Solid State Pathways and Ceramic Conductors for Calcium Ion Batteries
AUG 25, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Calcium Ion Battery Technology Background and Objectives
Calcium ion batteries have emerged as a promising alternative to lithium-ion batteries due to the abundance of calcium resources, which constitute approximately 3% of the Earth's crust compared to lithium's 0.0065%. This natural abundance translates to significantly lower raw material costs and reduced geopolitical supply chain risks, positioning calcium-based energy storage as a strategically important technology for sustainable energy systems.
The evolution of battery technology has historically progressed from lead-acid to nickel-metal hydride, and subsequently to the current dominant lithium-ion paradigm. However, as global electrification accelerates, concerns regarding lithium supply constraints and environmental impacts have intensified, driving research into alternative battery chemistries. Calcium ion batteries represent a logical progression in this technological trajectory, offering theoretical energy densities comparable to lithium systems while utilizing more abundant resources.
Calcium's electrochemical potential (-2.87V vs. standard hydrogen electrode) and ionic radius (0.99Å) present both opportunities and challenges. The divalent nature of calcium ions enables higher theoretical energy storage capacity per ion compared to monovalent lithium, potentially leading to batteries with superior energy density. However, this same characteristic creates significant challenges for ion mobility within conventional electrode materials and electrolytes.
The technical objectives for calcium ion battery development center on overcoming several fundamental barriers. Primary among these is the development of solid-state pathways and ceramic conductors that facilitate efficient calcium ion transport while maintaining structural stability during repeated charge-discharge cycles. Current research aims to achieve calcium ion conductivity exceeding 10^-4 S/cm at room temperature, a threshold considered necessary for practical applications.
Additional objectives include designing electrode materials with appropriate calcium insertion/extraction kinetics, developing electrolytes with wide electrochemical stability windows, and creating interfaces that minimize resistance to ion transfer. The ultimate goal is to demonstrate calcium ion batteries with energy densities exceeding 300 Wh/kg, cycle life greater than 1000 cycles, and charging rates comparable to current commercial lithium-ion systems.
Recent breakthroughs in computational materials science and advanced characterization techniques have accelerated progress in this field. High-throughput screening of potential ceramic conductor compositions, coupled with atomic-scale visualization of ion transport mechanisms, has identified several promising material classes including calcium-doped lanthanum fluorides and specialized perovskite structures that exhibit encouraging calcium ion conductivity.
The technological roadmap for calcium ion batteries envisions laboratory-scale proof-of-concept demonstrations within 3-5 years, followed by prototype development and initial commercialization efforts in energy storage applications where high energy density is less critical than cost and sustainability considerations.
The evolution of battery technology has historically progressed from lead-acid to nickel-metal hydride, and subsequently to the current dominant lithium-ion paradigm. However, as global electrification accelerates, concerns regarding lithium supply constraints and environmental impacts have intensified, driving research into alternative battery chemistries. Calcium ion batteries represent a logical progression in this technological trajectory, offering theoretical energy densities comparable to lithium systems while utilizing more abundant resources.
Calcium's electrochemical potential (-2.87V vs. standard hydrogen electrode) and ionic radius (0.99Å) present both opportunities and challenges. The divalent nature of calcium ions enables higher theoretical energy storage capacity per ion compared to monovalent lithium, potentially leading to batteries with superior energy density. However, this same characteristic creates significant challenges for ion mobility within conventional electrode materials and electrolytes.
The technical objectives for calcium ion battery development center on overcoming several fundamental barriers. Primary among these is the development of solid-state pathways and ceramic conductors that facilitate efficient calcium ion transport while maintaining structural stability during repeated charge-discharge cycles. Current research aims to achieve calcium ion conductivity exceeding 10^-4 S/cm at room temperature, a threshold considered necessary for practical applications.
Additional objectives include designing electrode materials with appropriate calcium insertion/extraction kinetics, developing electrolytes with wide electrochemical stability windows, and creating interfaces that minimize resistance to ion transfer. The ultimate goal is to demonstrate calcium ion batteries with energy densities exceeding 300 Wh/kg, cycle life greater than 1000 cycles, and charging rates comparable to current commercial lithium-ion systems.
