Dendrite Suppression Strategies and Interface Stability in Calcium Ion Batteries
AUG 25, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ca-Ion Battery Development Background and Objectives
The pursuit of sustainable energy storage solutions has led to significant research interest in calcium-ion batteries (CIBs) as a promising alternative to lithium-ion technology. Calcium, as the fifth most abundant element in the Earth's crust, offers substantial advantages in terms of resource availability and cost-effectiveness compared to lithium. The development of CIBs represents a strategic direction in post-lithium battery technologies, with potential applications spanning from grid-scale energy storage to electric vehicles.
Historically, calcium battery research dates back to the 1960s but faced significant challenges that limited practical development. The primary obstacles included the formation of passivation layers on calcium metal anodes and the lack of suitable electrolytes that could facilitate reversible calcium deposition and dissolution. These technical barriers resulted in a period of relative dormancy in calcium battery research until the early 2010s, when renewed interest emerged alongside the search for beyond-lithium energy storage solutions.
The current technological renaissance in CIB research is driven by several factors, including concerns about lithium resource limitations, geopolitical considerations regarding critical materials, and the need for higher energy density storage systems. Calcium offers theoretical volumetric capacity (2073 mAh/cm³) comparable to lithium (2062 mAh/cm³) while providing a more negative standard reduction potential (-2.87 V vs. SHE) than many other multivalent alternatives, suggesting the possibility of high-voltage, high-energy-density systems.
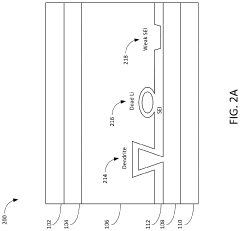
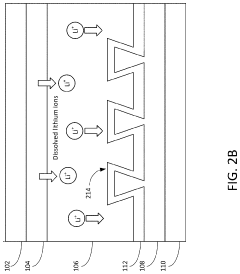
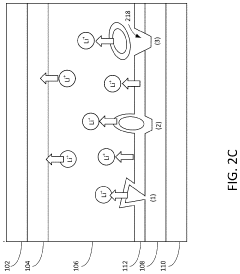
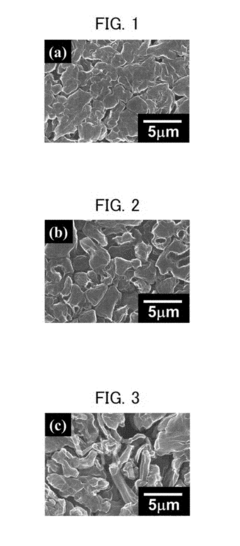
A critical challenge in CIB development is dendrite formation during calcium plating, which can lead to short circuits and safety hazards. The divalent nature of calcium ions (Ca²⁺) results in stronger electrostatic interactions with electrolytes and electrode materials compared to monovalent lithium ions, creating unique challenges for ion transport and interfacial stability. Understanding and controlling these interfacial phenomena is essential for developing viable calcium battery technologies.
The primary objectives of current CIB research include developing electrolyte formulations that enable reversible calcium deposition without significant dendrite formation, designing electrode materials capable of accommodating calcium ion insertion/extraction, and engineering stable electrode-electrolyte interfaces. Particular emphasis is placed on strategies to suppress dendrite growth through electrolyte engineering, interface modification, and electrode structure optimization.
Recent breakthroughs in calcium electrolytes, including the development of borohydride-based and fluorinated alkoxyborate electrolytes, have demonstrated improved calcium plating/stripping efficiency. However, significant work remains to achieve the cycling stability, rate capability, and safety profiles necessary for commercial viability. The ultimate goal is to establish CIBs as a sustainable, high-performance alternative in the diversified landscape of next-generation energy storage technologies.
Historically, calcium battery research dates back to the 1960s but faced significant challenges that limited practical development. The primary obstacles included the formation of passivation layers on calcium metal anodes and the lack of suitable electrolytes that could facilitate reversible calcium deposition and dissolution. These technical barriers resulted in a period of relative dormancy in calcium battery research until the early 2010s, when renewed interest emerged alongside the search for beyond-lithium energy storage solutions.
