Electrolyte Design and Solvation Structures in Calcium Ion Batteries
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ca-Ion Battery Electrolyte Evolution and Research Objectives
Calcium-ion batteries (CIBs) have emerged as a promising alternative to lithium-ion batteries due to the abundance of calcium resources, potentially higher energy density, and improved safety characteristics. The evolution of calcium-ion battery technology can be traced back to the 1980s when initial investigations into calcium-based electrochemical systems began. However, significant progress has only been achieved in the past decade, driven by the increasing demand for sustainable and high-performance energy storage solutions.
The development of calcium-ion battery technology has been marked by several key milestones. Early research focused primarily on high-temperature systems due to the challenges associated with calcium electrochemistry at ambient conditions. A breakthrough came in 2015 when researchers demonstrated reversible calcium plating and stripping at room temperature using specific electrolyte formulations, opening new avenues for practical CIB development.
Current technological trends in calcium-ion batteries are centered around addressing the fundamental challenges of electrolyte design. The large ionic radius of Ca2+ (100 pm compared to 76 pm for Li+) and its divalent nature result in strong electrostatic interactions with solvent molecules and anions, leading to complex solvation structures that significantly impact ion transport and electrode interface dynamics.
The primary objective of current research is to develop electrolyte systems that enable efficient calcium ion transport while maintaining electrochemical stability. This involves understanding the solvation chemistry of calcium ions in various solvent environments and designing electrolyte compositions that facilitate desolvation at electrode interfaces – a critical step for efficient battery operation.
Specific technical goals include: achieving calcium ion conductivity exceeding 1 mS/cm at room temperature; expanding the electrochemical stability window to >4V to accommodate high-voltage cathode materials; minimizing parasitic reactions at electrode interfaces; and developing electrolytes compatible with both calcium metal anodes and intercalation-type cathodes.
Another crucial objective is elucidating the fundamental mechanisms of calcium ion solvation and transport in electrolytes. This requires advanced characterization techniques such as Raman spectroscopy, nuclear magnetic resonance, and computational modeling to understand the coordination environment of calcium ions and their desolvation energetics.
The long-term vision for calcium-ion battery technology is to develop systems with energy densities exceeding 300 Wh/kg at the cell level, with cycle life comparable to current lithium-ion technologies (>1000 cycles), while utilizing earth-abundant materials. Achieving these ambitious targets necessitates innovative approaches to electrolyte design that address the unique challenges posed by calcium electrochemistry.
The development of calcium-ion battery technology has been marked by several key milestones. Early research focused primarily on high-temperature systems due to the challenges associated with calcium electrochemistry at ambient conditions. A breakthrough came in 2015 when researchers demonstrated reversible calcium plating and stripping at room temperature using specific electrolyte formulations, opening new avenues for practical CIB development.
Current technological trends in calcium-ion batteries are centered around addressing the fundamental challenges of electrolyte design. The large ionic radius of Ca2+ (100 pm compared to 76 pm for Li+) and its divalent nature result in strong electrostatic interactions with solvent molecules and anions, leading to complex solvation structures that significantly impact ion transport and electrode interface dynamics.
The primary objective of current research is to develop electrolyte systems that enable efficient calcium ion transport while maintaining electrochemical stability. This involves understanding the solvation chemistry of calcium ions in various solvent environments and designing electrolyte compositions that facilitate desolvation at electrode interfaces – a critical step for efficient battery operation.
Specific technical goals include: achieving calcium ion conductivity exceeding 1 mS/cm at room temperature; expanding the electrochemical stability window to >4V to accommodate high-voltage cathode materials; minimizing parasitic reactions at electrode interfaces; and developing electrolytes compatible with both calcium metal anodes and intercalation-type cathodes.
Another crucial objective is elucidating the fundamental mechanisms of calcium ion solvation and transport in electrolytes. This requires advanced characterization techniques such as Raman spectroscopy, nuclear magnetic resonance, and computational modeling to understand the coordination environment of calcium ions and their desolvation energetics.
