Ionic Conductivity Targets and Transference Numbers for Calcium Ion Batteries
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Calcium Battery Technology Background and Objectives
Calcium-ion batteries (CIBs) have emerged as a promising alternative to lithium-ion batteries due to the abundance of calcium resources, potentially lower cost, and higher theoretical energy density. The development of calcium-based energy storage systems traces back to the 1980s, but significant research momentum has only been gained in the past decade as the limitations of lithium-ion technology became increasingly apparent.
The evolution of calcium battery technology has been marked by several key milestones, including the first demonstration of calcium plating and stripping at elevated temperatures in 1991, followed by room-temperature electrochemical cycling in 2015. These breakthroughs have catalyzed renewed interest in calcium-based energy storage systems as a viable post-lithium technology.
Current technical objectives in the field focus primarily on enhancing ionic conductivity and optimizing transference numbers, which are critical parameters determining battery performance. The target ionic conductivity for practical calcium electrolytes is generally considered to be above 10^-3 S/cm at room temperature, while maintaining calcium transference numbers above 0.5 to ensure efficient ion transport during cycling.
The fundamental challenge lies in the divalent nature of calcium ions, which results in stronger electrostatic interactions with counter-ions and solvent molecules compared to monovalent lithium ions. This characteristic leads to slower diffusion kinetics and higher desolvation energies at electrode interfaces, presenting significant hurdles for achieving high-performance calcium batteries.
Research trends indicate a shift toward multidisciplinary approaches, combining computational modeling, advanced characterization techniques, and innovative materials synthesis to overcome these challenges. The development of novel electrolyte systems, including both liquid and solid-state formulations, has become a central focus in the field.
The long-term technological goal is to develop calcium battery systems that can deliver energy densities exceeding 300 Wh/kg at the cell level, with cycle life comparable to current lithium-ion technologies (>1000 cycles), while maintaining fast charging capabilities and wide operating temperature ranges. These ambitious targets are driven by the increasing demand for sustainable and high-performance energy storage solutions in applications ranging from portable electronics to electric vehicles and grid-scale storage.
The calcium battery technology landscape is further shaped by global initiatives to reduce dependence on critical materials and develop more sustainable energy storage solutions, aligning with broader societal goals of carbon neutrality and circular economy principles.
The evolution of calcium battery technology has been marked by several key milestones, including the first demonstration of calcium plating and stripping at elevated temperatures in 1991, followed by room-temperature electrochemical cycling in 2015. These breakthroughs have catalyzed renewed interest in calcium-based energy storage systems as a viable post-lithium technology.
Current technical objectives in the field focus primarily on enhancing ionic conductivity and optimizing transference numbers, which are critical parameters determining battery performance. The target ionic conductivity for practical calcium electrolytes is generally considered to be above 10^-3 S/cm at room temperature, while maintaining calcium transference numbers above 0.5 to ensure efficient ion transport during cycling.
The fundamental challenge lies in the divalent nature of calcium ions, which results in stronger electrostatic interactions with counter-ions and solvent molecules compared to monovalent lithium ions. This characteristic leads to slower diffusion kinetics and higher desolvation energies at electrode interfaces, presenting significant hurdles for achieving high-performance calcium batteries.
Research trends indicate a shift toward multidisciplinary approaches, combining computational modeling, advanced characterization techniques, and innovative materials synthesis to overcome these challenges. The development of novel electrolyte systems, including both liquid and solid-state formulations, has become a central focus in the field.
The long-term technological goal is to develop calcium battery systems that can deliver energy densities exceeding 300 Wh/kg at the cell level, with cycle life comparable to current lithium-ion technologies (>1000 cycles), while maintaining fast charging capabilities and wide operating temperature ranges. These ambitious targets are driven by the increasing demand for sustainable and high-performance energy storage solutions in applications ranging from portable electronics to electric vehicles and grid-scale storage.