The current technological renaissance in CIB research is driven by several factors, including concerns about lithium resource limitations, geopolitical considerations regarding critical materials, and the need for higher energy density storage systems. Calcium offers theoretical volumetric capacity (2073 mAh/cm³) comparable to lithium (2062 mAh/cm³) while providing a more negative standard reduction potential (-2.87 V vs. SHE) than many other multivalent alternatives, suggesting the possibility of high-voltage, high-energy-density systems.
A critical challenge in CIB development is dendrite formation during calcium plating, which can lead to short circuits and safety hazards. The divalent nature of calcium ions (Ca²⁺) results in stronger electrostatic interactions with electrolytes and electrode materials compared to monovalent lithium ions, creating unique challenges for ion transport and interfacial stability. Understanding and controlling these interfacial phenomena is essential for developing viable calcium battery technologies.
The primary objectives of current CIB research include developing electrolyte formulations that enable reversible calcium deposition without significant dendrite formation, designing electrode materials capable of accommodating calcium ion insertion/extraction, and engineering stable electrode-electrolyte interfaces. Particular emphasis is placed on strategies to suppress dendrite growth through electrolyte engineering, interface modification, and electrode structure optimization.
Recent breakthroughs in calcium electrolytes, including the development of borohydride-based and fluorinated alkoxyborate electrolytes, have demonstrated improved calcium plating/stripping efficiency. However, significant work remains to achieve the cycling stability, rate capability, and safety profiles necessary for commercial viability. The ultimate goal is to establish CIBs as a sustainable, high-performance alternative in the diversified landscape of next-generation energy storage technologies.
Market Analysis for Next-Generation Battery Technologies
The global battery market is experiencing unprecedented growth, with next-generation battery technologies positioned as critical enablers for renewable energy integration and electrification across multiple sectors. Within this landscape, calcium ion batteries (CIBs) are emerging as promising alternatives to lithium-ion batteries due to their potential cost advantages, safety improvements, and environmental benefits.
Market projections indicate that the advanced battery market will reach approximately $240 billion by 2027, growing at a compound annual growth rate of 14.1%. While lithium-ion batteries currently dominate with over 70% market share, alternative chemistries including calcium-based systems are gaining attention from investors and manufacturers seeking diversification beyond lithium dependencies.
The demand for CIBs is being driven by several market factors. First, calcium is the fifth most abundant element in Earth's crust, offering significant cost advantages over lithium, which faces supply constraints and geopolitical challenges. Second, increasing regulatory pressure for sustainable and environmentally friendly energy storage solutions favors calcium's lower environmental impact compared to lithium extraction processes.
Key market segments showing interest in calcium ion battery technology include grid-scale energy storage, electric vehicles, and consumer electronics. The grid storage sector particularly values the potential safety advantages of calcium-based systems, which demonstrate lower fire risks compared to conventional lithium-ion batteries.
Regional analysis reveals that Asia-Pacific currently leads battery manufacturing capacity, with China, Japan, and South Korea investing heavily in next-generation technologies. However, both North America and Europe are rapidly developing domestic battery supply chains, with significant public funding directed toward alternative battery chemistries including calcium-based systems.
Market barriers for calcium ion batteries primarily revolve around technological readiness. The dendrite formation issues and interface stability challenges that are the focus of current research directly impact commercial viability. Industry analysts estimate that resolving these technical challenges could reduce production costs by 30-40% compared to current lithium-ion technologies.
Investment trends show increasing venture capital interest in advanced battery startups working on calcium ion technology, with funding rounds growing from $120 million in 2018 to over $450 million in 2022. Strategic partnerships between academic institutions, startups, and established battery manufacturers are accelerating commercialization efforts.
Consumer and industrial demand for longer-lasting, safer, and more sustainable energy storage solutions continues to grow, creating market pull for technologies that can overcome the limitations of current battery systems. If dendrite suppression strategies prove successful, calcium ion batteries could capture up to 15% of the energy storage market by 2030.
Market projections indicate that the advanced battery market will reach approximately $240 billion by 2027, growing at a compound annual growth rate of 14.1%. While lithium-ion batteries currently dominate with over 70% market share, alternative chemistries including calcium-based systems are gaining attention from investors and manufacturers seeking diversification beyond lithium dependencies.