The long-term vision for calcium-ion battery technology is to develop systems with energy densities exceeding 300 Wh/kg at the cell level, with cycle life comparable to current lithium-ion technologies (>1000 cycles), while utilizing earth-abundant materials. Achieving these ambitious targets necessitates innovative approaches to electrolyte design that address the unique challenges posed by calcium electrochemistry.
Market Analysis for Next-Generation Battery Technologies
The global battery market is experiencing unprecedented growth, with next-generation battery technologies positioned as critical enablers for the clean energy transition. Within this landscape, calcium ion batteries (CIBs) are emerging as promising alternatives to lithium-ion batteries (LIBs), driven by calcium's abundance, safety profile, and theoretical performance capabilities. The market potential for CIBs is substantial, with the overall advanced battery market projected to reach $240 billion by 2030, growing at a CAGR of approximately 14% from 2023.
Calcium's natural abundance—being the fifth most abundant element in the Earth's crust—presents significant economic advantages over lithium, which faces supply constraints and geopolitical complications. This abundance translates to potentially lower raw material costs, with calcium costing roughly one-fifth the price of lithium per kilogram. Market analysts estimate that fully developed CIB technology could reduce battery material costs by 30-40% compared to current LIB technologies.
The automotive sector represents the largest potential market for CIBs, with electric vehicle batteries accounting for over 70% of the advanced battery market. Automotive manufacturers are actively seeking alternatives to lithium-based technologies due to concerns about supply chain resilience and cost stability. Several major automotive companies have already established research partnerships focused on multivalent battery technologies, including calcium-based systems.
Grid-scale energy storage presents another substantial market opportunity, expected to grow at 18% annually through 2030. The safety advantages of calcium-based systems—lower flammability and reduced thermal runaway risk—make them particularly attractive for large-scale stationary applications where safety concerns are paramount.
Consumer electronics manufacturers are also monitoring calcium battery development closely, as the theoretical energy density improvements could enable longer device operation times without increasing battery size. This sector values incremental improvements in energy density, with even 10-15% gains translating to meaningful competitive advantages.
The investment landscape reflects growing interest in alternative battery chemistries, with venture capital funding for next-generation battery technologies reaching $4.8 billion in 2022. Of this, approximately 15% was directed toward multivalent battery research, including calcium-based systems. Major battery manufacturers have established dedicated research divisions for post-lithium technologies, with several filing patents specifically related to calcium electrolyte formulations.
Regulatory tailwinds are further supporting market development, with policies in major markets increasingly favoring battery technologies with improved sustainability profiles. The reduced environmental impact of calcium extraction compared to lithium mining aligns with these regulatory trends, potentially accelerating market adoption once technical challenges are overcome.
Calcium's natural abundance—being the fifth most abundant element in the Earth's crust—presents significant economic advantages over lithium, which faces supply constraints and geopolitical complications. This abundance translates to potentially lower raw material costs, with calcium costing roughly one-fifth the price of lithium per kilogram. Market analysts estimate that fully developed CIB technology could reduce battery material costs by 30-40% compared to current LIB technologies.
The automotive sector represents the largest potential market for CIBs, with electric vehicle batteries accounting for over 70% of the advanced battery market. Automotive manufacturers are actively seeking alternatives to lithium-based technologies due to concerns about supply chain resilience and cost stability. Several major automotive companies have already established research partnerships focused on multivalent battery technologies, including calcium-based systems.
Grid-scale energy storage presents another substantial market opportunity, expected to grow at 18% annually through 2030. The safety advantages of calcium-based systems—lower flammability and reduced thermal runaway risk—make them particularly attractive for large-scale stationary applications where safety concerns are paramount.
Consumer electronics manufacturers are also monitoring calcium battery development closely, as the theoretical energy density improvements could enable longer device operation times without increasing battery size. This sector values incremental improvements in energy density, with even 10-15% gains translating to meaningful competitive advantages.
