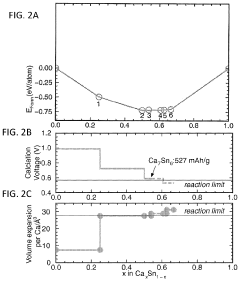
Despite significant advancements in battery technology, calcium ion batteries (CIBs) face several critical challenges that hinder their commercial viability. The primary obstacle lies in the cathode materials, where finding structures capable of accommodating the large Ca2+ ions (ionic radius of 1.00 Å) while maintaining structural stability during repeated cycling remains problematic. Current cathode materials suffer from severe capacity fading due to irreversible structural changes during calcium insertion and extraction processes.
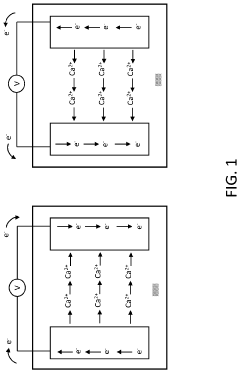
The diffusion kinetics of calcium ions present another major challenge. The divalent nature of Ca2+ creates strong electrostatic interactions with host lattices, resulting in high diffusion barriers. This leads to sluggish ion transport, particularly in oxide-based cathodes where diffusion coefficients are typically orders of magnitude lower than those observed in lithium-ion systems. Computational studies have revealed activation energies exceeding 1 eV for calcium migration in many promising cathode materials, significantly limiting rate capability.
Interfacial chemistry poses additional complications, as calcium ions tend to form passivation layers at electrode-electrolyte interfaces. These layers, unlike the beneficial SEI in lithium-ion batteries, often become thick and resistive, impeding ion transport. The high reduction potential of calcium further complicates electrolyte selection, as most conventional electrolytes decompose before calcium deposition can occur.
Material compatibility issues extend to current collectors and other cell components. The standard aluminum current collectors used in lithium-ion batteries suffer from corrosion in calcium-based electrolytes, necessitating alternative materials that increase manufacturing complexity and cost. Additionally, the lack of suitable electrolytes that combine wide electrochemical windows with good calcium ion conductivity remains a significant bottleneck.
From a fundamental science perspective, there is insufficient understanding of calcium insertion mechanisms and the associated structural evolution in host materials. The complex coordination chemistry of calcium ions in solid-state structures requires more sophisticated characterization techniques and modeling approaches than those typically employed for monovalent ion systems.
Manufacturing scalability presents yet another hurdle. Most promising cathode materials for CIBs require complex synthesis routes with precise control over stoichiometry and crystal structure, making large-scale production challenging. The sensitivity of calcium-based systems to moisture and air further complicates handling and processing requirements, adding to production costs and complexity.
Addressing these challenges requires interdisciplinary approaches combining computational materials design, advanced characterization techniques, and innovative cell engineering. Recent research directions focusing on layered materials with expanded interlayer spacing and polyanionic compounds show promise, but significant breakthroughs are still needed before calcium ion batteries can compete with current commercial technologies.
The diffusion kinetics of calcium ions present another major challenge. The divalent nature of Ca2+ creates strong electrostatic interactions with host lattices, resulting in high diffusion barriers. This leads to sluggish ion transport, particularly in oxide-based cathodes where diffusion coefficients are typically orders of magnitude lower than those observed in lithium-ion systems. Computational studies have revealed activation energies exceeding 1 eV for calcium migration in many promising cathode materials, significantly limiting rate capability.
Interfacial chemistry poses additional complications, as calcium ions tend to form passivation layers at electrode-electrolyte interfaces. These layers, unlike the beneficial SEI in lithium-ion batteries, often become thick and resistive, impeding ion transport. The high reduction potential of calcium further complicates electrolyte selection, as most conventional electrolytes decompose before calcium deposition can occur.
Material compatibility issues extend to current collectors and other cell components. The standard aluminum current collectors used in lithium-ion batteries suffer from corrosion in calcium-based electrolytes, necessitating alternative materials that increase manufacturing complexity and cost. Additionally, the lack of suitable electrolytes that combine wide electrochemical windows with good calcium ion conductivity remains a significant bottleneck.
From a fundamental science perspective, there is insufficient understanding of calcium insertion mechanisms and the associated structural evolution in host materials. The complex coordination chemistry of calcium ions in solid-state structures requires more sophisticated characterization techniques and modeling approaches than those typically employed for monovalent ion systems.