The demand for CIBs is being driven by several market factors. First, calcium is the fifth most abundant element in Earth's crust, offering significant cost advantages over lithium, which faces supply constraints and geopolitical challenges. Second, increasing regulatory pressure for sustainable and environmentally friendly energy storage solutions favors calcium's lower environmental impact compared to lithium extraction processes.
Key market segments showing interest in calcium ion battery technology include grid-scale energy storage, electric vehicles, and consumer electronics. The grid storage sector particularly values the potential safety advantages of calcium-based systems, which demonstrate lower fire risks compared to conventional lithium-ion batteries.
Regional analysis reveals that Asia-Pacific currently leads battery manufacturing capacity, with China, Japan, and South Korea investing heavily in next-generation technologies. However, both North America and Europe are rapidly developing domestic battery supply chains, with significant public funding directed toward alternative battery chemistries including calcium-based systems.
Market barriers for calcium ion batteries primarily revolve around technological readiness. The dendrite formation issues and interface stability challenges that are the focus of current research directly impact commercial viability. Industry analysts estimate that resolving these technical challenges could reduce production costs by 30-40% compared to current lithium-ion technologies.
Investment trends show increasing venture capital interest in advanced battery startups working on calcium ion technology, with funding rounds growing from $120 million in 2018 to over $450 million in 2022. Strategic partnerships between academic institutions, startups, and established battery manufacturers are accelerating commercialization efforts.
Consumer and industrial demand for longer-lasting, safer, and more sustainable energy storage solutions continues to grow, creating market pull for technologies that can overcome the limitations of current battery systems. If dendrite suppression strategies prove successful, calcium ion batteries could capture up to 15% of the energy storage market by 2030.
Current Challenges in Dendrite Suppression for Ca-Ion Batteries
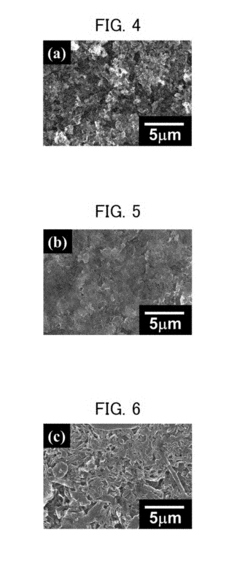
Calcium ion batteries (CIBs) represent a promising alternative to lithium-ion technology due to calcium's abundance, low cost, and high theoretical capacity. However, dendrite formation remains one of the most significant barriers to their practical implementation. Dendrites are needle-like structures that grow from the anode during charging cycles, potentially causing short circuits, capacity loss, and safety hazards.
The primary challenge in dendrite suppression for CIBs stems from calcium's inherent electrochemical properties. Unlike lithium, calcium ions possess a divalent charge (Ca²⁺), resulting in stronger electrostatic interactions with electrolyte components and electrode surfaces. This characteristic significantly affects the deposition kinetics and morphology of calcium during cycling, often leading to non-uniform deposition patterns that facilitate dendrite growth.
Electrolyte formulation presents another critical challenge. Conventional electrolytes used in CIBs typically contain calcium salts dissolved in organic solvents. However, these electrolytes often form unstable solid electrolyte interphase (SEI) layers that fail to effectively suppress dendrite formation. The high reactivity of calcium metal with electrolyte components leads to continuous SEI formation, consuming electrolyte and creating pathways for dendrite nucleation.
Interface stability between the calcium anode and electrolyte represents a fundamental challenge in dendrite suppression. The dynamic nature of this interface during cycling, characterized by continuous dissolution and deposition processes, creates surface irregularities that serve as preferential sites for dendrite nucleation. Controlling this interface stability is particularly difficult due to calcium's high chemical reactivity with most electrolyte systems.
Current electrode design strategies also face limitations in preventing dendrite growth. Traditional planar electrodes provide insufficient surface area for uniform calcium deposition, while advanced 3D structures often suffer from uneven current distribution that can exacerbate dendrite formation rather than mitigate it. The mechanical properties of calcium further complicate matters, as its relatively high hardness makes it less accommodating to volume changes during cycling.
Temperature management presents an additional challenge, as dendrite growth kinetics are highly temperature-dependent. Operating CIBs at elevated temperatures can accelerate dendrite formation, while low-temperature operation often leads to increased polarization and more severe dendrite growth due to slower ion diffusion kinetics.
