Electrolyte Additives and Passivation Chemistry in Calcium Ion Batteries
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Calcium Battery Electrolyte Development Background and Objectives
Calcium-ion batteries (CIBs) have emerged as a promising alternative to lithium-ion batteries due to the abundance of calcium resources, potentially higher energy density, and improved safety characteristics. The development of calcium battery technology can be traced back to the 1960s when initial attempts to utilize calcium metal anodes were made. However, progress was hindered by significant challenges, particularly the formation of passivation layers on calcium metal surfaces and the lack of suitable electrolytes that could facilitate reversible calcium deposition and dissolution.
The evolution of calcium battery technology has accelerated in the past decade, driven by increasing demands for sustainable and high-performance energy storage solutions. Research has shifted from primary to secondary calcium batteries, with a particular focus on developing electrolytes that can enable efficient calcium ion transport. The breakthrough came in 2015 when researchers demonstrated reversible calcium plating and stripping at elevated temperatures, marking a significant milestone in the field.
Current technological trends in calcium battery development are centered around electrolyte engineering, with particular emphasis on additives that can modify the passivation layer chemistry. The scientific community has recognized that understanding and controlling the solid electrolyte interphase (SEI) formation is crucial for achieving practical calcium batteries. This has led to increased research efforts in developing novel electrolyte formulations with functional additives that can promote favorable passivation characteristics.
The primary technical objectives in this field include developing electrolytes with wide electrochemical stability windows, high ionic conductivity, and compatibility with calcium metal anodes. Researchers aim to identify additives that can form stable and calcium-ion conductive passivation layers, preventing continuous electrolyte decomposition while allowing efficient calcium ion transport. Additionally, there is a focus on understanding the fundamental mechanisms of additive interactions with electrode surfaces and their role in SEI formation.
Another critical goal is to achieve room-temperature operation of calcium batteries with high Coulombic efficiency and long cycle life. This requires electrolyte systems that can effectively suppress dendrite formation and minimize side reactions. The development of computational models to predict electrolyte-additive behaviors and their impact on passivation layer properties is also becoming increasingly important in accelerating the discovery of optimal formulations.
The ultimate objective of this research direction is to establish design principles for calcium battery electrolytes that can guide the systematic development of next-generation energy storage systems with performance metrics comparable or superior to current lithium-ion technologies, while offering advantages in terms of sustainability, safety, and cost.
The evolution of calcium battery technology has accelerated in the past decade, driven by increasing demands for sustainable and high-performance energy storage solutions. Research has shifted from primary to secondary calcium batteries, with a particular focus on developing electrolytes that can enable efficient calcium ion transport. The breakthrough came in 2015 when researchers demonstrated reversible calcium plating and stripping at elevated temperatures, marking a significant milestone in the field.
Current technological trends in calcium battery development are centered around electrolyte engineering, with particular emphasis on additives that can modify the passivation layer chemistry. The scientific community has recognized that understanding and controlling the solid electrolyte interphase (SEI) formation is crucial for achieving practical calcium batteries. This has led to increased research efforts in developing novel electrolyte formulations with functional additives that can promote favorable passivation characteristics.
The primary technical objectives in this field include developing electrolytes with wide electrochemical stability windows, high ionic conductivity, and compatibility with calcium metal anodes. Researchers aim to identify additives that can form stable and calcium-ion conductive passivation layers, preventing continuous electrolyte decomposition while allowing efficient calcium ion transport. Additionally, there is a focus on understanding the fundamental mechanisms of additive interactions with electrode surfaces and their role in SEI formation.
Another critical goal is to achieve room-temperature operation of calcium batteries with high Coulombic efficiency and long cycle life. This requires electrolyte systems that can effectively suppress dendrite formation and minimize side reactions. The development of computational models to predict electrolyte-additive behaviors and their impact on passivation layer properties is also becoming increasingly important in accelerating the discovery of optimal formulations.
The ultimate objective of this research direction is to establish design principles for calcium battery electrolytes that can guide the systematic development of next-generation energy storage systems with performance metrics comparable or superior to current lithium-ion technologies, while offering advantages in terms of sustainability, safety, and cost.
Market Analysis for Next-Generation Battery Technologies
The global battery market is experiencing unprecedented growth, with next-generation battery technologies positioned as critical enablers for renewable energy integration, electric mobility, and portable electronics advancement. Calcium ion batteries (CIBs) represent an emerging segment within this landscape, attracting increasing attention as a potential alternative to lithium-ion technology due to calcium's abundance, safety profile, and theoretical performance capabilities.