Recent breakthroughs in computational materials science and advanced characterization techniques have accelerated progress in this field. High-throughput screening of potential ceramic conductor compositions, coupled with atomic-scale visualization of ion transport mechanisms, has identified several promising material classes including calcium-doped lanthanum fluorides and specialized perovskite structures that exhibit encouraging calcium ion conductivity.
The technological roadmap for calcium ion batteries envisions laboratory-scale proof-of-concept demonstrations within 3-5 years, followed by prototype development and initial commercialization efforts in energy storage applications where high energy density is less critical than cost and sustainability considerations.
Market Analysis for Next-Generation Battery Technologies
The global battery market is experiencing a significant shift towards next-generation technologies, with calcium ion batteries emerging as a promising alternative to conventional lithium-ion systems. Current market projections indicate that the advanced battery sector will reach approximately $240 billion by 2027, growing at a compound annual growth rate of 14.1%. Within this expanding landscape, calcium ion batteries utilizing solid-state pathways and ceramic conductors represent an emerging segment with substantial growth potential.
Market demand for calcium ion battery technology is primarily driven by several key factors. The increasing concerns regarding lithium supply chain vulnerabilities have prompted industries to seek alternative battery chemistries. Calcium, being the fifth most abundant element in Earth's crust, offers significant advantages in terms of resource availability and geographical distribution compared to lithium. This abundance translates to potentially lower raw material costs and reduced supply chain risks.
The automotive sector represents the largest potential market for calcium ion batteries, as manufacturers actively seek battery technologies that offer higher energy density, improved safety profiles, and lower costs. Current market research indicates that electric vehicle manufacturers are willing to adopt alternative battery technologies that can deliver performance improvements of at least 30% over conventional lithium-ion batteries while maintaining competitive pricing structures.
Energy storage systems constitute another significant market opportunity, with grid-scale storage installations projected to increase by 27% annually through 2030. Calcium ion batteries with solid-state pathways could capture a substantial portion of this market if they can demonstrate long cycle life and competitive cost metrics. The stationary storage sector particularly values safety characteristics inherent to ceramic conductors.
Consumer electronics manufacturers have also expressed interest in calcium-based battery technologies, especially those that can offer improved safety profiles and higher energy densities. This market segment values miniaturization capabilities and fast-charging features, areas where solid-state calcium ion systems could potentially excel.
Regional market analysis reveals varying levels of investment and research activity. Asia-Pacific leads in terms of manufacturing capacity development, with significant investments in solid-state battery production facilities. North America demonstrates strong research initiatives, particularly in ceramic conductor development, while Europe shows balanced activity across both research and commercialization efforts.
Market barriers include the current technological maturity gap between calcium ion and lithium-ion batteries, with the latter benefiting from decades of optimization and established manufacturing infrastructure. Additionally, customer perception and risk aversion in critical applications may slow initial adoption rates despite the theoretical advantages of calcium-based systems.
Market demand for calcium ion battery technology is primarily driven by several key factors. The increasing concerns regarding lithium supply chain vulnerabilities have prompted industries to seek alternative battery chemistries. Calcium, being the fifth most abundant element in Earth's crust, offers significant advantages in terms of resource availability and geographical distribution compared to lithium. This abundance translates to potentially lower raw material costs and reduced supply chain risks.
The automotive sector represents the largest potential market for calcium ion batteries, as manufacturers actively seek battery technologies that offer higher energy density, improved safety profiles, and lower costs. Current market research indicates that electric vehicle manufacturers are willing to adopt alternative battery technologies that can deliver performance improvements of at least 30% over conventional lithium-ion batteries while maintaining competitive pricing structures.
Energy storage systems constitute another significant market opportunity, with grid-scale storage installations projected to increase by 27% annually through 2030. Calcium ion batteries with solid-state pathways could capture a substantial portion of this market if they can demonstrate long cycle life and competitive cost metrics. The stationary storage sector particularly values safety characteristics inherent to ceramic conductors.
