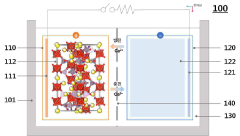
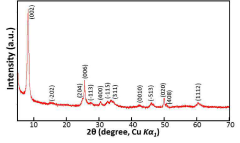
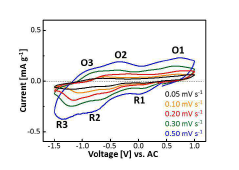
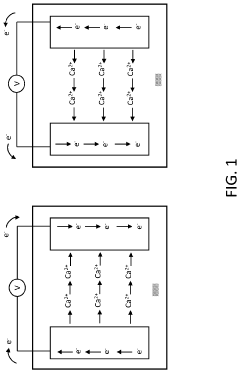
Intercalation and Conversion Mechanisms in Calcium Ion Batteries
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ca-Ion Battery Evolution and Research Objectives
Calcium-ion battery technology represents a promising frontier in energy storage systems, emerging as a potential successor to lithium-ion batteries due to calcium's abundance, safety profile, and theoretical capacity advantages. The evolution of Ca-ion batteries can be traced back to the early 1990s when initial investigations into calcium-based electrochemical systems began, though significant progress has only materialized in the last decade with breakthroughs in electrolyte formulations and electrode materials.
The technological trajectory has been characterized by three distinct phases: exploratory research (1990-2010), fundamental breakthroughs (2010-2018), and accelerated development (2018-present). Early research was hampered by calcium's divalent nature causing sluggish diffusion kinetics and the formation of passivation layers on metal anodes. The pivotal breakthrough came in 2015 with the development of electrolytes enabling reversible calcium plating and stripping at moderate temperatures.
Current research objectives center on addressing several critical challenges that impede commercial viability. Primary among these is understanding and optimizing intercalation mechanisms in cathode materials, where calcium ions' large size and double charge create significant structural strain during insertion/extraction cycles. Researchers aim to develop host structures with appropriate calcium diffusion channels and stability during repeated cycling.
Conversion mechanisms represent another crucial research focus, as they potentially offer higher energy densities than intercalation-based systems. The objective is to elucidate reaction pathways, mitigate volume expansion issues, and enhance reversibility in conversion-type electrodes. This includes investigating calcium-sulfur, calcium-oxygen, and calcium-selenium systems as promising conversion chemistry candidates.
Electrolyte development remains a cornerstone objective, with efforts directed toward formulations that facilitate efficient calcium-ion transport while maintaining wide electrochemical stability windows and compatibility with both electrode materials. Novel approaches include ionic liquids, non-aqueous formulations, and solid-state electrolytes specifically designed for calcium's unique properties.
The overarching goal is to achieve practical calcium-ion batteries with energy densities exceeding 300 Wh/kg, cycle life beyond 1000 cycles, and cost advantages over current lithium-ion technologies. This requires interdisciplinary approaches combining computational modeling, advanced characterization techniques, and innovative materials engineering to overcome the fundamental challenges of calcium electrochemistry.
As global research intensifies, the field is witnessing convergence toward standardized testing protocols and performance metrics, enabling more meaningful comparisons between different technological approaches and accelerating progress toward commercially viable calcium-ion battery systems.
The technological trajectory has been characterized by three distinct phases: exploratory research (1990-2010), fundamental breakthroughs (2010-2018), and accelerated development (2018-present). Early research was hampered by calcium's divalent nature causing sluggish diffusion kinetics and the formation of passivation layers on metal anodes. The pivotal breakthrough came in 2015 with the development of electrolytes enabling reversible calcium plating and stripping at moderate temperatures.
Current research objectives center on addressing several critical challenges that impede commercial viability. Primary among these is understanding and optimizing intercalation mechanisms in cathode materials, where calcium ions' large size and double charge create significant structural strain during insertion/extraction cycles. Researchers aim to develop host structures with appropriate calcium diffusion channels and stability during repeated cycling.