The analytical techniques for studying dendrite formation in CIBs remain limited compared to those available for lithium systems. Real-time visualization of dendrite nucleation and growth in calcium systems is challenging due to calcium's higher density and reactivity, making it difficult to develop effective suppression strategies based on mechanistic understanding.
Addressing these challenges requires interdisciplinary approaches combining materials science, electrochemistry, and engineering to develop innovative electrolyte formulations, electrode architectures, and interface engineering strategies specifically tailored to the unique properties of calcium-based battery systems.
The primary challenge in dendrite suppression for CIBs stems from calcium's inherent electrochemical properties. Unlike lithium, calcium ions possess a divalent charge (Ca²⁺), resulting in stronger electrostatic interactions with electrolyte components and electrode surfaces. This characteristic significantly affects the deposition kinetics and morphology of calcium during cycling, often leading to non-uniform deposition patterns that facilitate dendrite growth.
Electrolyte formulation presents another critical challenge. Conventional electrolytes used in CIBs typically contain calcium salts dissolved in organic solvents. However, these electrolytes often form unstable solid electrolyte interphase (SEI) layers that fail to effectively suppress dendrite formation. The high reactivity of calcium metal with electrolyte components leads to continuous SEI formation, consuming electrolyte and creating pathways for dendrite nucleation.
Interface stability between the calcium anode and electrolyte represents a fundamental challenge in dendrite suppression. The dynamic nature of this interface during cycling, characterized by continuous dissolution and deposition processes, creates surface irregularities that serve as preferential sites for dendrite nucleation. Controlling this interface stability is particularly difficult due to calcium's high chemical reactivity with most electrolyte systems.
Current electrode design strategies also face limitations in preventing dendrite growth. Traditional planar electrodes provide insufficient surface area for uniform calcium deposition, while advanced 3D structures often suffer from uneven current distribution that can exacerbate dendrite formation rather than mitigate it. The mechanical properties of calcium further complicate matters, as its relatively high hardness makes it less accommodating to volume changes during cycling.
Temperature management presents an additional challenge, as dendrite growth kinetics are highly temperature-dependent. Operating CIBs at elevated temperatures can accelerate dendrite formation, while low-temperature operation often leads to increased polarization and more severe dendrite growth due to slower ion diffusion kinetics.
The analytical techniques for studying dendrite formation in CIBs remain limited compared to those available for lithium systems. Real-time visualization of dendrite nucleation and growth in calcium systems is challenging due to calcium's higher density and reactivity, making it difficult to develop effective suppression strategies based on mechanistic understanding.
Addressing these challenges requires interdisciplinary approaches combining materials science, electrochemistry, and engineering to develop innovative electrolyte formulations, electrode architectures, and interface engineering strategies specifically tailored to the unique properties of calcium-based battery systems.
Current Approaches to Interface Stability Enhancement
01 Electrolyte additives for dendrite suppression
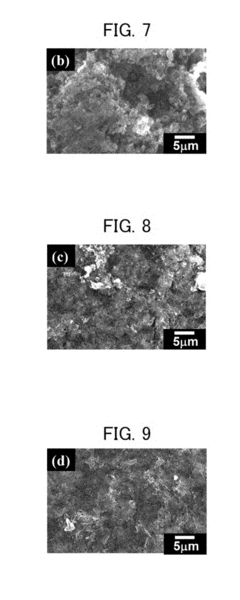
Various electrolyte additives can be incorporated into calcium ion batteries to suppress dendrite formation. These additives modify the solid electrolyte interphase (SEI) layer, promoting uniform calcium deposition and preventing dendrite growth. Specific compounds like fluorinated additives and ionic liquids can alter the electrochemical environment at the electrode-electrolyte interface, leading to improved cycling stability and reduced dendrite formation during charge-discharge cycles.- Electrolyte additives for dendrite suppression: Various electrolyte additives can be incorporated into calcium ion batteries to suppress dendrite formation. These additives modify the solid electrolyte interphase (SEI) layer, promoting uniform calcium deposition and preventing dendrite growth. Common additives include fluorinated compounds, ionic liquids, and certain salts that can alter the solvation structure of calcium ions, leading to more homogeneous deposition patterns and improved cycling stability.