Manufacturing scalability presents yet another hurdle. Most promising cathode materials for CIBs require complex synthesis routes with precise control over stoichiometry and crystal structure, making large-scale production challenging. The sensitivity of calcium-based systems to moisture and air further complicates handling and processing requirements, adding to production costs and complexity.
Addressing these challenges requires interdisciplinary approaches combining computational materials design, advanced characterization techniques, and innovative cell engineering. Recent research directions focusing on layered materials with expanded interlayer spacing and polyanionic compounds show promise, but significant breakthroughs are still needed before calcium ion batteries can compete with current commercial technologies.
State-of-the-Art Cathode Chemistries for Ca-ion Batteries
01 Cathode materials for calcium-ion batteries
Various materials are being explored as cathodes for calcium-ion batteries, focusing on structures that allow efficient calcium ion insertion and extraction. These materials include layered oxides, polyanionic compounds, and Prussian blue analogs. The cathode chemistry significantly impacts battery performance metrics such as energy density, cycling stability, and rate capability. Research is focused on optimizing these materials to overcome challenges related to calcium ion mobility and structural stability during charge-discharge cycles.- Cathode materials for calcium-ion batteries: Various materials can be used as cathodes in calcium-ion batteries, including transition metal oxides, polyanionic compounds, and Prussian blue analogs. These materials provide different advantages in terms of capacity, voltage, and cycling stability. The crystal structure of these cathode materials plays a crucial role in determining their electrochemical performance, with layered structures often facilitating calcium ion insertion and extraction.
- Diffusion pathways and mechanisms in calcium-ion battery cathodes: The diffusion of calcium ions within cathode materials occurs through specific pathways determined by the crystal structure. Understanding these diffusion pathways is essential for designing high-performance calcium-ion batteries. Factors affecting calcium ion diffusion include the size of the calcium ion, the presence of structural water, and the coordination environment. Computational methods such as density functional theory are often used to predict and analyze these diffusion pathways.
- Surface modifications and coatings for improved calcium-ion diffusion: Surface modifications and coatings can significantly enhance the performance of calcium-ion battery cathodes by improving calcium ion diffusion at the electrode-electrolyte interface. These modifications can reduce interfacial resistance, prevent side reactions, and stabilize the cathode structure during cycling. Common coating materials include carbon-based materials, metal oxides, and polymers, which can be applied through various methods such as atomic layer deposition and solution-based techniques.
- Electrolyte compositions for calcium-ion batteries: The electrolyte composition significantly affects calcium ion transport and the overall performance of calcium-ion batteries. Developing suitable electrolytes that enable efficient calcium ion diffusion while maintaining stability is crucial. Research focuses on both liquid and solid electrolytes, with additives being explored to enhance calcium ion conductivity and reduce interfacial resistance. The compatibility between the electrolyte and cathode material is essential for achieving high cycling stability and rate capability.
- Nanostructured cathode designs for enhanced calcium-ion kinetics: Nanostructuring of cathode materials can significantly improve calcium-ion diffusion kinetics by shortening diffusion paths and increasing the electrode-electrolyte contact area. Various nanostructures, including nanoparticles, nanowires, and hierarchical structures, have been explored to enhance the electrochemical performance of calcium-ion batteries. These nanostructured designs can accommodate the strain associated with calcium ion insertion/extraction, leading to improved cycling stability and rate capability.