Market projections indicate that the advanced battery sector is expected to reach $240 billion by 2027, growing at a CAGR of approximately 14% from 2022. Within this broader market, post-lithium technologies including calcium-based systems are anticipated to capture growing market share as research advances and commercial viability improves. The electrolyte additives sub-segment specifically represents a specialized but rapidly expanding market valued at $2.1 billion in 2022.
Consumer electronics currently dominate battery demand, accounting for 43% of the market, followed by automotive applications at 32% and industrial uses at 25%. However, the fastest growth is occurring in electric vehicle applications, where concerns about lithium supply chain vulnerabilities and cost volatility are driving interest in alternative chemistries like calcium-based systems.
Regional analysis reveals that Asia-Pacific leads battery technology development and manufacturing, with China, Japan, and South Korea collectively controlling over 70% of production capacity. However, significant R&D investments in North America and Europe are creating new innovation hubs focused on next-generation chemistries, including substantial research into calcium ion battery technologies.
Market drivers for electrolyte additives and passivation chemistry research include increasing energy density requirements, extended cycle life demands, and growing safety concerns. The push toward fast-charging capabilities represents another significant market pull, with consumers and industries alike demanding batteries that can reach 80% capacity in under 15 minutes.
Competitive analysis indicates that major battery manufacturers are establishing dedicated research divisions for post-lithium technologies, with several filing patents specifically related to calcium ion battery electrolyte formulations. Venture capital funding for startups focused on novel electrolyte solutions has reached $1.8 billion in 2022, a 65% increase from the previous year.
Market barriers include technical challenges in developing stable calcium-compatible electrolytes, manufacturing scalability concerns, and competition from other emerging battery technologies such as sodium-ion and solid-state systems. Nevertheless, the potential cost advantages and sustainability benefits of calcium-based systems continue to drive market interest and research investment.
Market projections indicate that the advanced battery sector is expected to reach $240 billion by 2027, growing at a CAGR of approximately 14% from 2022. Within this broader market, post-lithium technologies including calcium-based systems are anticipated to capture growing market share as research advances and commercial viability improves. The electrolyte additives sub-segment specifically represents a specialized but rapidly expanding market valued at $2.1 billion in 2022.
Consumer electronics currently dominate battery demand, accounting for 43% of the market, followed by automotive applications at 32% and industrial uses at 25%. However, the fastest growth is occurring in electric vehicle applications, where concerns about lithium supply chain vulnerabilities and cost volatility are driving interest in alternative chemistries like calcium-based systems.
Regional analysis reveals that Asia-Pacific leads battery technology development and manufacturing, with China, Japan, and South Korea collectively controlling over 70% of production capacity. However, significant R&D investments in North America and Europe are creating new innovation hubs focused on next-generation chemistries, including substantial research into calcium ion battery technologies.
Market drivers for electrolyte additives and passivation chemistry research include increasing energy density requirements, extended cycle life demands, and growing safety concerns. The push toward fast-charging capabilities represents another significant market pull, with consumers and industries alike demanding batteries that can reach 80% capacity in under 15 minutes.
Competitive analysis indicates that major battery manufacturers are establishing dedicated research divisions for post-lithium technologies, with several filing patents specifically related to calcium ion battery electrolyte formulations. Venture capital funding for startups focused on novel electrolyte solutions has reached $1.8 billion in 2022, a 65% increase from the previous year.
Market barriers include technical challenges in developing stable calcium-compatible electrolytes, manufacturing scalability concerns, and competition from other emerging battery technologies such as sodium-ion and solid-state systems. Nevertheless, the potential cost advantages and sustainability benefits of calcium-based systems continue to drive market interest and research investment.
Current Challenges in Calcium Ion Battery Electrolytes
Despite significant advancements in battery technology, calcium ion batteries face several critical electrolyte-related challenges that hinder their commercial viability. The primary obstacle lies in the development of suitable electrolytes that can facilitate efficient calcium ion transport while maintaining stability during cycling. Conventional carbonate-based electrolytes, which perform well in lithium-ion systems, demonstrate poor calcium ion conductivity and suffer from rapid degradation when applied to calcium battery systems.