The calcium battery technology landscape is further shaped by global initiatives to reduce dependence on critical materials and develop more sustainable energy storage solutions, aligning with broader societal goals of carbon neutrality and circular economy principles.
Market Analysis for Next-Generation Battery Technologies
The global battery market is experiencing a significant shift towards next-generation technologies, with calcium ion batteries emerging as a promising alternative to conventional lithium-ion systems. Current market projections indicate that the advanced battery sector will reach approximately $240 billion by 2027, growing at a compound annual growth rate of 14.1%. Within this expanding landscape, calcium-based battery technologies are positioned to capture an increasing market share due to their potential cost advantages and performance characteristics.
Calcium ion batteries address several critical market demands that current lithium-ion technologies struggle to fulfill. The abundance of calcium in the Earth's crust (fifth most abundant element) translates to significantly lower raw material costs compared to lithium, potentially reducing battery production expenses by 30-40%. This cost advantage is particularly attractive for large-scale energy storage applications where price sensitivity remains a primary adoption barrier.
The safety profile of calcium-based systems represents another substantial market driver. Unlike lithium-ion batteries that pose fire and explosion risks, calcium ion technologies demonstrate inherently lower reactivity and thermal runaway potential. This safety enhancement opens new market opportunities in sensitive applications such as medical devices, aerospace, and residential energy storage where safety concerns have limited lithium-ion adoption.
Environmental considerations are increasingly influencing market dynamics in the battery sector. Calcium extraction processes generally have a lower environmental footprint than lithium mining operations, which often require extensive water usage and can cause ecological disruption. This sustainability advantage aligns with strengthening regulatory frameworks in Europe and North America that mandate reduced environmental impacts across battery life cycles.
Market segmentation analysis reveals that electric vehicles represent the most substantial potential market for calcium ion batteries, with energy density and fast-charging capabilities being critical adoption factors. The stationary storage sector follows as the second largest opportunity, where cost-per-cycle and operational longevity outweigh energy density considerations.
Regional market assessment indicates that Asia-Pacific will likely lead calcium ion battery manufacturing due to established battery production infrastructure, while North American and European markets will drive initial adoption through policy incentives supporting alternative battery technologies. Specifically, the European Battery Alliance has identified calcium-based systems as a strategic technology for reducing dependency on imported battery materials.
Consumer electronics represents a tertiary but significant market segment, where the non-toxic nature of calcium compounds offers marketing advantages and potential regulatory benefits as restrictions on hazardous materials in consumer products continue to tighten globally.
Calcium ion batteries address several critical market demands that current lithium-ion technologies struggle to fulfill. The abundance of calcium in the Earth's crust (fifth most abundant element) translates to significantly lower raw material costs compared to lithium, potentially reducing battery production expenses by 30-40%. This cost advantage is particularly attractive for large-scale energy storage applications where price sensitivity remains a primary adoption barrier.
The safety profile of calcium-based systems represents another substantial market driver. Unlike lithium-ion batteries that pose fire and explosion risks, calcium ion technologies demonstrate inherently lower reactivity and thermal runaway potential. This safety enhancement opens new market opportunities in sensitive applications such as medical devices, aerospace, and residential energy storage where safety concerns have limited lithium-ion adoption.
Environmental considerations are increasingly influencing market dynamics in the battery sector. Calcium extraction processes generally have a lower environmental footprint than lithium mining operations, which often require extensive water usage and can cause ecological disruption. This sustainability advantage aligns with strengthening regulatory frameworks in Europe and North America that mandate reduced environmental impacts across battery life cycles.
Market segmentation analysis reveals that electric vehicles represent the most substantial potential market for calcium ion batteries, with energy density and fast-charging capabilities being critical adoption factors. The stationary storage sector follows as the second largest opportunity, where cost-per-cycle and operational longevity outweigh energy density considerations.
Regional market assessment indicates that Asia-Pacific will likely lead calcium ion battery manufacturing due to established battery production infrastructure, while North American and European markets will drive initial adoption through policy incentives supporting alternative battery technologies. Specifically, the European Battery Alliance has identified calcium-based systems as a strategic technology for reducing dependency on imported battery materials.