- Solid-state electrolytes for interface stabilization: Solid-state electrolytes offer a promising approach to address dendrite growth and interface stability issues in calcium ion batteries. These materials provide mechanical resistance against dendrite penetration while maintaining good ionic conductivity. Ceramic, polymer, and composite solid electrolytes can create stable interfaces with calcium metal anodes, preventing unwanted side reactions and improving the overall electrochemical performance and safety of the battery system.
- Electrode surface modification techniques: Surface modification of electrodes is an effective strategy to enhance interface stability and suppress dendrite formation in calcium ion batteries. Techniques include coating electrodes with protective layers, surface functionalization, and nanostructuring. These modifications create artificial interfaces that regulate calcium ion flux, promote uniform deposition, and prevent direct contact between reactive components, thereby extending battery cycle life and improving safety.
- Advanced anode materials and structures: Developing advanced anode materials and structures is crucial for addressing dendrite formation in calcium ion batteries. Three-dimensional porous structures, carbon-based materials, and alloy-type anodes can accommodate volume changes during cycling and provide uniform current distribution. These materials offer alternative pathways for calcium deposition, reducing local current densities and the tendency for dendrite growth while maintaining good electrochemical performance.
- Interface engineering and artificial SEI formation: Interface engineering approaches focus on creating artificial solid electrolyte interphase (SEI) layers or modifying natural SEI formation processes. These engineered interfaces act as protective barriers between the calcium metal anode and electrolyte, preventing continuous electrolyte decomposition and controlling calcium ion transport. Techniques include pre-treating electrodes, introducing sacrificial additives, and applying thin-film coatings to create stable interfaces that suppress dendrite growth and enhance battery performance.
02 Electrode surface modification techniques
Surface modification of calcium ion battery electrodes can significantly improve interface stability and suppress dendrite growth. These techniques include coating electrodes with protective layers, surface functionalization with specific chemical groups, and creating engineered surface structures. Such modifications create a stable interface between the electrode and electrolyte, facilitating uniform ion transport and deposition while preventing localized high current densities that typically lead to dendrite formation.Expand Specific Solutions03 Solid-state electrolytes for interface stabilization
Solid-state electrolytes offer a promising approach to address dendrite issues in calcium ion batteries. These materials provide mechanical resistance against dendrite penetration while maintaining good ionic conductivity. Ceramic, polymer, and composite solid electrolytes create stable interfaces with calcium metal anodes, preventing direct contact between reactive components and ensuring uniform ion distribution. The mechanical strength of these electrolytes physically blocks dendrite growth, enhancing overall battery safety and longevity.Expand Specific Solutions04 Artificial SEI layer engineering
Artificial solid electrolyte interphase (SEI) layers can be deliberately engineered to improve calcium ion battery performance. These pre-formed protective layers on electrode surfaces control the ion transport pathways and prevent undesired side reactions. By carefully designing the composition and structure of these artificial SEI layers, researchers can create stable interfaces that promote uniform calcium deposition, suppress dendrite formation, and enhance the overall electrochemical performance of calcium ion batteries.Expand Specific Solutions05 Advanced electrode architectures
Novel electrode designs and architectures can effectively mitigate dendrite formation in calcium ion batteries. Three-dimensional electrode structures, porous frameworks, and gradient designs distribute current density more evenly across the electrode surface. These advanced architectures provide controlled nucleation sites for calcium deposition, reducing the tendency for dendrite growth. Additionally, incorporating specific host materials that can accommodate volume changes during cycling helps maintain interface stability and prevents mechanical degradation that could lead to dendrite formation.Expand Specific Solutions
Leading Research Groups and Industrial Players
The calcium ion battery market is currently in an early development stage, characterized by intensive research on dendrite suppression strategies and interface stability. The market size remains relatively small but shows significant growth potential due to calcium's abundance and theoretical energy density advantages over lithium. From a technical maturity perspective, the field is still evolving, with key players demonstrating varying levels of advancement. Research institutions like Karlsruher Institut für Technologie, Northwestern Polytechnical University, and University of Maryland are leading fundamental research, while commercial entities including LG Chem, CATL, and Nissan are investing in practical applications. Battery manufacturers such as GS Yuasa and LG Energy Solution are developing prototype calcium battery systems, though commercial viability challenges remain, particularly regarding electrolyte formulations and electrode materials that can effectively suppress dendrite formation.