The high charge density of calcium ions (Ca²⁺) creates strong coordination with solvent molecules and anions, resulting in large solvation shells that impede ion mobility through electrolyte solutions and across electrode interfaces. This phenomenon significantly reduces ionic conductivity and increases the energy barrier for desolvation at electrode surfaces, leading to sluggish kinetics during charging and discharging processes.
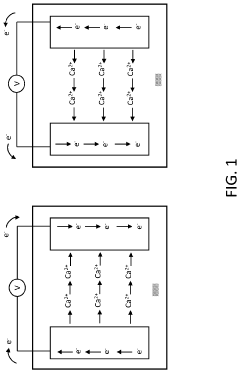
Another major challenge is the formation of passivation layers on calcium metal anodes. Unlike the beneficial solid electrolyte interphase (SEI) in lithium-ion batteries, calcium-based systems often develop non-uniform, resistive passivation films that block ion transport. These layers typically consist of CaF₂, CaCO₃, and other insoluble calcium compounds that form through reactions between the calcium metal and electrolyte components, particularly at high voltages.
Electrolyte stability presents an additional hurdle, as many potential calcium electrolytes exhibit narrow electrochemical stability windows. This limitation restricts the operating voltage range of calcium batteries and consequently their energy density. The decomposition of electrolytes at higher voltages not only compromises battery performance but also generates gas evolution and safety concerns.
Calcium salt solubility in organic solvents remains problematic, with many conventional calcium salts showing limited solubility compared to their lithium counterparts. This constraint restricts the concentration of charge carriers in the electrolyte, directly affecting power capability and rate performance of calcium batteries.
Water contamination poses a particular challenge for calcium-based systems, as even trace amounts of moisture can trigger parasitic reactions with both the electrolyte and electrode materials. These reactions accelerate capacity fading and reduce cycle life, necessitating stringent manufacturing conditions that add complexity and cost to production processes.
Current research efforts focus on developing novel electrolyte formulations incorporating specialized additives to address these challenges. Boron-based compounds, fluorinated additives, and chelating agents are being investigated for their ability to modify solvation structures, stabilize interfaces, and enhance calcium ion transport properties. However, a comprehensive understanding of the complex passivation chemistry and additive mechanisms remains elusive, highlighting the need for advanced characterization techniques and computational modeling approaches.
The high charge density of calcium ions (Ca²⁺) creates strong coordination with solvent molecules and anions, resulting in large solvation shells that impede ion mobility through electrolyte solutions and across electrode interfaces. This phenomenon significantly reduces ionic conductivity and increases the energy barrier for desolvation at electrode surfaces, leading to sluggish kinetics during charging and discharging processes.
Another major challenge is the formation of passivation layers on calcium metal anodes. Unlike the beneficial solid electrolyte interphase (SEI) in lithium-ion batteries, calcium-based systems often develop non-uniform, resistive passivation films that block ion transport. These layers typically consist of CaF₂, CaCO₃, and other insoluble calcium compounds that form through reactions between the calcium metal and electrolyte components, particularly at high voltages.
Electrolyte stability presents an additional hurdle, as many potential calcium electrolytes exhibit narrow electrochemical stability windows. This limitation restricts the operating voltage range of calcium batteries and consequently their energy density. The decomposition of electrolytes at higher voltages not only compromises battery performance but also generates gas evolution and safety concerns.
Calcium salt solubility in organic solvents remains problematic, with many conventional calcium salts showing limited solubility compared to their lithium counterparts. This constraint restricts the concentration of charge carriers in the electrolyte, directly affecting power capability and rate performance of calcium batteries.
Water contamination poses a particular challenge for calcium-based systems, as even trace amounts of moisture can trigger parasitic reactions with both the electrolyte and electrode materials. These reactions accelerate capacity fading and reduce cycle life, necessitating stringent manufacturing conditions that add complexity and cost to production processes.
Current research efforts focus on developing novel electrolyte formulations incorporating specialized additives to address these challenges. Boron-based compounds, fluorinated additives, and chelating agents are being investigated for their ability to modify solvation structures, stabilize interfaces, and enhance calcium ion transport properties. However, a comprehensive understanding of the complex passivation chemistry and additive mechanisms remains elusive, highlighting the need for advanced characterization techniques and computational modeling approaches.