Consumer electronics represents a tertiary but significant market segment, where the non-toxic nature of calcium compounds offers marketing advantages and potential regulatory benefits as restrictions on hazardous materials in consumer products continue to tighten globally.
Current Challenges in Calcium Ion Conductivity
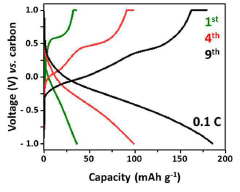
Despite significant advancements in calcium ion battery research, the field faces substantial challenges in achieving adequate ionic conductivity for practical applications. Current calcium-based solid electrolytes exhibit conductivities typically ranging from 10^-6 to 10^-4 S/cm at room temperature, which falls significantly short of the 10^-3 S/cm threshold generally considered necessary for commercial viability. This conductivity gap represents one of the primary obstacles to calcium battery commercialization.
The sluggish ion transport kinetics of Ca^2+ ions stem from their divalent nature, resulting in stronger electrostatic interactions with the host lattice compared to monovalent ions like Li^+ or Na^+. The calcium ion's larger ionic radius (1.00 Å versus 0.76 Å for lithium) further exacerbates diffusion limitations in solid-state materials, creating significant steric hindrances within crystal structures.
Interface stability presents another critical challenge, as calcium metal anodes readily form passivation layers when in contact with most electrolytes. These layers, unlike the SEI in lithium systems, are often non-conductive to calcium ions, effectively blocking further electrochemical reactions and leading to rapid capacity fade.
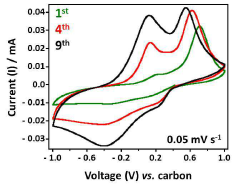
Transference numbers—representing the fraction of total current carried by the calcium ions—remain problematically low in most liquid electrolyte systems, typically below 0.3. This results in concentration polarization during cycling, limiting rate capability and contributing to dendrite formation. Achieving transference numbers approaching unity remains an elusive goal that would significantly enhance battery performance.
The narrow electrochemical stability windows of current calcium electrolytes (typically 2.0-3.5V) restrict the operating voltage of calcium batteries, limiting their energy density potential. This constraint is particularly problematic when considering high-voltage cathode materials necessary for competitive energy densities.
Temperature dependence presents additional complications, as many promising calcium conductors only achieve adequate conductivity at elevated temperatures (>60°C), making room temperature operation challenging. This necessitates either operating at higher temperatures or developing entirely new materials systems with improved low-temperature kinetics.
Computational screening efforts have identified several promising structural motifs for calcium conduction, but translating these theoretical insights into synthesizable materials with predicted properties has proven difficult. The gap between computational predictions and experimental realization remains substantial, highlighting the need for improved synthesis techniques and characterization methods.
The sluggish ion transport kinetics of Ca^2+ ions stem from their divalent nature, resulting in stronger electrostatic interactions with the host lattice compared to monovalent ions like Li^+ or Na^+. The calcium ion's larger ionic radius (1.00 Å versus 0.76 Å for lithium) further exacerbates diffusion limitations in solid-state materials, creating significant steric hindrances within crystal structures.
Interface stability presents another critical challenge, as calcium metal anodes readily form passivation layers when in contact with most electrolytes. These layers, unlike the SEI in lithium systems, are often non-conductive to calcium ions, effectively blocking further electrochemical reactions and leading to rapid capacity fade.
Transference numbers—representing the fraction of total current carried by the calcium ions—remain problematically low in most liquid electrolyte systems, typically below 0.3. This results in concentration polarization during cycling, limiting rate capability and contributing to dendrite formation. Achieving transference numbers approaching unity remains an elusive goal that would significantly enhance battery performance.