Consumer electronics manufacturers have also expressed interest in calcium-based battery technologies, especially those that can offer improved safety profiles and higher energy densities. This market segment values miniaturization capabilities and fast-charging features, areas where solid-state calcium ion systems could potentially excel.
Regional market analysis reveals varying levels of investment and research activity. Asia-Pacific leads in terms of manufacturing capacity development, with significant investments in solid-state battery production facilities. North America demonstrates strong research initiatives, particularly in ceramic conductor development, while Europe shows balanced activity across both research and commercialization efforts.
Market barriers include the current technological maturity gap between calcium ion and lithium-ion batteries, with the latter benefiting from decades of optimization and established manufacturing infrastructure. Additionally, customer perception and risk aversion in critical applications may slow initial adoption rates despite the theoretical advantages of calcium-based systems.
Current Challenges in Solid-State Calcium Ion Conductors
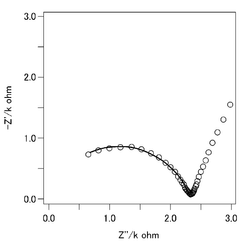
Despite significant advancements in calcium ion battery technology, solid-state calcium ion conductors face several critical challenges that impede their commercial viability. The primary obstacle remains the limited ionic conductivity at room temperature, with most current ceramic conductors exhibiting conductivity values below 10^-5 S/cm, significantly lower than the 10^-3 S/cm threshold considered necessary for practical applications. This conductivity limitation stems from the large ionic radius of Ca^2+ (1.00 Å) compared to Li^+ (0.76 Å), resulting in stronger electrostatic interactions with the host lattice and higher migration energy barriers.
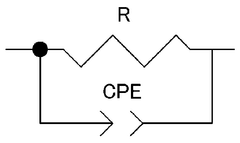
Interface stability presents another major challenge, as many solid-state calcium conductors form resistive interphases when in contact with calcium metal anodes. These interphases, often composed of mixed ionic-electronic conducting materials, lead to increased impedance and decreased battery performance over cycling. The high reactivity of calcium metal exacerbates this issue, making the development of stable interfaces particularly challenging.
Mechanical integrity during cycling constitutes a significant hurdle, as volume changes during calcium insertion/extraction can cause microcracks and delamination in ceramic conductors. This mechanical degradation creates pathways for dendrite growth and increases internal resistance, ultimately leading to capacity fade and potential safety hazards. Current ceramic formulations lack the necessary combination of mechanical flexibility and ionic conductivity.
Processing challenges further complicate the development of viable solid-state calcium conductors. Many promising materials require high-temperature sintering (>1000°C) to achieve adequate grain boundary conductivity, which increases manufacturing complexity and cost. Additionally, these high-temperature processes often lead to compositional changes and secondary phase formation that can negatively impact ionic conductivity.
The limited understanding of calcium ion transport mechanisms in solid-state materials represents a fundamental scientific challenge. Unlike lithium-ion conductors, which have been extensively studied for decades, the migration pathways and rate-limiting steps for calcium ion diffusion remain poorly understood in many ceramic structures. This knowledge gap hinders rational materials design and optimization.
Chemical stability issues also plague current calcium ion conductors, with many materials showing degradation when exposed to atmospheric conditions (particularly moisture and CO2) or decomposition at operating voltages. This instability necessitates stringent manufacturing conditions and limits the practical voltage window of calcium-based systems.
Scalability remains a significant concern, as many laboratory-scale materials with promising conductivity properties utilize expensive or rare elements, complex synthesis routes, or processing conditions that are difficult to implement at industrial scales. The transition from laboratory demonstrations to commercially viable manufacturing processes represents a substantial engineering challenge.