State-of-the-Art Electrolyte Formulations
01 Passivation layer formation in calcium ion batteries
Passivation layers play a crucial role in calcium ion batteries by protecting electrode surfaces from continuous electrolyte decomposition. These layers form through controlled chemical reactions between the electrode materials and electrolyte components, creating a stable interface that allows calcium ion transport while preventing further degradation. Proper formation of these passivation films is essential for improving battery cycle life and performance.- Electrolyte additives for passivation layer formation: Various electrolyte additives can be incorporated into calcium ion batteries to form stable passivation layers on electrode surfaces. These additives react with electrode materials to create protective films that prevent continuous electrolyte decomposition while allowing calcium ion transport. Effective additives include fluorinated compounds, carbonates, and specific salts that contribute to the formation of a calcium-containing solid electrolyte interphase (SEI) layer, improving battery cycling stability and efficiency.
- Surface coating technologies for electrode protection: Protective surface coatings can be applied to calcium ion battery electrodes to mitigate passivation issues. These coatings serve as artificial interfaces that stabilize the electrode-electrolyte boundary and prevent undesirable side reactions. Various coating materials including metal oxides, polymers, and composite materials can be engineered to specific thicknesses and compositions to enhance calcium ion diffusion while minimizing parasitic reactions that lead to capacity fade and increased internal resistance.
- Novel electrode materials with passivation resistance: Research has led to the development of novel electrode materials specifically designed to resist passivation in calcium ion battery environments. These materials feature optimized crystal structures, doping strategies, or surface modifications that minimize unwanted chemical reactions with the electrolyte. By engineering materials with inherent passivation resistance, batteries can achieve improved cycling performance, enhanced capacity retention, and extended operational lifetimes even under demanding charge-discharge conditions.
- Passivation layer characterization and analysis techniques: Advanced analytical techniques have been developed to characterize the composition, structure, and properties of passivation layers in calcium ion batteries. These methods include spectroscopic analysis, microscopy, and electrochemical testing protocols that provide insights into passivation layer formation mechanisms and their impact on battery performance. Understanding the chemical and physical properties of these layers enables researchers to develop targeted strategies for controlling passivation processes and optimizing battery design for improved performance and longevity.
- Battery management systems for passivation control: Specialized battery management systems have been designed to control and mitigate passivation effects in calcium ion batteries. These systems employ algorithms that monitor battery parameters and adjust charging/discharging protocols to minimize conditions that accelerate passivation. By implementing strategic voltage limits, current profiles, and temperature management, these systems can significantly reduce passivation layer growth while maintaining optimal battery performance. Some approaches also incorporate periodic conditioning cycles that help dissolve or restructure existing passivation layers.
02 Electrolyte additives for improved passivation chemistry
Specific electrolyte additives can be incorporated to enhance the formation of stable passivation layers in calcium ion batteries. These additives participate in controlled decomposition reactions at electrode surfaces, resulting in more uniform and functional passivation films. Carefully selected additives can modify the chemical composition of the passivation layer, improving calcium ion conductivity while maintaining protective properties against further electrolyte degradation.Expand Specific Solutions03 Electrode surface treatments for passivation enhancement
Various surface treatment methods can be applied to electrode materials to improve passivation layer properties in calcium ion batteries. These treatments include chemical modifications, coatings, and controlled pre-cycling protocols that facilitate the formation of more stable and functional passivation films. By engineering the electrode surface chemistry, the resulting passivation layers demonstrate better calcium ion transport properties while maintaining protective functions.Expand Specific Solutions04 Advanced characterization of passivation interfaces
Understanding the chemical composition and structure of passivation layers in calcium ion batteries requires advanced characterization techniques. Methods such as X-ray photoelectron spectroscopy, transmission electron microscopy, and electrochemical impedance spectroscopy provide insights into the formation mechanisms and properties of these interfaces. This knowledge enables the development of strategies to optimize passivation chemistry for improved battery performance and longevity.Expand Specific Solutions05 Calcium ion transport mechanisms through passivation layers
The transport of calcium ions through passivation layers involves specific mechanisms that differ from those in lithium-ion batteries due to the divalent nature of calcium ions. Research focuses on understanding how calcium ions navigate through these protective films and identifying chemical compositions that facilitate this transport while maintaining protective functions. Optimizing the passivation chemistry to enhance calcium ion conductivity while preserving electrode protection is crucial for developing high-performance calcium ion batteries.Expand Specific Solutions
Leading Research Groups and Industrial Players
The research on calcium ion battery electrolyte additives and passivation chemistry is currently in an early developmental stage, with the market still emerging but showing significant growth potential. The global landscape is characterized by academic-industrial collaborations, with research institutions like Nankai University, Shanghai University, and CNRS working alongside commercial players. Leading battery manufacturers including CATL, StoreDot, and BYD are investing in this technology as a promising alternative to lithium-ion batteries. Specialized chemical companies such as Shenzhen Capchem Technology and DuPont are developing advanced electrolyte formulations, while Wildcat Discovery Technologies is utilizing high-throughput methods to accelerate materials discovery. The technology remains pre-commercial, with challenges in electrolyte stability and calcium metal passivation still requiring significant breakthroughs before widespread commercialization.