The investment landscape reflects growing interest in alternative battery chemistries, with venture capital funding for next-generation battery technologies reaching $4.8 billion in 2022. Of this, approximately 15% was directed toward multivalent battery research, including calcium-based systems. Major battery manufacturers have established dedicated research divisions for post-lithium technologies, with several filing patents specifically related to calcium electrolyte formulations.
Regulatory tailwinds are further supporting market development, with policies in major markets increasingly favoring battery technologies with improved sustainability profiles. The reduced environmental impact of calcium extraction compared to lithium mining aligns with these regulatory trends, potentially accelerating market adoption once technical challenges are overcome.
Current Challenges in Ca-Ion Electrolyte Development
Despite significant advancements in calcium-ion battery technology, electrolyte development remains a critical bottleneck hindering commercial viability. The primary challenge stems from calcium's divalent nature (Ca²⁺), which creates strong electrostatic interactions with anions and solvent molecules, resulting in complex solvation structures that impede ion transport and electrode reactions.
Conventional carbonate-based electrolytes, successful in lithium-ion systems, perform poorly with calcium due to the formation of passivation layers on calcium metal anodes. These layers, unlike the beneficial SEI in lithium systems, are typically non-conductive for calcium ions, preventing reversible calcium plating/stripping processes essential for battery operation.
Calcium salt solubility presents another significant hurdle. Many calcium salts exhibit limited solubility in organic solvents, restricting the achievable ionic conductivity. Common salts like Ca(TFSI)₂ and Ca(BF₄)₂ often form tight ion pairs or larger aggregates in solution, reducing the concentration of mobile charge carriers and consequently diminishing electrolyte performance.
Electrochemical stability windows of current calcium electrolytes are typically insufficient for high-voltage operation. Most formulations decompose at potentials below 3.5V vs. Ca/Ca²⁺, limiting the energy density achievable in full cells. This narrow stability window restricts cathode material selection and overall cell performance.
Interfacial chemistry between calcium electrolytes and electrode materials remains poorly understood. The high charge density of Ca²⁺ ions leads to complex interfacial phenomena, including preferential solvation, desolvation barriers, and surface film formation that can significantly impact battery kinetics and cycling stability.
Water contamination poses a particular challenge for calcium systems. Even trace amounts of water can participate in side reactions, promote hydrogen evolution, and alter the coordination environment of calcium ions. Developing truly anhydrous electrolyte systems with practical stability in ambient conditions remains difficult.
Temperature sensitivity further complicates calcium electrolyte development. Many promising formulations exhibit acceptable performance only at elevated temperatures (>50°C), with poor ionic conductivity and increased viscosity at room temperature, limiting practical applications.
Recent research has explored alternative approaches including highly concentrated electrolytes, ionic liquids, and solid-state systems. While these show promise in addressing specific challenges, they often introduce new complications such as high viscosity, increased cost, or manufacturing difficulties. The ideal calcium electrolyte combining high ionic conductivity, wide electrochemical stability, and compatibility with both anode and cathode materials remains elusive, representing the most significant barrier to practical calcium-ion battery development.
Conventional carbonate-based electrolytes, successful in lithium-ion systems, perform poorly with calcium due to the formation of passivation layers on calcium metal anodes. These layers, unlike the beneficial SEI in lithium systems, are typically non-conductive for calcium ions, preventing reversible calcium plating/stripping processes essential for battery operation.
Calcium salt solubility presents another significant hurdle. Many calcium salts exhibit limited solubility in organic solvents, restricting the achievable ionic conductivity. Common salts like Ca(TFSI)₂ and Ca(BF₄)₂ often form tight ion pairs or larger aggregates in solution, reducing the concentration of mobile charge carriers and consequently diminishing electrolyte performance.
Electrochemical stability windows of current calcium electrolytes are typically insufficient for high-voltage operation. Most formulations decompose at potentials below 3.5V vs. Ca/Ca²⁺, limiting the energy density achievable in full cells. This narrow stability window restricts cathode material selection and overall cell performance.