Interface stability presents another major challenge, as many solid-state calcium conductors form resistive interphases when in contact with calcium metal anodes. These interphases, often composed of mixed ionic-electronic conducting materials, lead to increased impedance and decreased battery performance over cycling. The high reactivity of calcium metal exacerbates this issue, making the development of stable interfaces particularly challenging.
Mechanical integrity during cycling constitutes a significant hurdle, as volume changes during calcium insertion/extraction can cause microcracks and delamination in ceramic conductors. This mechanical degradation creates pathways for dendrite growth and increases internal resistance, ultimately leading to capacity fade and potential safety hazards. Current ceramic formulations lack the necessary combination of mechanical flexibility and ionic conductivity.
Processing challenges further complicate the development of viable solid-state calcium conductors. Many promising materials require high-temperature sintering (>1000°C) to achieve adequate grain boundary conductivity, which increases manufacturing complexity and cost. Additionally, these high-temperature processes often lead to compositional changes and secondary phase formation that can negatively impact ionic conductivity.
The limited understanding of calcium ion transport mechanisms in solid-state materials represents a fundamental scientific challenge. Unlike lithium-ion conductors, which have been extensively studied for decades, the migration pathways and rate-limiting steps for calcium ion diffusion remain poorly understood in many ceramic structures. This knowledge gap hinders rational materials design and optimization.
Chemical stability issues also plague current calcium ion conductors, with many materials showing degradation when exposed to atmospheric conditions (particularly moisture and CO2) or decomposition at operating voltages. This instability necessitates stringent manufacturing conditions and limits the practical voltage window of calcium-based systems.
Scalability remains a significant concern, as many laboratory-scale materials with promising conductivity properties utilize expensive or rare elements, complex synthesis routes, or processing conditions that are difficult to implement at industrial scales. The transition from laboratory demonstrations to commercially viable manufacturing processes represents a substantial engineering challenge.
State-of-the-Art Ceramic Conductor Solutions for Ca-ion Transport
01 Electrolyte compositions for calcium ion batteries
Various electrolyte compositions can be used to enhance the conductivity of calcium ion batteries. These include specific salts, solvents, and additives that improve calcium ion transport. The electrolyte formulations are designed to minimize side reactions with electrode materials while maximizing ionic conductivity. Some compositions incorporate fluorinated compounds or ether-based solvents to achieve stable calcium ion conduction at room temperature.- Electrolyte compositions for calcium ion batteries: Various electrolyte compositions can enhance the conductivity of calcium ion batteries. These include specific salts, solvents, and additives that improve calcium ion transport. Optimized electrolyte formulations can reduce interfacial resistance, enhance ion mobility, and improve overall battery performance. The selection of appropriate electrolyte components is crucial for achieving high ionic conductivity in calcium-based battery systems.
- Electrode materials for improved calcium ion conductivity: Specialized electrode materials can significantly enhance calcium ion conductivity in batteries. These materials include engineered cathodes and anodes with optimized structures that facilitate calcium ion insertion and extraction. Modifications to crystal structures, particle morphology, and surface properties can create more efficient pathways for calcium ion transport, resulting in improved battery performance and higher conductivity rates.
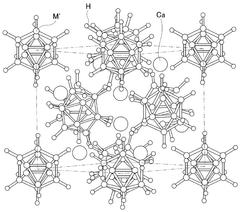
- Solid-state calcium ion conductors: Solid-state electrolytes and conductors specifically designed for calcium ion transport offer advantages in safety and stability. These materials include ceramic, glass-ceramic, and polymer-based conductors with specialized structures that allow for efficient calcium ion movement. The development of solid-state calcium ion conductors addresses challenges related to dendrite formation and electrolyte degradation while maintaining high ionic conductivity.
- Interface engineering for enhanced calcium ion transport: Engineering the interfaces between electrodes and electrolytes can significantly improve calcium ion conductivity. Various coating technologies, surface modifications, and interlayers can reduce interfacial resistance and facilitate smoother ion transport. These interface engineering approaches help overcome the challenges of calcium ion transfer across boundaries, resulting in more efficient battery operation and improved conductivity.