StoreDot Ltd.
Technical Solution: StoreDot has pioneered a novel approach to calcium ion battery electrolytes focusing on rapid charging capabilities while addressing passivation issues. Their technology centers on a proprietary blend of boron-based additives that modify the solvation structure of calcium ions in the electrolyte, facilitating faster ion transport through the electrode-electrolyte interface. StoreDot's research has shown that their additives can effectively disrupt the formation of passivation layers by creating alternative reaction pathways during the initial cycles. The company has developed a dual-function additive system where one component sacrificially reacts to form a thin, calcium-ion conductive protective layer, while the second component maintains long-term electrolyte stability. This approach has enabled StoreDot to achieve calcium plating/stripping efficiencies exceeding 85% over 100 cycles in prototype cells. Their electrolyte formulation also incorporates crown ether derivatives that selectively coordinate with calcium ions, reducing their effective charge density and enhancing their mobility through the electrolyte bulk and interfaces.
Strengths: StoreDot's technology enables exceptionally fast charging capabilities while maintaining reasonable cycle life. Their additives show excellent compatibility with various cathode materials being developed for calcium ion systems. Weaknesses: The specialized additives may be costly to produce at scale, and the long-term stability of the formed interfacial layers remains a concern under extreme operating conditions.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: Contemporary Amperex Technology Co., Ltd. (CATL) has developed innovative electrolyte additive systems specifically for calcium ion batteries that address the key challenges of calcium plating/stripping and electrode passivation. Their approach involves a multi-component additive strategy using fluorinated organic compounds and calcium salt complexing agents to modify the solid electrolyte interphase (SEI) formation. CATL's research has demonstrated that their proprietary blend of additives containing fluoroethylene carbonate (FEC) and calcium hexafluorophosphate can significantly reduce the overpotential for calcium deposition and dissolution, improving the reversibility of the calcium electrochemistry. Their technology also incorporates chelating agents that help solubilize calcium ions in conventional carbonate-based electrolytes, addressing the poor solubility issues that have historically limited calcium battery performance. CATL has reported cycle life improvements of over 300% compared to conventional electrolyte formulations, with initial Coulombic efficiencies reaching 90% for calcium metal anodes.
Strengths: CATL's extensive manufacturing infrastructure allows for rapid scaling of their electrolyte technologies. Their additive packages demonstrate superior calcium ion transport properties and significantly improved cycling stability. Weaknesses: The technology still faces challenges with long-term stability at elevated temperatures and may require specialized manufacturing processes that increase production costs.
Key Passivation Mechanisms and Interface Chemistry
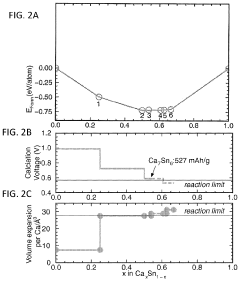
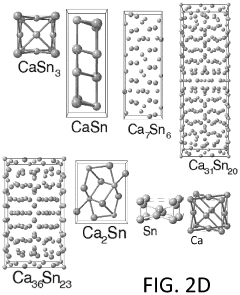
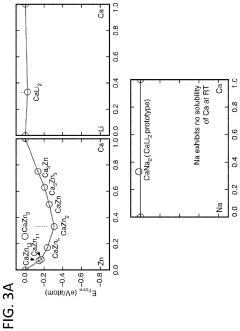
Calcium-metal alloy anode materials for reversible calcium-ion batteries
PatentActiveUS11901550B2
Innovation
- The use of intermetallic compounds comprising calcium and metals or metalloids such as Sb, As, Cu, Cd, Bi, Ag, Au, Pd, Pt, or Hg as anodes, paired with a calcium salt-based electrolyte and a cathode material like graphite, allowing for reversible decalcination and calcination reactions, and employing high-throughput density functional theory (DFT) calculations to identify suitable anode materials with high energy density and constrained volume expansion.