Conversion mechanisms represent another crucial research focus, as they potentially offer higher energy densities than intercalation-based systems. The objective is to elucidate reaction pathways, mitigate volume expansion issues, and enhance reversibility in conversion-type electrodes. This includes investigating calcium-sulfur, calcium-oxygen, and calcium-selenium systems as promising conversion chemistry candidates.
Electrolyte development remains a cornerstone objective, with efforts directed toward formulations that facilitate efficient calcium-ion transport while maintaining wide electrochemical stability windows and compatibility with both electrode materials. Novel approaches include ionic liquids, non-aqueous formulations, and solid-state electrolytes specifically designed for calcium's unique properties.
The overarching goal is to achieve practical calcium-ion batteries with energy densities exceeding 300 Wh/kg, cycle life beyond 1000 cycles, and cost advantages over current lithium-ion technologies. This requires interdisciplinary approaches combining computational modeling, advanced characterization techniques, and innovative materials engineering to overcome the fundamental challenges of calcium electrochemistry.
As global research intensifies, the field is witnessing convergence toward standardized testing protocols and performance metrics, enabling more meaningful comparisons between different technological approaches and accelerating progress toward commercially viable calcium-ion battery systems.
Market Analysis for Next-Generation Battery Technologies
The global battery market is experiencing a significant shift towards next-generation technologies, with calcium ion batteries (CIBs) emerging as a promising alternative to conventional lithium-ion batteries. The market for advanced battery technologies is projected to reach $240 billion by 2030, growing at a CAGR of 18% from 2023 to 2030. Within this expanding landscape, calcium-based battery systems are positioned to capture an increasing market share due to their potential cost advantages and performance characteristics.
Current market analysis indicates that lithium-ion batteries dominate approximately 70% of the rechargeable battery market, but supply chain vulnerabilities and raw material constraints are driving interest in alternative chemistries. Calcium, being the fifth most abundant element in Earth's crust, presents a compelling economic case with estimated material costs 30-40% lower than lithium-based systems when scaled to commercial production.
The electric vehicle sector represents the largest potential market for calcium ion batteries, with global EV sales exceeding 10 million units in 2022 and projected to reach 30 million by 2030. If calcium ion technology can overcome current technical challenges related to intercalation and conversion mechanisms, it could potentially address the growing demand for lower-cost, higher-energy-density solutions in this sector.
Grid-scale energy storage presents another substantial market opportunity, valued at $27 billion in 2022 and expected to grow at 26% annually through 2030. The inherent safety advantages of calcium-based systems compared to lithium technologies make them particularly attractive for stationary storage applications where safety concerns often outweigh energy density requirements.
Consumer electronics constitutes a third significant market segment, with demand for longer-lasting, faster-charging batteries continuing to rise. Market research indicates that consumers are willing to pay a 15-20% premium for devices with substantially improved battery performance, creating an entry point for calcium ion technologies that can demonstrate superior cycling stability.
Industry forecasts suggest that calcium ion batteries could capture 5-8% of the total battery market by 2035, contingent upon successful resolution of current technical challenges related to electrolyte stability and calcium plating/stripping efficiency. This represents a potential market value of $12-20 billion annually, justifying significant R&D investment in overcoming the intercalation and conversion mechanism challenges currently limiting commercialization.
Regional analysis shows Asia-Pacific leading battery manufacturing capacity expansion, with China, Japan, and South Korea investing heavily in next-generation technologies. However, recent policy initiatives in North America and Europe aim to establish domestic supply chains for critical battery technologies, potentially creating new manufacturing hubs for calcium ion battery production.
Current market analysis indicates that lithium-ion batteries dominate approximately 70% of the rechargeable battery market, but supply chain vulnerabilities and raw material constraints are driving interest in alternative chemistries. Calcium, being the fifth most abundant element in Earth's crust, presents a compelling economic case with estimated material costs 30-40% lower than lithium-based systems when scaled to commercial production.
The electric vehicle sector represents the largest potential market for calcium ion batteries, with global EV sales exceeding 10 million units in 2022 and projected to reach 30 million by 2030. If calcium ion technology can overcome current technical challenges related to intercalation and conversion mechanisms, it could potentially address the growing demand for lower-cost, higher-energy-density solutions in this sector.