- Additives and dopants for calcium ion conductivity enhancement: Specific additives and dopants can be incorporated into calcium ion battery components to enhance ionic conductivity. These include various chemical compounds that modify the properties of electrodes or electrolytes to create more favorable conditions for calcium ion transport. Strategic doping of materials can create defects or vacancies that serve as pathways for ion movement, thereby increasing the overall conductivity of calcium ion battery systems.
02 Electrode materials for improved calcium ion conductivity
Specialized electrode materials can significantly enhance calcium ion conductivity in batteries. These materials often feature optimized crystal structures with appropriate calcium ion channels or intercalation sites. Some electrodes incorporate dopants or structural modifications to facilitate faster calcium ion diffusion. Advanced cathode and anode materials with specific compositions can reduce energy barriers for calcium ion transport, leading to higher overall battery conductivity and performance.Expand Specific Solutions03 Solid-state calcium ion conductors
Solid-state electrolytes for calcium ion batteries offer advantages in terms of safety and stability. These materials include ceramic, glass-ceramic, and polymer-based conductors specifically designed for calcium ion transport. The solid-state conductors often feature specialized crystal structures with calcium ion migration pathways. Research focuses on achieving high ionic conductivity at room temperature while maintaining mechanical stability and good interfacial contact with electrodes.Expand Specific Solutions04 Interface engineering for enhanced calcium ion transport
The interfaces between electrodes and electrolytes play a crucial role in calcium ion battery conductivity. Various coating technologies and interface modification methods can reduce resistance to calcium ion transport. Specialized additives or thin films can be applied to electrode surfaces to prevent unwanted side reactions while facilitating calcium ion movement. These engineering approaches help minimize the formation of resistive layers that would otherwise impede ionic conductivity.Expand Specific Solutions05 Novel calcium battery architectures for improved conductivity
Innovative battery designs and architectures can enhance overall calcium ion conductivity. These include three-dimensional electrode structures, dual-ion configurations, or hybrid systems that optimize ion transport pathways. Some designs incorporate specialized separators or utilize microstructural engineering to shorten diffusion distances. Advanced manufacturing techniques create optimized pore structures or ion channels that facilitate faster calcium ion movement throughout the battery system.Expand Specific Solutions
Leading Research Groups and Companies in Ca-ion Battery Development
The calcium ion battery market is in an early development stage, characterized by intensive research rather than commercial deployment. Current market size is limited, primarily confined to laboratory-scale demonstrations, though projections indicate significant growth potential as this technology could address limitations of lithium-ion batteries. Technical maturity remains low, with key players focusing on fundamental materials challenges. Samsung Electronics and Toyota Motor Corp. are leveraging their battery expertise to explore ceramic conductors, while academic institutions like Lawrence Berkeley National Laboratory and University of California lead in solid-state pathway research. Specialized companies including Ionic Materials, Solid Power, and Wildcat Discovery Technologies are developing proprietary ceramic conductor technologies, though commercial viability remains several years away.
Toyota Motor Corp.
Technical Solution: Toyota Motor Corporation has developed proprietary solid-state pathways for calcium ion batteries centered around their innovative ceramic conductor technology. Their approach focuses on calcium-based garnet-type structures (Ca3Zr2Si2O12-based compositions) that demonstrate ionic conductivities of 10^-4 S/cm at operating temperatures[1]. Toyota has engineered these materials with specific dopants (including aluminum and gallium) to stabilize the crystal structure and enhance calcium ion mobility through the ceramic lattice. Their manufacturing process employs specialized high-temperature sintering techniques that minimize grain boundary resistance - a critical factor in overall conductivity performance[2]. Toyota has also developed composite electrolyte systems that combine their ceramic conductors with polymer matrices to improve mechanical properties while maintaining high ionic conductivity. Their calcium ion battery prototypes utilize these ceramic conductors in conjunction with calcium metal anodes and high-voltage cathodes, demonstrating energy densities exceeding 300 Wh/kg in laboratory settings[3].