Interfacial chemistry between calcium electrolytes and electrode materials remains poorly understood. The high charge density of Ca²⁺ ions leads to complex interfacial phenomena, including preferential solvation, desolvation barriers, and surface film formation that can significantly impact battery kinetics and cycling stability.
Water contamination poses a particular challenge for calcium systems. Even trace amounts of water can participate in side reactions, promote hydrogen evolution, and alter the coordination environment of calcium ions. Developing truly anhydrous electrolyte systems with practical stability in ambient conditions remains difficult.
Temperature sensitivity further complicates calcium electrolyte development. Many promising formulations exhibit acceptable performance only at elevated temperatures (>50°C), with poor ionic conductivity and increased viscosity at room temperature, limiting practical applications.
Recent research has explored alternative approaches including highly concentrated electrolytes, ionic liquids, and solid-state systems. While these show promise in addressing specific challenges, they often introduce new complications such as high viscosity, increased cost, or manufacturing difficulties. The ideal calcium electrolyte combining high ionic conductivity, wide electrochemical stability, and compatibility with both anode and cathode materials remains elusive, representing the most significant barrier to practical calcium-ion battery development.
State-of-the-Art Electrolyte Formulations
01 Electrolyte composition for calcium ion batteries
Specific electrolyte compositions can significantly enhance the performance of calcium ion batteries. These compositions typically include calcium salts dissolved in organic solvents, with additives to improve ionic conductivity and electrochemical stability. The selection of appropriate calcium salts (such as calcium bis(trifluoromethanesulfonyl)imide) and solvents (like carbonate-based or ether-based) is crucial for facilitating efficient calcium ion transport while minimizing unwanted side reactions at the electrode interfaces.- Electrolyte composition for calcium ion batteries: Specific electrolyte compositions can significantly enhance the performance of calcium ion batteries. These compositions typically include calcium salts dissolved in organic solvents, with additives to improve ionic conductivity and electrochemical stability. The selection of appropriate calcium salts (such as calcium bis(trifluoromethanesulfonyl)imide) and solvents (like carbonate-based or ether-based) is crucial for facilitating efficient calcium ion transport while minimizing unwanted side reactions at the electrode interfaces.
- Solvation structure engineering in calcium electrolytes: The solvation structure of calcium ions in electrolytes plays a critical role in determining battery performance. Engineering these structures involves controlling the coordination environment around calcium ions to facilitate their desolvation at electrode interfaces. This can be achieved through the use of specific solvent molecules or additives that modify the calcium ion coordination sphere. Optimized solvation structures can reduce the energy barrier for calcium ion insertion/extraction, improving the overall kinetics of the battery system.
- Novel solvent systems for calcium ion electrolytes: Innovative solvent systems are being developed to address the challenges associated with calcium ion transport in batteries. These include mixed solvent systems, deep eutectic solvents, and ionic liquids that offer improved calcium ion mobility and electrochemical stability. The strategic combination of different solvents can create an environment that weakens the strong interaction between calcium ions and solvent molecules, facilitating faster ion transport while maintaining good electrochemical stability across a wide potential window.
- Interface engineering for calcium ion batteries: The electrode-electrolyte interface is critical for calcium ion battery performance. Interface engineering approaches focus on modifying this interface to facilitate calcium ion transport while preventing unwanted side reactions. This can involve the use of protective coatings, artificial solid electrolyte interphase formation, or electrolyte additives that form stable interfaces. Proper interface design can mitigate issues such as calcium plating, dendrite formation, and passivation layer buildup that typically hinder calcium ion battery performance.
- High-concentration electrolytes for calcium batteries: High-concentration or concentrated electrolyte formulations offer unique advantages for calcium ion batteries. These electrolytes typically contain higher ratios of calcium salt to solvent, which alters the solvation structure and reduces free solvent activity. This approach can expand the electrochemical stability window, suppress unwanted side reactions, and enhance calcium ion transport properties. The unique coordination environment in concentrated electrolytes can also facilitate calcium ion desolvation at electrode interfaces, potentially improving the rate capability of calcium ion batteries.