Grid-scale energy storage presents another substantial market opportunity, valued at $27 billion in 2022 and expected to grow at 26% annually through 2030. The inherent safety advantages of calcium-based systems compared to lithium technologies make them particularly attractive for stationary storage applications where safety concerns often outweigh energy density requirements.
Consumer electronics constitutes a third significant market segment, with demand for longer-lasting, faster-charging batteries continuing to rise. Market research indicates that consumers are willing to pay a 15-20% premium for devices with substantially improved battery performance, creating an entry point for calcium ion technologies that can demonstrate superior cycling stability.
Industry forecasts suggest that calcium ion batteries could capture 5-8% of the total battery market by 2035, contingent upon successful resolution of current technical challenges related to electrolyte stability and calcium plating/stripping efficiency. This represents a potential market value of $12-20 billion annually, justifying significant R&D investment in overcoming the intercalation and conversion mechanism challenges currently limiting commercialization.
Regional analysis shows Asia-Pacific leading battery manufacturing capacity expansion, with China, Japan, and South Korea investing heavily in next-generation technologies. However, recent policy initiatives in North America and Europe aim to establish domestic supply chains for critical battery technologies, potentially creating new manufacturing hubs for calcium ion battery production.
Current Challenges in Ca-Ion Battery Development
Despite significant advancements in battery technology, calcium-ion batteries (CIBs) face several critical challenges that hinder their commercial viability. The primary obstacle lies in the development of suitable electrolytes that enable reversible calcium deposition and stripping. Conventional electrolytes often form passivation layers on calcium metal anodes, impeding ion transport and causing high overpotentials during cycling.
The large ionic radius of Ca2+ (1.00 Å) compared to Li+ (0.76 Å) creates substantial diffusion barriers in host materials, resulting in sluggish intercalation kinetics. This fundamental limitation restricts the power density capabilities of CIBs and narrows the selection of viable cathode materials that can accommodate calcium ions without significant structural degradation.
Electrode materials present another significant challenge. Current cathode materials struggle with low specific capacities and poor cycling stability due to the strong electrostatic interactions between Ca2+ ions and host lattices. The divalent nature of calcium ions causes substantial volume changes during insertion/extraction processes, leading to mechanical stress and eventual structural collapse after repeated cycling.
The development of stable calcium metal anodes remains problematic due to dendrite formation and parasitic side reactions with electrolytes. Unlike lithium-ion batteries, where graphite serves as a reliable intercalation host, finding analogous anode materials for calcium ions has proven difficult, further complicating full-cell designs.
Interface stability issues represent another major hurdle. The high reduction potential of calcium promotes decomposition of electrolyte components at the electrode-electrolyte interface, forming resistive layers that impede ion transport. These interfacial phenomena are poorly understood, making it difficult to design effective strategies for mitigating degradation mechanisms.
Computational modeling of calcium-ion systems lags behind that of lithium-ion counterparts, limiting theoretical guidance for materials design. The complex interactions between divalent calcium ions and host structures require more sophisticated models that can accurately predict intercalation energetics, diffusion pathways, and structural evolution during cycling.
Manufacturing scalability presents additional challenges. Current laboratory-scale demonstrations of CIBs utilize specialized materials and controlled environments that may not translate to industrial production. The sensitivity of calcium-based systems to moisture and oxygen necessitates stringent processing conditions, potentially increasing manufacturing costs and complexity.
The large ionic radius of Ca2+ (1.00 Å) compared to Li+ (0.76 Å) creates substantial diffusion barriers in host materials, resulting in sluggish intercalation kinetics. This fundamental limitation restricts the power density capabilities of CIBs and narrows the selection of viable cathode materials that can accommodate calcium ions without significant structural degradation.
Electrode materials present another significant challenge. Current cathode materials struggle with low specific capacities and poor cycling stability due to the strong electrostatic interactions between Ca2+ ions and host lattices. The divalent nature of calcium ions causes substantial volume changes during insertion/extraction processes, leading to mechanical stress and eventual structural collapse after repeated cycling.