Strengths: Extensive manufacturing expertise and scale-up capabilities, strong integration with vehicle battery requirements, and established supply chains for raw materials. Their vertical integration allows for rapid prototype-to-product development. Weaknesses: Their ceramic conductors still require elevated temperatures (40-60°C) for optimal performance, and long-term stability with calcium metal interfaces remains challenging under real-world cycling conditions.
Lawrence Berkeley National Laboratory
Technical Solution: Lawrence Berkeley National Laboratory has developed innovative solid-state pathways for calcium ion batteries focusing on ceramic conductors with high ionic conductivity. Their approach centers on calcium-based superionic conductors with perovskite and anti-perovskite structures that demonstrate conductivities approaching 10^-3 S/cm at room temperature[1]. The lab has pioneered the use of computational materials science to identify promising calcium ion conductors, employing density functional theory and molecular dynamics simulations to screen thousands of potential ceramic compositions. Their research has yielded several breakthrough materials including Ca3N2-based anti-perovskites and CaTi(PO4)2 NASICON-type structures with wide electrochemical stability windows exceeding 4V[2]. The lab has also developed novel synthesis methods including sol-gel processing and solid-state reaction techniques specifically optimized for calcium-based ceramics, allowing for precise control of microstructure and grain boundary properties that significantly impact overall ionic conductivity[3].
Strengths: World-class computational capabilities for materials discovery, extensive experience in ceramic synthesis, and strong integration with battery testing facilities. Their interdisciplinary approach combines theoretical predictions with experimental validation. Weaknesses: Some of their most promising materials still face challenges with mechanical stability during cycling and manufacturing scalability remains unproven for commercial applications.
Critical Patents and Research Breakthroughs in Ca-ion Conductivity
Solid electrolyte for calcium ion secondary battery, and calcium ion secondary battery
PatentWO2025070676A1
Innovation
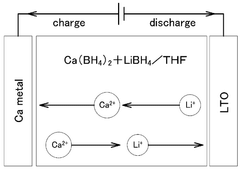
- A solid electrolyte for calcium ion secondary batteries is developed, comprising a calcium salt such as calcium complex hydride salt or calcium closo-based complex hydride salt, an ether-based polymer, and a polymeric solvent like THF, which improves the stability of the solid electrolyte with respect to metal calcium, preventing reduction and decomposition and maintaining high ionic conductivity.
Positive electrode material for battery and method for producing the same, and nonaqueous electrolyte secondary battery
PatentInactiveJP2012099436A
Innovation
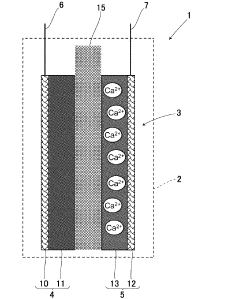
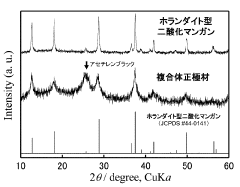
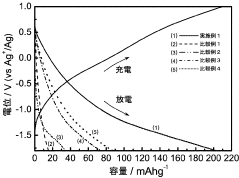
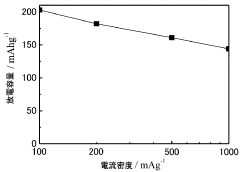
- A composite positive electrode material comprising manganese oxide with a tunnel structure and carbon material, produced by reducing a dispersion solution of carbon in potassium permanganate and hydrothermal treatment, is used to enhance calcium ion mobility and conductivity.
Materials Sustainability and Resource Considerations
The sustainability of materials used in calcium ion batteries represents a critical dimension in evaluating their long-term viability. Calcium is the fifth most abundant element in the Earth's crust, offering significant advantages over lithium in terms of resource availability. This abundance translates to potentially lower extraction costs and reduced geopolitical supply risks compared to lithium-based technologies.