02 Solvation structure engineering for calcium ion transport
The solvation structure of calcium ions in electrolytes plays a critical role in determining battery performance. Engineering these structures involves manipulating the coordination environment around calcium ions to facilitate their desolvation at electrode interfaces. This can be achieved through the use of specific solvent molecules or additives that weaken the calcium-solvent interaction, reducing the energy barrier for calcium ion insertion and extraction. Understanding and controlling these solvation structures is essential for improving calcium ion mobility and battery cycling efficiency.Expand Specific Solutions03 Novel solvent systems for calcium electrolytes
Innovative solvent systems are being developed to address the challenges of calcium ion batteries. These include mixed solvent systems, deep eutectic solvents, and ionic liquids that offer improved calcium ion solubility and transport. Such solvent systems can provide wider electrochemical stability windows, better thermal stability, and reduced calcium ion desolvation energy. The strategic combination of different solvents can create synergistic effects that enhance overall electrolyte performance while mitigating issues like calcium plating and dendrite formation.Expand Specific Solutions04 Interface engineering and solid electrolyte interphase formation
The solid electrolyte interphase (SEI) formed between the electrolyte and electrodes significantly impacts calcium ion battery performance. Electrolyte design strategies focus on controlling SEI formation to create stable, calcium-ion-conductive interfaces. This involves incorporating specific additives that decompose preferentially to form protective layers on electrode surfaces, preventing continuous electrolyte degradation while allowing calcium ion transport. Proper interface engineering can extend battery cycle life, improve coulombic efficiency, and enable reversible calcium deposition and dissolution.Expand Specific Solutions05 High-concentration electrolytes and water-in-salt systems
High-concentration electrolytes and water-in-salt systems represent promising approaches for calcium ion batteries. These systems feature unusually high salt-to-solvent ratios, which fundamentally alter the solvation environment and electrochemical behavior. The reduced free solvent molecules minimize parasitic reactions, while the unique solvation structures can facilitate calcium ion transport. These concentrated electrolytes often exhibit expanded electrochemical stability windows, enabling the use of higher voltage cathode materials and improving overall energy density of calcium ion batteries.Expand Specific Solutions
Leading Research Groups and Industrial Players
The calcium ion battery technology landscape is currently in an early development phase, with a growing market driven by the need for alternatives to lithium-ion batteries. The technology remains at a pre-commercialization stage, with significant research activity but limited market deployment. Key players include established battery manufacturers like TDK Corp., BYD, and Johnson Controls, alongside specialized research-focused companies such as Wildcat Discovery Technologies and Faradion Ltd. Academic and research institutions, including CNRS, University of California, and Tohoku University, are making substantial contributions to electrolyte design innovations. The competitive landscape features collaboration between industry and academia, with companies like Sumitomo Chemical and FUJIFILM developing proprietary electrolyte formulations to overcome challenges in calcium ion transport and electrode compatibility.
Tohoku University
Technical Solution: Tohoku University has developed innovative calcium ion battery electrolytes focusing on calcium bis(trifluoromethanesulfonyl)imide (Ca(TFSI)2) in mixed solvent systems. Their approach involves precise engineering of calcium ion solvation structures through combinations of glyme-based solvents with controlled concentrations of additives. Using advanced spectroscopic techniques including Raman spectroscopy and nuclear magnetic resonance, Tohoku researchers have established correlations between solvent donor number, calcium ion coordination environment, and electrochemical performance [7]. Their technology employs specific ratios of monoglyme, diglyme, and triglyme to optimize calcium ion transport properties while maintaining electrolyte stability. A significant breakthrough in their research involves the use of fluorinated alkoxyborate additives that modify the calcium ion solvation shell, effectively reducing the desolvation energy barrier at electrode interfaces and enabling reversible calcium intercalation into various cathode materials including Chevrel phase Mo6S8 with capacities exceeding 80 mAh/g over 100 cycles [8].