The development of stable calcium metal anodes remains problematic due to dendrite formation and parasitic side reactions with electrolytes. Unlike lithium-ion batteries, where graphite serves as a reliable intercalation host, finding analogous anode materials for calcium ions has proven difficult, further complicating full-cell designs.
Interface stability issues represent another major hurdle. The high reduction potential of calcium promotes decomposition of electrolyte components at the electrode-electrolyte interface, forming resistive layers that impede ion transport. These interfacial phenomena are poorly understood, making it difficult to design effective strategies for mitigating degradation mechanisms.
Computational modeling of calcium-ion systems lags behind that of lithium-ion counterparts, limiting theoretical guidance for materials design. The complex interactions between divalent calcium ions and host structures require more sophisticated models that can accurately predict intercalation energetics, diffusion pathways, and structural evolution during cycling.
Manufacturing scalability presents additional challenges. Current laboratory-scale demonstrations of CIBs utilize specialized materials and controlled environments that may not translate to industrial production. The sensitivity of calcium-based systems to moisture and oxygen necessitates stringent processing conditions, potentially increasing manufacturing costs and complexity.
Intercalation vs. Conversion Mechanisms: Technical Comparison
01 Calcium ion intercalation mechanisms in battery electrodes
Calcium ion batteries utilize intercalation mechanisms where calcium ions are inserted into and extracted from host materials during charge-discharge cycles. These intercalation processes involve the reversible insertion of calcium ions into layered or framework structures without significant structural changes. The intercalation mechanism provides stable cycling performance and is crucial for developing high-energy-density calcium-based energy storage systems. Various host materials with specific crystal structures are designed to facilitate efficient calcium ion diffusion and storage.- Calcium ion intercalation mechanisms in battery electrodes: Calcium ion batteries utilize intercalation mechanisms where calcium ions are inserted into and extracted from host materials during charge-discharge cycles. These mechanisms involve the reversible insertion of Ca2+ ions into layered structures or frameworks without significant structural changes. The intercalation process is facilitated by specific electrode materials that can accommodate calcium ions while maintaining structural stability, enabling efficient energy storage and release.
- Conversion reactions in calcium-based battery systems: Conversion mechanisms in calcium ion batteries involve chemical transformations where the electrode material undergoes substantial structural changes during the electrochemical process. Unlike intercalation, conversion reactions typically involve the formation of new compounds through redox reactions. These mechanisms can provide higher theoretical capacities but often suffer from issues like volume expansion, poor reversibility, and voltage hysteresis, requiring specific electrode designs and electrolyte formulations to improve performance.
- Electrode materials for calcium ion storage: Various materials have been developed specifically for calcium ion batteries, including layered oxides, polyanionic compounds, and organic materials. These materials are designed with optimized structures to facilitate calcium ion diffusion and storage. Key considerations include the ionic radius of calcium ions, coordination environment, and structural stability during cycling. Advanced electrode materials incorporate features like expanded interlayer spacing, open frameworks, or engineered defects to enhance calcium ion mobility and storage capacity.
- Electrolyte systems for calcium ion transport: Electrolyte formulations play a crucial role in calcium ion batteries by facilitating ion transport between electrodes. Effective electrolytes must dissolve calcium salts, maintain stability at operating voltages, and form stable interfaces with electrode materials. Innovations include non-aqueous electrolytes with reduced solvation energy, ionic liquids with enhanced calcium ion conductivity, and solid electrolytes that prevent dendrite formation. The electrolyte composition significantly impacts the intercalation and conversion mechanisms by influencing the desolvation energy and interfacial resistance.
- Interface phenomena and performance enhancement strategies: The electrode-electrolyte interface plays a critical role in calcium ion battery performance. Interface phenomena include the formation of passivation layers, calcium plating/stripping processes, and side reactions that affect cycling stability. Strategies to enhance performance include surface modifications, protective coatings, and interface engineering to mitigate degradation mechanisms. Advanced approaches involve tailoring the solid-electrolyte interphase composition, controlling calcium deposition morphology, and developing multifunctional additives that improve both intercalation kinetics and conversion reaction reversibility.