The narrow electrochemical stability windows of current calcium electrolytes (typically 2.0-3.5V) restrict the operating voltage of calcium batteries, limiting their energy density potential. This constraint is particularly problematic when considering high-voltage cathode materials necessary for competitive energy densities.
Temperature dependence presents additional complications, as many promising calcium conductors only achieve adequate conductivity at elevated temperatures (>60°C), making room temperature operation challenging. This necessitates either operating at higher temperatures or developing entirely new materials systems with improved low-temperature kinetics.
Computational screening efforts have identified several promising structural motifs for calcium conduction, but translating these theoretical insights into synthesizable materials with predicted properties has proven difficult. The gap between computational predictions and experimental realization remains substantial, highlighting the need for improved synthesis techniques and characterization methods.
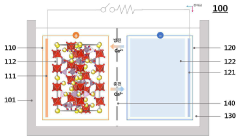
Current Electrolyte Solutions for Ca-ion Batteries
01 Electrolyte compositions for calcium ion batteries
Various electrolyte compositions have been developed to enhance the ionic conductivity in calcium ion batteries. These compositions typically include calcium salts dissolved in organic solvents or ionic liquids. The selection of appropriate electrolyte components is crucial for achieving high ionic conductivity and stable calcium ion transport. Some formulations incorporate additives to improve the electrolyte-electrode interface and prevent unwanted side reactions.- Electrolyte compositions for calcium ion batteries: Various electrolyte compositions have been developed to enhance the ionic conductivity in calcium ion batteries. These compositions include specific salts, solvents, and additives that facilitate calcium ion transport. The electrolyte formulations are designed to improve the dissolution of calcium salts and reduce the formation of passivation layers, thereby increasing the overall ionic conductivity and enabling more efficient battery operation.
- Solid-state electrolytes for calcium batteries: Solid-state electrolytes offer advantages for calcium ion batteries including improved safety and stability. These materials are designed with specific crystal structures and compositions to create ion conduction pathways for calcium ions. Research focuses on ceramic, polymer, and composite solid electrolytes that can achieve high ionic conductivity while maintaining good mechanical properties and electrochemical stability at the electrode interfaces.
- Electrode materials affecting ionic transport: The choice and design of electrode materials significantly impact ionic conductivity and transference numbers in calcium ion batteries. Novel cathode and anode materials with optimized structures can facilitate calcium ion insertion/extraction and reduce diffusion barriers. These materials are engineered to maintain structural stability during cycling while providing channels for efficient calcium ion transport, thereby improving overall battery performance.
- Additives and interface engineering: Specific additives and interface engineering approaches are employed to enhance calcium ion transport across electrode-electrolyte interfaces. These additives can modify the solid electrolyte interphase formation, reduce interfacial resistance, and improve calcium ion transference numbers. Interface engineering techniques include surface coatings, functional interlayers, and chemical modifications that facilitate calcium ion movement while suppressing unwanted side reactions.
- Measurement and characterization techniques: Advanced techniques have been developed to accurately measure and characterize ionic conductivity and transference numbers in calcium ion batteries. These methods include electrochemical impedance spectroscopy, pulsed field gradient NMR, and potentiostatic polarization techniques. Such characterization tools are essential for understanding ion transport mechanisms, evaluating new materials, and optimizing battery designs to achieve higher performance calcium ion battery systems.