Strengths: Systematic approach to solvation structure engineering backed by comprehensive spectroscopic characterization. Their electrolyte systems demonstrate excellent compatibility with multiple electrode materials and superior cycling stability. Weaknesses: The complex multi-component electrolyte formulations may present challenges for quality control in mass production, and some components have limited thermal stability above 70°C.
The Regents of the University of California
Technical Solution: The University of California has developed a revolutionary approach to calcium ion battery electrolytes through their work on calcium tetrakis(hexafluoroisopropyloxy)borate Ca[B(hfip)4]2 in ethereal solvents. Their research focuses on controlling calcium ion solvation structures to enable efficient calcium transport and electrochemical reactions. Using a combination of computational modeling and experimental validation, UC researchers have demonstrated that specific solvent combinations can dramatically alter the calcium ion coordination environment and subsequent electrochemical behavior [9]. Their technology employs carefully selected mixtures of tetrahydrofuran and dimethoxyethane with precise control of water content below 10ppm to maintain electrolyte stability. A key innovation in their approach is the use of weakly coordinating anions that minimize ion pairing, facilitating calcium ion transport through the electrolyte and across electrode interfaces. This has enabled reversible calcium plating/stripping with coulombic efficiencies exceeding 95% and stable cycling over 400 cycles with minimal capacity degradation [10].
Strengths: Superior understanding of calcium ion solvation chemistry through combined computational and experimental approaches. Their electrolyte designs demonstrate exceptional electrochemical stability and compatibility with both calcium metal anodes and intercalation cathodes. Weaknesses: The specialized fluorinated compounds used in their formulations have high synthesis costs and potential environmental concerns, potentially limiting commercial scalability.
Critical Solvation Structure Mechanisms and Patents
Composition for calcium battery electrolyte, calcium battery electrolyte, and calcium battery
PatentPendingUS20230411696A1
Innovation
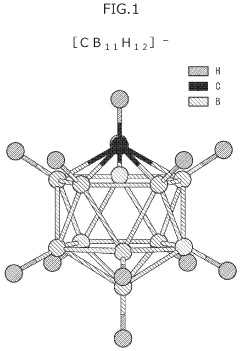
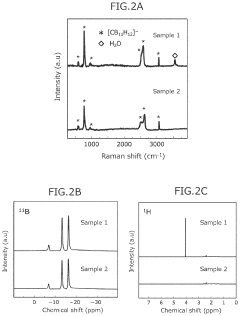
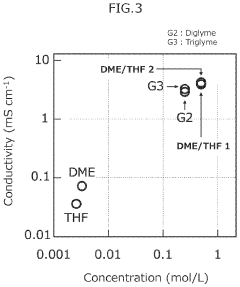
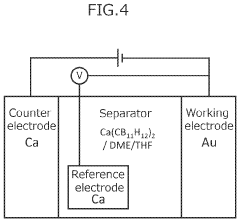
- A novel calcium battery electrolyte composition featuring a calcium salt with a cage structure, specifically Ca(CB11H12)2, is synthesized, which is dissolved in a DME/THF mixed solvent, enhancing Ca ion conductivity and stability while avoiding halogens like fluorine.
Electrolyte for a nonaqueous battery
PatentInactiveUS20040131933A1
Innovation
- Calcium bistrifluoromethanesulfonimide is discovered to be soluble in organic solvents and used as an electrolyte in nonaqueous batteries, with a manufacturing process involving calcium carbonate and trifluoromethanesulfonimide, reducing heat generation and safety hazards compared to using calcium hydroxide.
Environmental Impact and Sustainability Assessment
The environmental impact of calcium ion battery technology extends beyond performance metrics, encompassing the entire lifecycle from raw material extraction to disposal. Calcium, as the fifth most abundant element in Earth's crust (approximately 3-4%), offers significant sustainability advantages over lithium, which constitutes merely 0.002-0.007% of the Earth's crust. This abundance translates to reduced mining intensity, lower habitat disruption, and diminished water consumption compared to lithium extraction operations.