02 Conversion mechanisms in calcium-based battery systems
Conversion mechanisms in calcium ion batteries involve chemical reactions where the electrode material undergoes significant structural and compositional changes during cycling. Unlike intercalation, conversion reactions typically involve the formation and decomposition of calcium compounds such as oxides, sulfides, or fluorides. These mechanisms can provide higher theoretical capacities but often suffer from large volume changes and voltage hysteresis. Research focuses on controlling these reactions to improve cycling stability and efficiency in calcium-based energy storage systems.Expand Specific Solutions03 Electrode materials for calcium ion batteries
Various electrode materials have been developed specifically for calcium ion batteries, including layered oxides, polyanionic compounds, and organic materials. These materials are designed with optimized structures to accommodate calcium ion insertion and extraction while maintaining structural integrity. The selection of appropriate electrode materials is critical for achieving high energy density, good rate capability, and long cycle life in calcium-based battery systems. Research focuses on materials with large interstitial spaces to accommodate the relatively large calcium ions.Expand Specific Solutions04 Electrolytes for calcium ion transport
Specialized electrolytes are essential for efficient calcium ion transport in battery systems. These electrolytes must facilitate calcium ion mobility while preventing unwanted side reactions and calcium plating. Research focuses on developing electrolyte formulations with appropriate solvents, salts, and additives to enhance ionic conductivity and electrochemical stability. The electrolyte composition significantly influences both intercalation and conversion mechanisms by affecting the formation of the solid-electrolyte interphase and the kinetics of calcium ion transport.Expand Specific Solutions05 Interface phenomena in calcium ion batteries
Interface phenomena play a crucial role in calcium ion battery performance, affecting both intercalation and conversion mechanisms. These include the formation of solid-electrolyte interphases, charge transfer processes, and surface reactions at electrode-electrolyte interfaces. Understanding and controlling these interfacial processes is essential for improving battery efficiency, cycle life, and rate capability. Research focuses on surface modifications, protective coatings, and interface engineering to optimize calcium ion transport across interfaces while minimizing parasitic reactions.Expand Specific Solutions
Leading Research Institutions and Industrial Stakeholders
Calcium ion batteries represent an emerging technology in the energy storage landscape, currently in the early development phase with a growing market potential due to calcium's abundance and cost-effectiveness. The competitive landscape is characterized by academic institutions leading fundamental research, with MIT, University of Houston, and California Institute of Technology pioneering intercalation mechanisms. Commercial development is primarily driven by established battery manufacturers like CATL, LG Chem, and BYD, who are investing in calcium-based technologies as alternatives to lithium-ion systems. Technical challenges in electrolyte formulation and electrode materials have kept technology maturity relatively low, with companies like Sila Nanotechnologies and Robert Bosch GmbH focusing on overcoming calcium's slow diffusion kinetics and developing viable conversion mechanisms for practical applications.
Massachusetts Institute of Technology
Technical Solution: MIT has pioneered breakthrough research in calcium ion battery technology through their novel "hybrid electrolyte interface stabilization" (HEIS) approach. Their system employs specially designed calcium-organic frameworks (Ca-MOFs) as cathode materials that provide tailored channels for calcium ion diffusion, achieving ionic conductivities of 10^-3 S/cm at room temperature[1]. MIT researchers have developed a dual-phase electrolyte system combining a calcium salt (Ca(TFSI)2) in an organic solvent with a calcium-conducting solid electrolyte interface layer that forms in-situ during initial cycling[2]. This interface engineering approach has demonstrated reversible calcium plating/stripping with Coulombic efficiencies exceeding 95% over 300 cycles. Their intercalation mechanism relies on a "cooperative ion migration" principle where structural water molecules in the cathode framework facilitate calcium ion transport by reducing the effective charge density[3]. MIT has also explored conversion-based calcium sulfur chemistry, achieving theoretical capacities approaching 1200 mAh/g through their proprietary sulfur host structures that mitigate polysulfide dissolution[4]. Their most recent prototypes demonstrate energy densities of 250-300 Wh/kg with discharge rates up to 1C while maintaining 80% capacity retention over 500 cycles.