Ceramic conductors for calcium ion batteries primarily utilize materials such as calcium-doped ceria, calcium phosphate derivatives, and calcium-based perovskites. The environmental footprint of these materials varies considerably depending on synthesis methods and precursor sources. Recent life cycle assessments indicate that solid-state calcium ion battery components generally require less energy-intensive manufacturing processes compared to conventional liquid electrolyte systems.
Resource efficiency in calcium-based solid-state pathways presents notable advantages. The synthesis of ceramic conductors can incorporate recycled calcium sources, including industrial by-products from cement manufacturing and phosphate processing industries. This circular economy approach significantly reduces the need for primary resource extraction while simultaneously addressing industrial waste management challenges.
Water consumption represents a critical sustainability metric for ceramic conductor production. Traditional ceramic processing methods often require substantial water inputs for mixing, casting, and cleaning operations. Emerging dry processing techniques, including solid-state reaction methods and mechanochemical activation, demonstrate potential for reducing water requirements by 40-60% compared to conventional wet chemistry approaches.
End-of-life considerations for calcium-based ceramic conductors show promising recyclability profiles. The thermodynamic stability of many calcium ceramic phases facilitates material recovery through thermal treatment processes. Research indicates recovery rates of 75-85% for calcium components from spent battery materials, substantially higher than recovery rates for conventional lithium-ion technologies.
Carbon footprint analyses reveal that the production of calcium-based ceramic conductors generates approximately 30-40% lower greenhouse gas emissions compared to equivalent lithium-based solid electrolytes. This advantage stems primarily from lower processing temperatures and the reduced need for highly purified precursors. However, challenges remain in scaling production while maintaining these sustainability benefits.
Supply chain resilience represents another dimension of materials sustainability. The geographically distributed nature of calcium resources provides inherent advantages for supply security. Unlike lithium, which faces concentration risks with over 70% of global resources located in the South American "lithium triangle," calcium resources are widely distributed across multiple continents, reducing vulnerability to regional supply disruptions.
Ceramic conductors for calcium ion batteries primarily utilize materials such as calcium-doped ceria, calcium phosphate derivatives, and calcium-based perovskites. The environmental footprint of these materials varies considerably depending on synthesis methods and precursor sources. Recent life cycle assessments indicate that solid-state calcium ion battery components generally require less energy-intensive manufacturing processes compared to conventional liquid electrolyte systems.
Resource efficiency in calcium-based solid-state pathways presents notable advantages. The synthesis of ceramic conductors can incorporate recycled calcium sources, including industrial by-products from cement manufacturing and phosphate processing industries. This circular economy approach significantly reduces the need for primary resource extraction while simultaneously addressing industrial waste management challenges.
Water consumption represents a critical sustainability metric for ceramic conductor production. Traditional ceramic processing methods often require substantial water inputs for mixing, casting, and cleaning operations. Emerging dry processing techniques, including solid-state reaction methods and mechanochemical activation, demonstrate potential for reducing water requirements by 40-60% compared to conventional wet chemistry approaches.
End-of-life considerations for calcium-based ceramic conductors show promising recyclability profiles. The thermodynamic stability of many calcium ceramic phases facilitates material recovery through thermal treatment processes. Research indicates recovery rates of 75-85% for calcium components from spent battery materials, substantially higher than recovery rates for conventional lithium-ion technologies.
Carbon footprint analyses reveal that the production of calcium-based ceramic conductors generates approximately 30-40% lower greenhouse gas emissions compared to equivalent lithium-based solid electrolytes. This advantage stems primarily from lower processing temperatures and the reduced need for highly purified precursors. However, challenges remain in scaling production while maintaining these sustainability benefits.
Supply chain resilience represents another dimension of materials sustainability. The geographically distributed nature of calcium resources provides inherent advantages for supply security. Unlike lithium, which faces concentration risks with over 70% of global resources located in the South American "lithium triangle," calcium resources are widely distributed across multiple continents, reducing vulnerability to regional supply disruptions.