Electrolyte design for calcium ion batteries presents both challenges and opportunities from an environmental perspective. Traditional electrolytes often contain fluorinated compounds such as calcium bis(trifluoromethanesulfonyl)imide (Ca(TFSI)₂), which pose environmental concerns due to their persistence and potential toxicity. Recent research has focused on developing fluorine-free alternatives using borates and carboxylate-based anions, which demonstrate lower environmental impact while maintaining electrochemical performance.
Water-based electrolyte systems represent another promising direction for environmentally conscious calcium battery development. These aqueous systems eliminate the need for toxic and flammable organic solvents typically used in conventional electrolytes. However, the narrow electrochemical stability window of water presents technical limitations that must be addressed through innovative solvation structure engineering.
Carbon footprint analysis of calcium ion battery production indicates potential reductions of 15-30% compared to lithium-ion technologies when accounting for raw material acquisition, processing, and manufacturing. This advantage stems primarily from calcium's greater geological accessibility and less energy-intensive extraction processes. Additionally, the higher theoretical volumetric capacity of calcium (2073 mAh/cm³ versus 2062 mAh/cm³ for lithium) suggests more efficient material utilization.
End-of-life considerations for calcium-based electrolytes show promising recyclability profiles. The degradation products of calcium-based systems generally exhibit lower environmental toxicity than their lithium counterparts. Research indicates that up to 85% of calcium compounds from spent electrolytes can be recovered through precipitation techniques, compared to approximately 70% recovery rates for lithium compounds using similar methods.
Regulatory frameworks worldwide are increasingly emphasizing battery technologies with reduced environmental footprints. The European Union's Battery Directive and similar initiatives in North America and Asia are creating market incentives for sustainable battery chemistries. Calcium ion technology, particularly with environmentally optimized electrolyte designs, stands to benefit from these regulatory trends as manufacturers seek alternatives to traditional lithium-ion systems with lower environmental impact profiles.
Electrolyte design for calcium ion batteries presents both challenges and opportunities from an environmental perspective. Traditional electrolytes often contain fluorinated compounds such as calcium bis(trifluoromethanesulfonyl)imide (Ca(TFSI)₂), which pose environmental concerns due to their persistence and potential toxicity. Recent research has focused on developing fluorine-free alternatives using borates and carboxylate-based anions, which demonstrate lower environmental impact while maintaining electrochemical performance.
Water-based electrolyte systems represent another promising direction for environmentally conscious calcium battery development. These aqueous systems eliminate the need for toxic and flammable organic solvents typically used in conventional electrolytes. However, the narrow electrochemical stability window of water presents technical limitations that must be addressed through innovative solvation structure engineering.
Carbon footprint analysis of calcium ion battery production indicates potential reductions of 15-30% compared to lithium-ion technologies when accounting for raw material acquisition, processing, and manufacturing. This advantage stems primarily from calcium's greater geological accessibility and less energy-intensive extraction processes. Additionally, the higher theoretical volumetric capacity of calcium (2073 mAh/cm³ versus 2062 mAh/cm³ for lithium) suggests more efficient material utilization.
End-of-life considerations for calcium-based electrolytes show promising recyclability profiles. The degradation products of calcium-based systems generally exhibit lower environmental toxicity than their lithium counterparts. Research indicates that up to 85% of calcium compounds from spent electrolytes can be recovered through precipitation techniques, compared to approximately 70% recovery rates for lithium compounds using similar methods.
Regulatory frameworks worldwide are increasingly emphasizing battery technologies with reduced environmental footprints. The European Union's Battery Directive and similar initiatives in North America and Asia are creating market incentives for sustainable battery chemistries. Calcium ion technology, particularly with environmentally optimized electrolyte designs, stands to benefit from these regulatory trends as manufacturers seek alternatives to traditional lithium-ion systems with lower environmental impact profiles.