Additives for increasing ion conductivity of molten salt type electrolyte in battery
PatentInactiveUS20080286649A1
Innovation
- Incorporating organic additives such as alkyl carbonates, phosphates, and other organic compounds into the molten salt electrolyte to enhance lithium ion conductivity without compromising safety by maintaining non-flammability under normal conditions.
Sustainability and Resource Considerations
The sustainability aspects of calcium ion battery development represent a critical dimension in evaluating their potential as alternatives to lithium-ion technologies. Calcium is the fifth most abundant element in the Earth's crust, with reserves approximately 2,000 times greater than lithium, offering significant advantages from a resource availability perspective. This abundance translates to lower extraction costs and reduced geopolitical supply risks compared to lithium, which is concentrated in specific regions like the "Lithium Triangle" of South America.
The environmental impact of calcium extraction is generally less severe than lithium mining, which often requires extensive water usage in arid regions and can lead to soil contamination. Calcium can be sourced through more environmentally benign processes, including from limestone and dolomite deposits that are widely distributed globally. However, the energy requirements and carbon footprint of calcium processing for battery-grade materials still require thorough life cycle assessment.
Electrolyte additives for calcium ion batteries present both challenges and opportunities from a sustainability standpoint. Many current research directions involve fluorinated compounds that may pose environmental concerns regarding persistence and degradation pathways. The development of bio-derived or readily biodegradable additives represents an important frontier in sustainable calcium battery chemistry, with recent research exploring natural polymers and ionic liquids derived from renewable sources.
The passivation chemistry in calcium batteries also intersects with sustainability considerations through the longevity it enables. Effective passivation layers can significantly extend battery cycle life, reducing the frequency of replacement and thereby decreasing the overall material demand. Research indicates that certain organic additives can form stable passivation films while being derived from sustainable precursors, offering a dual benefit.
Recycling infrastructure represents another critical sustainability consideration. The development of electrolyte systems and passivation chemistries should ideally account for end-of-life recovery and material reclamation. Current research suggests that calcium-based battery components may offer advantages in recyclability compared to some lithium technologies, particularly when designed with disassembly and material separation in mind.
Water sensitivity remains a challenge for many calcium electrolyte systems, requiring anhydrous processing environments that can be energy-intensive. Research into water-stable calcium electrolytes with appropriate additives could significantly reduce manufacturing energy requirements and associated carbon emissions, representing an important direction for sustainable technology development.
The environmental impact of calcium extraction is generally less severe than lithium mining, which often requires extensive water usage in arid regions and can lead to soil contamination. Calcium can be sourced through more environmentally benign processes, including from limestone and dolomite deposits that are widely distributed globally. However, the energy requirements and carbon footprint of calcium processing for battery-grade materials still require thorough life cycle assessment.
Electrolyte additives for calcium ion batteries present both challenges and opportunities from a sustainability standpoint. Many current research directions involve fluorinated compounds that may pose environmental concerns regarding persistence and degradation pathways. The development of bio-derived or readily biodegradable additives represents an important frontier in sustainable calcium battery chemistry, with recent research exploring natural polymers and ionic liquids derived from renewable sources.
The passivation chemistry in calcium batteries also intersects with sustainability considerations through the longevity it enables. Effective passivation layers can significantly extend battery cycle life, reducing the frequency of replacement and thereby decreasing the overall material demand. Research indicates that certain organic additives can form stable passivation films while being derived from sustainable precursors, offering a dual benefit.
Recycling infrastructure represents another critical sustainability consideration. The development of electrolyte systems and passivation chemistries should ideally account for end-of-life recovery and material reclamation. Current research suggests that calcium-based battery components may offer advantages in recyclability compared to some lithium technologies, particularly when designed with disassembly and material separation in mind.
Water sensitivity remains a challenge for many calcium electrolyte systems, requiring anhydrous processing environments that can be energy-intensive. Research into water-stable calcium electrolytes with appropriate additives could significantly reduce manufacturing energy requirements and associated carbon emissions, representing an important direction for sustainable technology development.