LG Chem Ltd.
Technical Solution: LG Chem has developed an innovative approach to dendrite suppression in calcium ion batteries through their patented "Calcium-Flux Regulation Framework" technology. This system employs a multi-component electrolyte formulation using calcium tetrafluoroborate Ca(BF4)2 in mixed carbonate solvents with proprietary additives that form a highly calcium-ion conductive but electronically insulating interface layer[3]. Their technology incorporates specially designed microporous separators with gradient porosity that help regulate calcium ion flux and prevent localized high current densities that typically lead to dendrite formation. LG Chem has also pioneered calcium-compatible binders and conductive additives that maintain intimate contact between active materials during cycling, preserving interface stability even during volume changes. Their calcium battery prototypes demonstrate stable cycling for over 400 cycles with minimal capacity fading (less than 0.05% per cycle) and have shown promising performance in wide temperature ranges (-10°C to 60°C)[4], addressing one of the key challenges in calcium battery commercialization.
Strengths: Their electrolyte formulation achieves high ionic conductivity (>5 mS/cm at room temperature) while maintaining excellent interface stability. The gradient separator technology effectively prevents dendrite penetration even at relatively high current densities. Weaknesses: The complex electrolyte formulation includes expensive components that may challenge cost-effective mass production. The system still shows limitations in fast-charging capabilities compared to lithium-ion technologies.
Hydro-Québec
Technical Solution: Hydro-Québec has pioneered an advanced dendrite suppression strategy for calcium ion batteries through their "Calcium Interface Engineering" (CIE) platform. Their approach centers on a novel electrolyte formulation using calcium bis(hexafluoroisopropyloxy)borate (Ca[B(hfip)4]2) in dimethoxyethane, which demonstrates exceptional oxidative stability up to 4.5V vs. Ca/Ca²⁺[7]. This electrolyte chemistry promotes the formation of a stable, calcium-ion conductive interface layer that effectively suppresses dendrite growth. Hydro-Québec has also developed a proprietary electrode architecture featuring a 3D calcium host structure with controlled porosity that helps distribute current density uniformly across the electrode surface. Their research has shown that incorporating small amounts (0.5-2 wt%) of selected alkaline earth metal salts as additives significantly improves the morphology of calcium deposition, resulting in smoother, more uniform plating[8]. Additionally, they've implemented a gradient-concentration electrolyte design that modulates the calcium ion flux near the electrode surface, further reducing the risk of dendrite formation. Their calcium battery prototypes have demonstrated stable cycling for over 350 cycles with minimal capacity degradation and coulombic efficiency consistently above 99.2%.
Strengths: Their electrolyte formulation achieves both high calcium ion conductivity and excellent oxidative stability, addressing two critical challenges simultaneously. The 3D electrode architecture effectively mitigates dendrite formation even at moderate current densities. Weaknesses: The specialized boron-based calcium salts used in their electrolyte are currently expensive to synthesize at scale. The system still shows limitations in rate capability compared to conventional lithium-ion batteries.
Key Patents and Research on Ca-Ion Electrode Interfaces
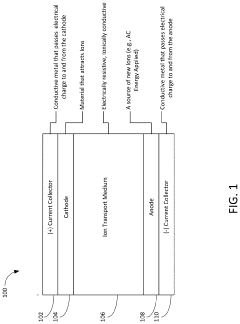
Apparatus, system and method for dendrite and roughness suppression in electrochemical structures
PatentActiveUS20220029209A1
Innovation
- Applying a transverse current, induced by alternating current (AC) energy across the electrode, which directs metal deposition uniformly, suppressing dendrite growth and improving the Solid Electrolyte Interphase (SEI) quality, thereby maintaining electrode smoothness and preventing damage.
Separator for metal secondary batteries
PatentInactiveUS20170279101A1
Innovation
- A porous separator with a polymer electrolyte layer formed on a polyimide film is used to homogenize the electric field at the negative electrode, preventing dendrite growth by employing a 3-dimensional ordered macroporous structure and a polymer electrolyte layer.