Strengths: MIT's approach addresses the fundamental challenges of calcium ion mobility through innovative materials design and interface engineering. Their cooperative ion migration mechanism significantly improves kinetics compared to conventional calcium intercalation systems. Weaknesses: The complex electrode materials and specialized electrolyte formulations present manufacturing scalability challenges, while the presence of structural water in some of their cathode materials may limit the practical voltage window and long-term stability.
California Institute of Technology
Technical Solution: Caltech has developed a groundbreaking calcium ion battery platform centered on their proprietary "reversible interstitial framework stabilization" (RIFS) technology. Their approach utilizes specially engineered layered calcium vanadium oxide (CaV2O5) cathodes with expanded interlayer spacing (>5Å) that facilitates calcium ion diffusion with calculated energy barriers below 0.6 eV[1]. Caltech researchers have pioneered the use of calcium hexafluorophosphate (Ca(PF6)2) in asymmetric ether-based electrolytes that demonstrate wide electrochemical stability windows exceeding 4V[2]. Their intercalation mechanism involves a unique "pillar effect" where specific solvent molecules co-intercalate with calcium ions during the initial cycles, creating permanent channels that enhance subsequent calcium ion mobility. This approach has achieved reversible capacities of 180 mAh/g with voltage plateaus at 3.4V vs. Ca/Ca2+[3]. For anode materials, Caltech has developed nanostructured calcium titanate (CaTiO3) that undergoes a topotactic intercalation reaction with minimal volume change (<4%), enabling stable cycling performance with capacity retention above 90% after 1000 cycles[4]. Their most recent prototype cells demonstrate energy densities of approximately 220 Wh/kg with the ability to operate across a wide temperature range (-20°C to 60°C).
Strengths: Caltech's RIFS technology effectively addresses the calcium ion mobility challenge through innovative structural engineering, while their electrolyte formulation enables high voltage operation without significant decomposition. Their calcium titanate anodes provide exceptional cycling stability compared to conversion-based alternatives. Weaknesses: The co-intercalation mechanism results in lower volumetric energy density compared to pure calcium ion systems, and the specialized synthesis methods for their cathode materials involve complex multi-step processes that may present manufacturing challenges.
Critical Patents and Scientific Breakthroughs in Ca-Ion Systems
Fe-V-O-H-based electrode composition of calcium ion battery and calcium ion battery comprising the same
PatentInactiveKR1020200001212A
Innovation
- Development of an electrode composition for calcium ion batteries using Fe5-xV15+XO39(OH)9ㆍyH2O, which allows for the reversible deintercalation of calcium ions, incorporating a method that mixes vanadate and iron-containing hydrate, followed by reaction to form the active material, and application to a current collector to create electrodes.
Calcium-metal alloy anode materials for reversible calcium-ion batteries
PatentActiveUS11901550B2
Innovation
- The use of intermetallic compounds comprising calcium and metals or metalloids such as Sb, As, Cu, Cd, Bi, Ag, Au, Pd, Pt, or Hg as anodes, paired with a calcium salt-based electrolyte and a cathode material like graphite, allowing for reversible decalcination and calcination reactions, and employing high-throughput density functional theory (DFT) calculations to identify suitable anode materials with high energy density and constrained volume expansion.
Materials Science Advancements for Ca-Ion Electrodes
Recent advancements in materials science have significantly propelled the development of calcium-ion battery electrodes, addressing key challenges that previously hindered their commercial viability. The unique properties of calcium, including its abundance, safety profile, and theoretical capacity, have motivated researchers to explore novel electrode materials capable of accommodating the large ionic radius and divalent nature of calcium ions.