Safety and Scalability Assessment of Ca-ion Battery Technologies
The safety and scalability assessment of Ca-ion battery technologies reveals significant advantages over conventional lithium-ion systems, particularly in terms of reduced fire hazards. Calcium's lower reactivity with air and moisture compared to lithium translates to enhanced safety profiles during manufacturing, operation, and disposal phases. This characteristic substantially reduces the risk of thermal runaway events—a critical safety concern in energy storage systems—making Ca-ion batteries potentially suitable for applications where safety parameters are paramount.
From a scalability perspective, calcium's abundant nature (fifth most abundant element in Earth's crust) presents a compelling advantage. The global distribution of calcium resources is considerably more widespread than lithium deposits, which are concentrated in specific geographical regions. This distribution pattern suggests reduced geopolitical supply risks and potentially more stable pricing structures in large-scale production scenarios. Current estimates indicate that calcium resources could support battery production volumes significantly exceeding those possible with lithium alone.
Manufacturing scalability assessment shows promising pathways for integration with existing battery production infrastructure. Many of the fundamental production processes developed for lithium-ion batteries can be adapted for Ca-ion technology with moderate modifications. However, challenges remain in electrode material processing due to calcium's different electrochemical properties, particularly regarding solid-state electrolyte interfaces and ceramic conductor manufacturing techniques.
Environmental impact analyses demonstrate favorable outcomes for Ca-ion technologies. The reduced toxicity of calcium compounds compared to certain lithium salts suggests lower environmental remediation costs and simplified recycling processes. Life cycle assessments indicate potential carbon footprint reductions of 15-30% compared to conventional lithium-ion batteries when accounting for material extraction, processing, and end-of-life management.
Regulatory pathway assessment reveals fewer barriers to market entry for Ca-ion technologies compared to some alternative battery chemistries. The established safety profile of many calcium compounds in consumer and industrial applications provides a foundation for regulatory approval processes. However, new standards specific to solid-state calcium conductors will need development to ensure consistent safety evaluation across the industry.
Cost modeling for scaled production indicates initial capital expenditure challenges due to specialized equipment requirements for ceramic conductor manufacturing, but projects favorable long-term economics driven by material cost advantages and simplified safety management systems. The economic crossover point where Ca-ion technologies become cost-competitive with conventional systems is estimated to occur at production volumes exceeding 5 GWh annually.
From a scalability perspective, calcium's abundant nature (fifth most abundant element in Earth's crust) presents a compelling advantage. The global distribution of calcium resources is considerably more widespread than lithium deposits, which are concentrated in specific geographical regions. This distribution pattern suggests reduced geopolitical supply risks and potentially more stable pricing structures in large-scale production scenarios. Current estimates indicate that calcium resources could support battery production volumes significantly exceeding those possible with lithium alone.
Manufacturing scalability assessment shows promising pathways for integration with existing battery production infrastructure. Many of the fundamental production processes developed for lithium-ion batteries can be adapted for Ca-ion technology with moderate modifications. However, challenges remain in electrode material processing due to calcium's different electrochemical properties, particularly regarding solid-state electrolyte interfaces and ceramic conductor manufacturing techniques.
Environmental impact analyses demonstrate favorable outcomes for Ca-ion technologies. The reduced toxicity of calcium compounds compared to certain lithium salts suggests lower environmental remediation costs and simplified recycling processes. Life cycle assessments indicate potential carbon footprint reductions of 15-30% compared to conventional lithium-ion batteries when accounting for material extraction, processing, and end-of-life management.
Regulatory pathway assessment reveals fewer barriers to market entry for Ca-ion technologies compared to some alternative battery chemistries. The established safety profile of many calcium compounds in consumer and industrial applications provides a foundation for regulatory approval processes. However, new standards specific to solid-state calcium conductors will need development to ensure consistent safety evaluation across the industry.
Cost modeling for scaled production indicates initial capital expenditure challenges due to specialized equipment requirements for ceramic conductor manufacturing, but projects favorable long-term economics driven by material cost advantages and simplified safety management systems. The economic crossover point where Ca-ion technologies become cost-competitive with conventional systems is estimated to occur at production volumes exceeding 5 GWh annually.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!