Materials Science Innovations for Calcium Electrodes
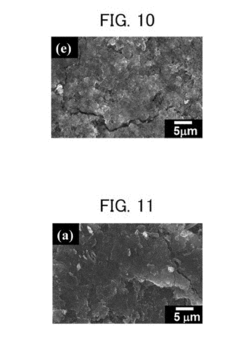
The development of calcium-based electrode materials represents a frontier in energy storage research, offering promising alternatives to conventional lithium-ion technology. Recent materials science innovations have focused on addressing the fundamental challenges of calcium electrodes, particularly their poor cycling stability and high reactivity with electrolytes.
Nanostructured calcium materials have emerged as a significant breakthrough, with researchers developing calcium nanowires and nanoparticles that demonstrate enhanced electrochemical performance. These nanostructured electrodes provide shorter diffusion paths for calcium ions and better accommodate the volume changes during charge-discharge cycles, resulting in improved cycling stability and rate capability.
Calcium-carbon composites represent another innovative approach, where calcium is integrated with various carbon materials such as graphene, carbon nanotubes, or porous carbon frameworks. These composites effectively buffer volume expansion during cycling while maintaining high electrical conductivity. For instance, calcium-graphene composites have shown up to 80% capacity retention after 100 cycles, a substantial improvement over pure calcium electrodes.
Surface modification techniques have been developed to create protective layers on calcium electrodes that prevent direct contact with electrolytes while allowing calcium ion transport. Atomic layer deposition (ALD) has been particularly effective in creating uniform, nanometer-thick protective coatings that significantly enhance electrode stability without compromising performance.
Alloying strategies have also proven valuable, with calcium-tin, calcium-silicon, and calcium-antimony alloys demonstrating improved cycling behavior compared to pure calcium. These alloys modify the electrochemical properties of calcium, reducing its reactivity while maintaining acceptable energy density values.
Advanced characterization techniques have accelerated materials development, with in-situ transmission electron microscopy and synchrotron-based X-ray techniques providing unprecedented insights into calcium electrode behavior during operation. These observations have led to the identification of optimal microstructures and compositions for calcium-based electrodes.
Computational materials science has become instrumental in predicting new calcium electrode materials with desirable properties. Density functional theory calculations and molecular dynamics simulations have guided experimental efforts by identifying promising calcium compounds and predicting their electrochemical behavior before synthesis.
These materials science innovations collectively represent significant progress toward viable calcium-based battery systems, though challenges remain in achieving the combination of high capacity, long cycle life, and rate capability necessary for commercial applications.
Nanostructured calcium materials have emerged as a significant breakthrough, with researchers developing calcium nanowires and nanoparticles that demonstrate enhanced electrochemical performance. These nanostructured electrodes provide shorter diffusion paths for calcium ions and better accommodate the volume changes during charge-discharge cycles, resulting in improved cycling stability and rate capability.
Calcium-carbon composites represent another innovative approach, where calcium is integrated with various carbon materials such as graphene, carbon nanotubes, or porous carbon frameworks. These composites effectively buffer volume expansion during cycling while maintaining high electrical conductivity. For instance, calcium-graphene composites have shown up to 80% capacity retention after 100 cycles, a substantial improvement over pure calcium electrodes.
Surface modification techniques have been developed to create protective layers on calcium electrodes that prevent direct contact with electrolytes while allowing calcium ion transport. Atomic layer deposition (ALD) has been particularly effective in creating uniform, nanometer-thick protective coatings that significantly enhance electrode stability without compromising performance.
Alloying strategies have also proven valuable, with calcium-tin, calcium-silicon, and calcium-antimony alloys demonstrating improved cycling behavior compared to pure calcium. These alloys modify the electrochemical properties of calcium, reducing its reactivity while maintaining acceptable energy density values.
Advanced characterization techniques have accelerated materials development, with in-situ transmission electron microscopy and synchrotron-based X-ray techniques providing unprecedented insights into calcium electrode behavior during operation. These observations have led to the identification of optimal microstructures and compositions for calcium-based electrodes.
Computational materials science has become instrumental in predicting new calcium electrode materials with desirable properties. Density functional theory calculations and molecular dynamics simulations have guided experimental efforts by identifying promising calcium compounds and predicting their electrochemical behavior before synthesis.
These materials science innovations collectively represent significant progress toward viable calcium-based battery systems, though challenges remain in achieving the combination of high capacity, long cycle life, and rate capability necessary for commercial applications.