Performance Benchmarking Against Lithium-ion Technology
When comparing calcium-ion batteries (CIBs) with the well-established lithium-ion technology, several key performance metrics must be evaluated to understand their relative advantages and limitations. Lithium-ion batteries currently dominate the commercial market with energy densities ranging from 150-265 Wh/kg and 250-670 Wh/L, power densities of 300-1500 W/kg, and cycle lives typically exceeding 1000 cycles at 80% capacity retention.
In contrast, calcium-ion batteries demonstrate theoretical advantages in several areas. The theoretical capacity of calcium metal (1340 mAh/g) exceeds that of lithium (3860 mAh/g), but when considering the divalent nature of calcium ions, the energy density potential becomes more comparable. Current experimental CIBs achieve only 30-120 Wh/kg, significantly lower than commercial lithium-ion systems.
Voltage efficiency represents another critical comparison point. While lithium-ion batteries typically operate at 3.6-3.9V with coulombic efficiencies exceeding 99.9% in optimized systems, calcium-ion batteries currently demonstrate lower operating voltages (2.0-3.4V) and substantially lower coulombic efficiencies (70-90%) due to parasitic reactions and incomplete passivation.
Rate capability presents a significant challenge for calcium-ion technology. The larger ionic radius of Ca²⁺ (1.00 Å) compared to Li⁺ (0.76 Å) results in slower diffusion kinetics, limiting power density. Current CIB prototypes demonstrate rate capabilities approximately 5-10 times lower than lithium-ion equivalents.
Temperature performance also favors lithium-ion technology, which maintains reasonable performance from -20°C to 60°C. Calcium-ion systems currently operate in a narrower range, with significant performance degradation below 10°C due to increased electrolyte resistance and slower calcium ion transport.
Cost projections potentially favor calcium-ion technology due to calcium's greater natural abundance (41,500 ppm in Earth's crust versus 20 ppm for lithium). However, this advantage remains theoretical until manufacturing processes mature and economies of scale develop.
Safety comparisons indicate potential advantages for calcium-ion systems due to calcium's lower reactivity with water compared to lithium. However, the higher operating temperatures required for efficient calcium ion transport may introduce different safety concerns that require further investigation.
The cycle life of current calcium-ion prototypes remains substantially below lithium-ion standards, with most systems demonstrating significant capacity fading after 50-200 cycles, compared to 1000+ cycles for commercial lithium-ion batteries. This limitation primarily stems from incomplete understanding of passivation layer chemistry and electrolyte stability.
In contrast, calcium-ion batteries demonstrate theoretical advantages in several areas. The theoretical capacity of calcium metal (1340 mAh/g) exceeds that of lithium (3860 mAh/g), but when considering the divalent nature of calcium ions, the energy density potential becomes more comparable. Current experimental CIBs achieve only 30-120 Wh/kg, significantly lower than commercial lithium-ion systems.
Voltage efficiency represents another critical comparison point. While lithium-ion batteries typically operate at 3.6-3.9V with coulombic efficiencies exceeding 99.9% in optimized systems, calcium-ion batteries currently demonstrate lower operating voltages (2.0-3.4V) and substantially lower coulombic efficiencies (70-90%) due to parasitic reactions and incomplete passivation.
Rate capability presents a significant challenge for calcium-ion technology. The larger ionic radius of Ca²⁺ (1.00 Å) compared to Li⁺ (0.76 Å) results in slower diffusion kinetics, limiting power density. Current CIB prototypes demonstrate rate capabilities approximately 5-10 times lower than lithium-ion equivalents.
Temperature performance also favors lithium-ion technology, which maintains reasonable performance from -20°C to 60°C. Calcium-ion systems currently operate in a narrower range, with significant performance degradation below 10°C due to increased electrolyte resistance and slower calcium ion transport.
Cost projections potentially favor calcium-ion technology due to calcium's greater natural abundance (41,500 ppm in Earth's crust versus 20 ppm for lithium). However, this advantage remains theoretical until manufacturing processes mature and economies of scale develop.
Safety comparisons indicate potential advantages for calcium-ion systems due to calcium's lower reactivity with water compared to lithium. However, the higher operating temperatures required for efficient calcium ion transport may introduce different safety concerns that require further investigation.
The cycle life of current calcium-ion prototypes remains substantially below lithium-ion standards, with most systems demonstrating significant capacity fading after 50-200 cycles, compared to 1000+ cycles for commercial lithium-ion batteries. This limitation primarily stems from incomplete understanding of passivation layer chemistry and electrolyte stability.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!