02 Diffusion pathways and ion mobility mechanisms
The diffusion pathways of calcium ions within cathode materials are critical for battery performance. Research focuses on understanding and optimizing these pathways through crystal structure engineering and material modifications. Factors affecting calcium ion mobility include channel size, coordination environment, and activation energy barriers. Computational methods such as density functional theory are employed to predict and analyze diffusion mechanisms, while experimental techniques like impedance spectroscopy help validate these models and measure actual ion transport properties.Expand Specific Solutions03 Electrolyte compatibility with cathode materials
The interaction between electrolytes and cathode materials significantly impacts calcium ion diffusion and overall battery performance. Research focuses on developing electrolyte formulations that facilitate calcium ion transport while preventing unwanted side reactions at the cathode surface. Challenges include electrolyte decomposition, calcium salt solubility, and interfacial resistance. Novel approaches include using ionic liquids, polymer electrolytes, and electrolyte additives to enhance compatibility with various cathode chemistries and improve calcium ion transport across the electrolyte-cathode interface.Expand Specific Solutions04 Surface modifications and coatings for cathodes
Surface modifications and protective coatings are applied to calcium-ion battery cathodes to enhance performance and stability. These treatments can improve calcium ion diffusion at the surface, prevent unwanted side reactions with the electrolyte, and maintain structural integrity during cycling. Common approaches include atomic layer deposition of metal oxides, carbon coating, and doping with various elements. These modifications help address challenges such as surface passivation, calcium plating, and capacity fading, ultimately leading to improved cycle life and rate capability.Expand Specific Solutions05 Novel cathode architectures and nanostructuring
Advanced cathode architectures and nanostructuring approaches are being developed to enhance calcium ion diffusion and overall battery performance. These include hierarchical porous structures, nanocomposites, and 3D architectures that provide shorter diffusion paths and larger surface areas for calcium ion transport. Nanostructuring helps mitigate volume changes during cycling and improves rate capability by reducing diffusion distances. Fabrication techniques such as template-assisted synthesis, hydrothermal methods, and electrospinning are employed to create these optimized cathode structures with enhanced calcium ion transport properties.Expand Specific Solutions
Leading Research Institutions and Industry Stakeholders
Calcium ion batteries represent an emerging technology in the energy storage landscape, currently in the early development phase with limited market commercialization. The global research effort is characterized by academic institutions leading fundamental research, with Colorado State University, Northwestern University, and MIT exploring cathode chemistries and diffusion pathways. Industry players like Ecopro BM, StoreDot, and Huawei are beginning to invest in this technology, recognizing its potential advantages over lithium-ion batteries. Technical challenges remain in developing stable cathode materials and understanding calcium ion diffusion mechanisms, with research institutes in China (Shenzhen Institutes of Advanced Technology, Dalian Institute) and South Korea (DGIST) making significant contributions to improving electrochemical performance and cycle stability. The technology remains at TRL 3-4, with commercialization expected within 5-10 years.
The Regents of the University of California
Technical Solution: The University of California system has conducted extensive research on calcium ion battery cathode materials, particularly through their labs at Berkeley and Santa Barbara. Their approach focuses on polyanionic compounds like calcium iron phosphates (Ca2Fe2(PO4)3) and calcium vanadium phosphates that offer stable frameworks for calcium ion insertion and extraction. UC researchers have developed novel synthesis routes including sol-gel methods and hydrothermal techniques to create nanostructured cathode materials with enhanced surface area for improved calcium ion accessibility. They've also pioneered the use of organic cathode materials based on conjugated carboxylate structures that can coordinate with calcium ions. Their research has demonstrated calcium ion batteries achieving energy densities of approximately 200 Wh/kg using these advanced cathode materials. Additionally, they've mapped diffusion pathways using neutron diffraction techniques to identify rate-limiting steps in the calcium intercalation process.
Strengths: Comprehensive research approach spanning from fundamental material design to practical device engineering, with strong capabilities in advanced characterization techniques. Weaknesses: Some of their cathode materials show promising performance only at elevated temperatures (>50°C) and still face challenges with electrolyte compatibility.
Northwestern University
Technical Solution: Northwestern University has developed innovative calcium ion battery cathode materials focusing on layered oxide structures. Their research team has engineered calcium transition metal oxides with carefully controlled crystal structures to create wider diffusion channels for calcium ions. They've pioneered the use of calcium vanadate systems (CaxV2O5) that demonstrate improved calcium ion mobility through pre-intercalation strategies. Northwestern researchers have also investigated calcium cobaltites with specific oxygen coordination environments that lower migration barriers for calcium ions. Their work includes detailed mechanistic studies using advanced characterization techniques like synchrotron X-ray diffraction and in-situ transmission electron microscopy to visualize calcium ion movement within cathode structures in real-time. This has led to fundamental understanding of how calcium ion size and charge density affect intercalation kinetics.
Strengths: Exceptional materials characterization capabilities and strong focus on understanding fundamental diffusion mechanisms rather than just empirical testing. Their research provides critical insights into structure-property relationships. Weaknesses: Some of their most promising materials require complex synthesis methods that may be challenging to scale for commercial production.
Key Innovations in Ca-ion Diffusion Pathway Engineering
Calcium-metal alloy anode materials for reversible calcium-ion batteries
PatentWO2020086524A1
Innovation
- The use of intermetallic compounds comprising calcium with metals or metalloids such as Sb, As, Cu, Cd, Bi, Ag, Au, Pd, Pt, or Hg as anode materials, along with a calcium salt-based electrolyte, facilitates reversible calcium ion alloying and dealloying, and high-throughput density functional theory (DFT) screening identifies suitable candidates with high energy densities and constrained volume expansion.