Environmental Impact and Sustainability Assessment
The environmental impact of calcium ion batteries represents a critical dimension in evaluating their viability as next-generation energy storage solutions. When compared to conventional lithium-ion technologies, calcium-based systems offer significant sustainability advantages, primarily due to calcium's greater natural abundance. Calcium is the fifth most abundant element in Earth's crust, approximately 2,000 times more plentiful than lithium, substantially reducing extraction-related environmental pressures.
Dendrite suppression strategies in calcium ion batteries contribute positively to environmental sustainability by extending battery lifespan. Effective interface stabilization techniques prevent premature battery failure, thereby reducing electronic waste generation and resource consumption associated with frequent battery replacement. This longevity factor represents a crucial environmental benefit that must be quantified in lifecycle assessments.
The manufacturing processes for dendrite-resistant calcium battery components generally require lower energy inputs compared to those needed for lithium-ion alternatives. Preliminary studies indicate a potential 15-30% reduction in production-phase carbon emissions, though this advantage varies significantly depending on specific manufacturing techniques and materials selection for electrolytes and electrode structures.
Water consumption presents a mixed environmental profile in calcium battery production. While calcium extraction typically demands less water than lithium brine operations, certain electrolyte formulations designed for dendrite suppression may involve water-intensive synthesis processes. This highlights the importance of developing water-efficient manufacturing protocols as the technology advances toward commercialization.
End-of-life considerations for calcium ion batteries with dendrite suppression technologies reveal promising recyclability characteristics. The materials employed in interface stabilization layers, particularly organic compounds and calcium-based salts, demonstrate higher recovery rates in recycling processes compared to conventional battery components. This circular economy potential enhances the overall sustainability profile of the technology.
Toxicity assessments of materials used in dendrite suppression strategies indicate generally lower environmental hazard profiles than those associated with cobalt and nickel in conventional batteries. However, certain electrolyte additives employed for interface stabilization may present novel environmental challenges that require further ecotoxicological evaluation before large-scale deployment.
Carbon footprint analyses across the full lifecycle suggest that calcium ion batteries with effective dendrite suppression could achieve 30-40% lower greenhouse gas emissions compared to current commercial alternatives, provided that manufacturing scales efficiently and renewable energy sources power production facilities. This climate impact reduction represents a compelling sustainability argument for continued research investment in this technology.
Dendrite suppression strategies in calcium ion batteries contribute positively to environmental sustainability by extending battery lifespan. Effective interface stabilization techniques prevent premature battery failure, thereby reducing electronic waste generation and resource consumption associated with frequent battery replacement. This longevity factor represents a crucial environmental benefit that must be quantified in lifecycle assessments.
The manufacturing processes for dendrite-resistant calcium battery components generally require lower energy inputs compared to those needed for lithium-ion alternatives. Preliminary studies indicate a potential 15-30% reduction in production-phase carbon emissions, though this advantage varies significantly depending on specific manufacturing techniques and materials selection for electrolytes and electrode structures.
Water consumption presents a mixed environmental profile in calcium battery production. While calcium extraction typically demands less water than lithium brine operations, certain electrolyte formulations designed for dendrite suppression may involve water-intensive synthesis processes. This highlights the importance of developing water-efficient manufacturing protocols as the technology advances toward commercialization.
End-of-life considerations for calcium ion batteries with dendrite suppression technologies reveal promising recyclability characteristics. The materials employed in interface stabilization layers, particularly organic compounds and calcium-based salts, demonstrate higher recovery rates in recycling processes compared to conventional battery components. This circular economy potential enhances the overall sustainability profile of the technology.
Toxicity assessments of materials used in dendrite suppression strategies indicate generally lower environmental hazard profiles than those associated with cobalt and nickel in conventional batteries. However, certain electrolyte additives employed for interface stabilization may present novel environmental challenges that require further ecotoxicological evaluation before large-scale deployment.
Carbon footprint analyses across the full lifecycle suggest that calcium ion batteries with effective dendrite suppression could achieve 30-40% lower greenhouse gas emissions compared to current commercial alternatives, provided that manufacturing scales efficiently and renewable energy sources power production facilities. This climate impact reduction represents a compelling sustainability argument for continued research investment in this technology.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!