Cathode materials have witnessed remarkable progress with the development of layered oxides, Prussian blue analogs, and polyanionic compounds. These structures offer improved calcium ion diffusion pathways and enhanced structural stability during repeated cycling. Notably, calcium cobaltites and manganese-based oxides have demonstrated promising electrochemical performance with reversible capacities exceeding 100 mAh/g and operating voltages around 3.5V vs. Ca/Ca²⁺.
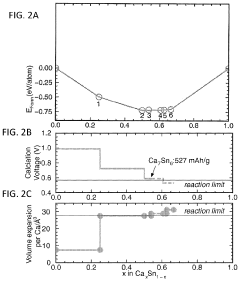
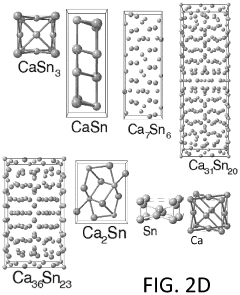
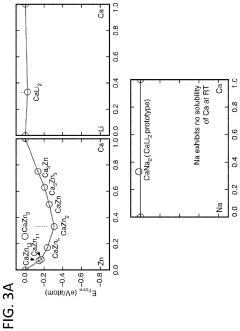
Anode development has focused on carbonaceous materials, alloys, and conversion-type electrodes. Expanded graphite and hard carbon derivatives have shown the ability to intercalate calcium ions with limited volume expansion. Calcium-tin and calcium-antimony alloys represent another promising direction, offering high theoretical capacities despite challenges with volume changes during cycling. Recent breakthroughs in nanostructuring these materials have significantly improved their cycling stability.
Interface engineering has emerged as a critical aspect of calcium-ion electrode development. The high reduction potential of calcium often leads to electrolyte decomposition and formation of passivation layers that impede ion transport. Surface modifications using atomic layer deposition and functional coatings have successfully mitigated these issues, enhancing the electrode-electrolyte interface stability and improving overall battery performance.
Computational materials design has accelerated the discovery of novel electrode materials through density functional theory calculations and molecular dynamics simulations. These approaches have enabled researchers to predict calcium ion diffusion barriers, structural stability, and electrochemical properties before experimental validation, significantly reducing development time and resources.
Nanostructuring strategies, including the synthesis of mesoporous architectures, nanoparticles, and hierarchical structures, have effectively addressed the kinetic limitations associated with calcium ion transport. These approaches provide shortened diffusion paths and increased active surface areas, resulting in enhanced rate capability and cycling stability of calcium-ion electrodes.
Cathode materials have witnessed remarkable progress with the development of layered oxides, Prussian blue analogs, and polyanionic compounds. These structures offer improved calcium ion diffusion pathways and enhanced structural stability during repeated cycling. Notably, calcium cobaltites and manganese-based oxides have demonstrated promising electrochemical performance with reversible capacities exceeding 100 mAh/g and operating voltages around 3.5V vs. Ca/Ca²⁺.
Anode development has focused on carbonaceous materials, alloys, and conversion-type electrodes. Expanded graphite and hard carbon derivatives have shown the ability to intercalate calcium ions with limited volume expansion. Calcium-tin and calcium-antimony alloys represent another promising direction, offering high theoretical capacities despite challenges with volume changes during cycling. Recent breakthroughs in nanostructuring these materials have significantly improved their cycling stability.
Interface engineering has emerged as a critical aspect of calcium-ion electrode development. The high reduction potential of calcium often leads to electrolyte decomposition and formation of passivation layers that impede ion transport. Surface modifications using atomic layer deposition and functional coatings have successfully mitigated these issues, enhancing the electrode-electrolyte interface stability and improving overall battery performance.
Computational materials design has accelerated the discovery of novel electrode materials through density functional theory calculations and molecular dynamics simulations. These approaches have enabled researchers to predict calcium ion diffusion barriers, structural stability, and electrochemical properties before experimental validation, significantly reducing development time and resources.
Nanostructuring strategies, including the synthesis of mesoporous architectures, nanoparticles, and hierarchical structures, have effectively addressed the kinetic limitations associated with calcium ion transport. These approaches provide shortened diffusion paths and increased active surface areas, resulting in enhanced rate capability and cycling stability of calcium-ion electrodes.