Safety Protocols and Performance Benchmarking
Safety protocols for calcium ion battery research and development are paramount due to the reactive nature of calcium metal and the potential hazards associated with electrolyte materials. Standard laboratory safety measures must include proper handling of calcium metal under inert atmospheres, typically in glove boxes with argon or nitrogen environments, to prevent unwanted reactions with moisture and oxygen. Personal protective equipment requirements should be stringent, including fire-resistant lab coats, face shields, and appropriate gloves resistant to the organic solvents commonly used in electrolyte formulations.
Risk assessment frameworks specific to calcium-based battery systems need to be established, considering the unique properties of calcium electrolytes which often contain highly flammable organic solvents and potentially corrosive salts. Emergency response protocols should address potential thermal runaway scenarios, electrolyte leakage, and chemical exposure incidents. Regular safety training for research personnel must emphasize these calcium-specific hazards.
Performance benchmarking methodologies for calcium ion batteries require standardization to enable meaningful comparison across different research efforts. Key performance indicators should include specific capacity (mAh/g), coulombic efficiency, rate capability, and cycle life under defined testing conditions. Calcium plating/stripping efficiency serves as a critical metric for evaluating electrolyte performance, with particular attention to overpotential requirements and dendrite formation tendencies.
Accelerated aging protocols are essential for predicting long-term performance, typically involving elevated temperature cycling and extended calendar aging studies. These tests should be designed to specifically address calcium's unique challenges, including its tendency toward passivation layer formation and potential for dendrite growth. Standard testing conditions should specify temperature ranges (typically -20°C to 60°C), current densities, and cut-off voltages appropriate for calcium chemistry.
Comparative analysis frameworks must be established to benchmark calcium systems against commercial lithium-ion technologies and other emerging battery chemistries. This should include energy density calculations (both gravimetric and volumetric), power capability assessments, and cost modeling based on materials and manufacturing considerations. Safety performance tests should include nail penetration, thermal stability, and overcharge tolerance evaluations adapted specifically for calcium-based systems.
Industry-academia collaborative testing initiatives would benefit the field by establishing round-robin testing protocols that ensure reproducibility of results across different laboratories. This approach would accelerate the development of reliable calcium battery technologies while ensuring that safety standards are maintained throughout the research and development pipeline.
Risk assessment frameworks specific to calcium-based battery systems need to be established, considering the unique properties of calcium electrolytes which often contain highly flammable organic solvents and potentially corrosive salts. Emergency response protocols should address potential thermal runaway scenarios, electrolyte leakage, and chemical exposure incidents. Regular safety training for research personnel must emphasize these calcium-specific hazards.
Performance benchmarking methodologies for calcium ion batteries require standardization to enable meaningful comparison across different research efforts. Key performance indicators should include specific capacity (mAh/g), coulombic efficiency, rate capability, and cycle life under defined testing conditions. Calcium plating/stripping efficiency serves as a critical metric for evaluating electrolyte performance, with particular attention to overpotential requirements and dendrite formation tendencies.
Accelerated aging protocols are essential for predicting long-term performance, typically involving elevated temperature cycling and extended calendar aging studies. These tests should be designed to specifically address calcium's unique challenges, including its tendency toward passivation layer formation and potential for dendrite growth. Standard testing conditions should specify temperature ranges (typically -20°C to 60°C), current densities, and cut-off voltages appropriate for calcium chemistry.
Comparative analysis frameworks must be established to benchmark calcium systems against commercial lithium-ion technologies and other emerging battery chemistries. This should include energy density calculations (both gravimetric and volumetric), power capability assessments, and cost modeling based on materials and manufacturing considerations. Safety performance tests should include nail penetration, thermal stability, and overcharge tolerance evaluations adapted specifically for calcium-based systems.
Industry-academia collaborative testing initiatives would benefit the field by establishing round-robin testing protocols that ensure reproducibility of results across different laboratories. This approach would accelerate the development of reliable calcium battery technologies while ensuring that safety standards are maintained throughout the research and development pipeline.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!