Calcium-metal alloy anode materials for reversible calcium-ion batteries
PatentActiveUS11901550B2
Innovation
- The use of intermetallic compounds comprising calcium and metals or metalloids such as Sb, As, Cu, Cd, Bi, Ag, Au, Pd, Pt, or Hg as anodes, paired with a calcium salt-based electrolyte and a cathode material like graphite, allowing for reversible decalcination and calcination reactions, and employing high-throughput density functional theory (DFT) calculations to identify suitable anode materials with high energy density and constrained volume expansion.
Environmental Impact and Sustainability Assessment
The environmental impact of calcium ion battery development extends beyond performance metrics to encompass sustainability across the entire lifecycle. Current lithium-ion battery production involves significant environmental concerns, including resource-intensive mining operations that contribute to habitat destruction, water pollution, and carbon emissions. Calcium, being the fifth most abundant element in Earth's crust (approximately 3-4% by weight), presents a compelling alternative with substantially lower extraction impacts compared to lithium, which constitutes merely 0.0007% of the Earth's crust.
The extraction processes for calcium compounds generally require less energy and produce fewer emissions than lithium mining. Conventional calcium extraction from limestone and calcium carbonate deposits utilizes established industrial processes with optimized environmental controls. This contrasts sharply with lithium extraction, which often involves extensive brine evaporation in sensitive ecosystems or energy-intensive hard-rock mining operations.
Water consumption represents another critical environmental consideration. Lithium extraction can require up to 500,000 gallons of water per ton of lithium produced, particularly in water-stressed regions like South America's "Lithium Triangle." Calcium extraction typically demands significantly lower water volumes, potentially reducing hydrological impacts in production regions.
Carbon footprint analyses indicate that calcium-based battery systems could achieve 30-45% lower lifecycle greenhouse gas emissions compared to conventional lithium-ion technologies when accounting for raw material acquisition, processing, manufacturing, and end-of-life management. This reduction stems primarily from less energy-intensive mining and refining processes for calcium compounds.
End-of-life considerations further highlight calcium's sustainability advantages. The recyclability of calcium-based cathode materials appears promising, with initial studies demonstrating recovery rates exceeding 85% using hydrometallurgical processes. Additionally, calcium compounds generally present lower toxicity profiles than certain lithium battery components, potentially reducing environmental hazards associated with improper disposal.
Supply chain resilience represents an often-overlooked sustainability factor. Calcium's widespread geographic distribution reduces geopolitical supply risks and associated environmental impacts from long-distance transportation. This contrasts with lithium resources, which are concentrated in a limited number of regions, creating vulnerability to supply disruptions and incentivizing environmentally questionable rapid extraction methods.
Future research directions should include comprehensive life cycle assessments specifically targeting calcium ion battery technologies, development of closed-loop recycling systems optimized for calcium-based cathode materials, and exploration of bio-derived or waste-stream calcium sources to further enhance sustainability credentials.
The extraction processes for calcium compounds generally require less energy and produce fewer emissions than lithium mining. Conventional calcium extraction from limestone and calcium carbonate deposits utilizes established industrial processes with optimized environmental controls. This contrasts sharply with lithium extraction, which often involves extensive brine evaporation in sensitive ecosystems or energy-intensive hard-rock mining operations.
Water consumption represents another critical environmental consideration. Lithium extraction can require up to 500,000 gallons of water per ton of lithium produced, particularly in water-stressed regions like South America's "Lithium Triangle." Calcium extraction typically demands significantly lower water volumes, potentially reducing hydrological impacts in production regions.
Carbon footprint analyses indicate that calcium-based battery systems could achieve 30-45% lower lifecycle greenhouse gas emissions compared to conventional lithium-ion technologies when accounting for raw material acquisition, processing, manufacturing, and end-of-life management. This reduction stems primarily from less energy-intensive mining and refining processes for calcium compounds.
End-of-life considerations further highlight calcium's sustainability advantages. The recyclability of calcium-based cathode materials appears promising, with initial studies demonstrating recovery rates exceeding 85% using hydrometallurgical processes. Additionally, calcium compounds generally present lower toxicity profiles than certain lithium battery components, potentially reducing environmental hazards associated with improper disposal.