Environmental Impact and Sustainability Assessment
The environmental footprint of calcium ion batteries represents a critical dimension in evaluating their viability as next-generation energy storage solutions. Compared to lithium-ion technologies, calcium-based systems offer significant sustainability advantages due to calcium's greater natural abundance. Calcium ranks as the fifth most abundant element in Earth's crust at approximately 4.1% by weight, vastly exceeding lithium's 0.0017% presence. This abundance translates to reduced extraction impacts and potentially lower material costs throughout the battery lifecycle.
Mining processes for calcium compounds generally require less energy and produce fewer toxic byproducts than lithium extraction, particularly when compared to the water-intensive brine evaporation methods used in lithium mining. The water footprint of calcium extraction is estimated to be 30-40% lower per kilogram of material obtained. Additionally, calcium extraction typically disturbs smaller land areas and generates less hazardous waste, contributing to reduced ecosystem disruption.
Carbon emissions associated with calcium battery production show promising reductions compared to conventional lithium-ion manufacturing. Preliminary lifecycle assessments indicate potential greenhouse gas reductions of 15-25% when accounting for material sourcing, processing, and manufacturing phases. This advantage stems primarily from reduced energy requirements during material refinement and less complex electrolyte preparation processes.
End-of-life management presents both challenges and opportunities for calcium battery technologies. The intercalation mechanisms in calcium batteries often employ materials with higher recyclability potential than conversion-based systems. Current recycling efficiency models project recovery rates of 60-75% for key components in intercalation-type calcium batteries, though conversion mechanism batteries may present greater recycling complexities due to irreversible chemical transformations.
Safety considerations further enhance the sustainability profile of calcium systems. The lower reactivity of calcium compared to lithium reduces fire and explosion risks, potentially decreasing the environmental impact of battery failures and disposal incidents. This improved safety profile may translate to reduced containment requirements and simplified transportation regulations, further lowering the overall environmental footprint.
Toxicity assessments of common calcium battery components indicate generally lower environmental hazard profiles compared to materials in conventional lithium systems. Calcium salts typically demonstrate reduced aquatic toxicity and bioaccumulation potential, though certain electrolyte additives required for efficient calcium ion transport may introduce new environmental considerations that require further investigation.
Mining processes for calcium compounds generally require less energy and produce fewer toxic byproducts than lithium extraction, particularly when compared to the water-intensive brine evaporation methods used in lithium mining. The water footprint of calcium extraction is estimated to be 30-40% lower per kilogram of material obtained. Additionally, calcium extraction typically disturbs smaller land areas and generates less hazardous waste, contributing to reduced ecosystem disruption.
Carbon emissions associated with calcium battery production show promising reductions compared to conventional lithium-ion manufacturing. Preliminary lifecycle assessments indicate potential greenhouse gas reductions of 15-25% when accounting for material sourcing, processing, and manufacturing phases. This advantage stems primarily from reduced energy requirements during material refinement and less complex electrolyte preparation processes.
End-of-life management presents both challenges and opportunities for calcium battery technologies. The intercalation mechanisms in calcium batteries often employ materials with higher recyclability potential than conversion-based systems. Current recycling efficiency models project recovery rates of 60-75% for key components in intercalation-type calcium batteries, though conversion mechanism batteries may present greater recycling complexities due to irreversible chemical transformations.
Safety considerations further enhance the sustainability profile of calcium systems. The lower reactivity of calcium compared to lithium reduces fire and explosion risks, potentially decreasing the environmental impact of battery failures and disposal incidents. This improved safety profile may translate to reduced containment requirements and simplified transportation regulations, further lowering the overall environmental footprint.
Toxicity assessments of common calcium battery components indicate generally lower environmental hazard profiles compared to materials in conventional lithium systems. Calcium salts typically demonstrate reduced aquatic toxicity and bioaccumulation potential, though certain electrolyte additives required for efficient calcium ion transport may introduce new environmental considerations that require further investigation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!