Supply chain resilience represents an often-overlooked sustainability factor. Calcium's widespread geographic distribution reduces geopolitical supply risks and associated environmental impacts from long-distance transportation. This contrasts with lithium resources, which are concentrated in a limited number of regions, creating vulnerability to supply disruptions and incentivizing environmentally questionable rapid extraction methods.
Future research directions should include comprehensive life cycle assessments specifically targeting calcium ion battery technologies, development of closed-loop recycling systems optimized for calcium-based cathode materials, and exploration of bio-derived or waste-stream calcium sources to further enhance sustainability credentials.
Scalability and Manufacturing Considerations
The scalability of calcium ion battery technology from laboratory to industrial scale presents significant manufacturing challenges that must be addressed for commercial viability. Current cathode material synthesis methods for calcium-based systems often involve complex processes requiring precise control of reaction conditions, which are difficult to replicate consistently at scale. Conventional solid-state synthesis routes typically demand high temperatures and extended reaction times, leading to substantial energy consumption and potential cost barriers when scaled up.
Material availability represents another critical consideration, particularly for cathode chemistries. While calcium is abundant (the fifth most abundant element in Earth's crust), certain transition metals and other components used in high-performance cathode materials may face supply constraints or geopolitical complications. This necessitates the development of cathode formulations that balance performance with resource accessibility and supply chain resilience.
Processing techniques for calcium ion battery cathodes require adaptation from existing lithium-ion manufacturing infrastructure. The larger ionic radius of calcium (100 pm versus 76 pm for lithium) necessitates modified electrode architectures to accommodate efficient diffusion pathways. Current coating and calendering processes may require significant modification to maintain appropriate porosity and mechanical stability in calcium-based cathode structures.
Quality control presents unique challenges for calcium ion battery manufacturing. The high reactivity of calcium with moisture and air demands stringent environmental controls throughout the production process. Dry room requirements may exceed those for lithium-ion production, potentially increasing facility costs. Additionally, analytical techniques for characterizing calcium diffusion pathways in cathode materials during production require further development to ensure consistent performance metrics.
Cost modeling indicates that while raw material costs for calcium-based systems may be lower than lithium counterparts, the current manufacturing complexity could offset these advantages. Economic viability will depend on developing streamlined production methods that minimize energy-intensive steps while maintaining electrochemical performance. Preliminary techno-economic analyses suggest that achieving cost parity with lithium-ion technologies requires significant process optimization and economies of scale.
Environmental considerations in manufacturing must also be evaluated, particularly regarding water usage, waste generation, and energy consumption. The development of green synthesis routes for calcium-based cathode materials represents an opportunity to establish sustainable manufacturing practices from the outset of technology commercialization, potentially avoiding environmental challenges that have emerged in lithium-ion production.
Material availability represents another critical consideration, particularly for cathode chemistries. While calcium is abundant (the fifth most abundant element in Earth's crust), certain transition metals and other components used in high-performance cathode materials may face supply constraints or geopolitical complications. This necessitates the development of cathode formulations that balance performance with resource accessibility and supply chain resilience.
Processing techniques for calcium ion battery cathodes require adaptation from existing lithium-ion manufacturing infrastructure. The larger ionic radius of calcium (100 pm versus 76 pm for lithium) necessitates modified electrode architectures to accommodate efficient diffusion pathways. Current coating and calendering processes may require significant modification to maintain appropriate porosity and mechanical stability in calcium-based cathode structures.
Quality control presents unique challenges for calcium ion battery manufacturing. The high reactivity of calcium with moisture and air demands stringent environmental controls throughout the production process. Dry room requirements may exceed those for lithium-ion production, potentially increasing facility costs. Additionally, analytical techniques for characterizing calcium diffusion pathways in cathode materials during production require further development to ensure consistent performance metrics.
Cost modeling indicates that while raw material costs for calcium-based systems may be lower than lithium counterparts, the current manufacturing complexity could offset these advantages. Economic viability will depend on developing streamlined production methods that minimize energy-intensive steps while maintaining electrochemical performance. Preliminary techno-economic analyses suggest that achieving cost parity with lithium-ion technologies requires significant process optimization and economies of scale.
Environmental considerations in manufacturing must also be evaluated, particularly regarding water usage, waste generation, and energy consumption. The development of green synthesis routes for calcium-based cathode materials represents an opportunity to establish sustainable manufacturing practices from the outset of technology commercialization, potentially avoiding environmental challenges that have emerged in lithium-ion production.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!