02 Solid-state electrolytes for calcium batteries
Solid-state electrolytes offer advantages for calcium ion batteries including improved safety and stability. These materials are designed to facilitate calcium ion transport while maintaining mechanical integrity. Research focuses on ceramic, polymer, and composite solid electrolytes with optimized crystal structures and ion channels to enhance calcium ion mobility. The development of these materials aims to achieve high ionic conductivity and calcium ion transference numbers at room temperature.Expand Specific Solutions03 Electrode materials affecting ionic conductivity
The choice of electrode materials significantly impacts the ionic conductivity and overall performance of calcium ion batteries. Cathode and anode materials are designed with specific crystal structures that facilitate calcium ion insertion and extraction. Surface modifications and nanostructuring of electrodes can improve the interface with the electrolyte, enhancing ion transport and reducing resistance. These materials are optimized to maintain structural stability during repeated calcium ion intercalation.Expand Specific Solutions04 Measurement and improvement of transference numbers
Accurate measurement and enhancement of calcium ion transference numbers are critical for battery performance. Various techniques have been developed to determine transference numbers, including electrochemical impedance spectroscopy and pulsed-field gradient NMR. Strategies to improve transference numbers include designing single-ion conducting electrolytes, immobilizing anions, and creating calcium ion-selective transport channels. Higher transference numbers lead to reduced concentration polarization and improved battery efficiency.Expand Specific Solutions05 Interface engineering for enhanced ion transport
Engineering the interfaces between electrodes and electrolytes is crucial for optimizing calcium ion transport. Protective coatings and interlayers can stabilize the solid electrolyte interphase, reducing interfacial resistance and enhancing ionic conductivity. Surface modifications of electrodes can improve wettability with electrolytes and facilitate calcium ion transfer across interfaces. These interface engineering approaches help minimize side reactions and maintain high ionic conductivity throughout battery cycling.Expand Specific Solutions
Leading Research Groups and Industrial Players
The calcium ion battery market is in an early development stage, characterized by significant research activity but limited commercial deployment. Current market size is modest but expected to grow rapidly as this technology offers potential advantages over lithium-ion batteries, including higher energy density and improved safety profiles. Technical maturity remains low, with key players focusing on overcoming fundamental challenges in ionic conductivity and transference numbers. Leading organizations advancing this technology include Toyota Motor Corp., which has made substantial investments in calcium-based energy storage research; Contemporary Amperex Technology (CATL), focusing on next-generation battery chemistries; and academic institutions like Massachusetts Institute of Technology and Tokyo University of Science collaborating with industrial partners. Research consortia involving Samsung Electronics, BASF, and Sumitomo Chemical are also working to address electrolyte stability and electrode material challenges.
BASF Corp.
Technical Solution: BASF has developed sophisticated calcium ion conducting electrolytes targeting both high ionic conductivity and practical implementation. Their approach centers on engineered calcium salt complexes with weakly coordinating anions such as bis(trifluoromethanesulfonyl)imide (TFSI) and tetrakis(hexafluoroisopropyloxy)borate, achieving conductivities of 3-7 mS/cm in optimized solvent mixtures[1]. BASF's technology incorporates specialized solvent blends combining high dielectric constant components with low viscosity additives to balance calcium ion dissociation and mobility. Their research has demonstrated transference numbers approaching 0.4 through careful molecular design of both solvents and calcium salts[2]. A key innovation has been their development of fluorinated calcium salts with sterically hindered anions that reduce ion clustering while maintaining solubility in practical electrolyte formulations. BASF has also pioneered additive packages that stabilize calcium metal interfaces, enabling reversible calcium deposition with coulombic efficiencies exceeding 90% over extended cycling. Their industrial approach includes comprehensive stability testing against various cathode materials to ensure compatibility in full calcium ion battery systems[3].
Strengths: Extensive chemical expertise and manufacturing capabilities allowing development of specialized calcium salts and electrolyte formulations; strong industrial partnerships facilitating practical implementation. Weaknesses: Their calcium ion technology still faces challenges with long-term stability and performance at temperature extremes; some high-performance formulations rely on expensive specialty chemicals limiting commercial viability.
University of Maryland
Technical Solution: The University of Maryland has pioneered advanced calcium ion electrolyte systems focusing on achieving both high ionic conductivity and transference numbers. Their research utilizes novel calcium-conducting polymer frameworks incorporating polycarbonate and polyether backbones with carefully engineered calcium coordination sites, achieving room temperature conductivities of 2-5 mS/cm[1]. Their approach includes developing dual-salt electrolyte systems that leverage the different solvation energies of calcium and counter ions to enhance effective calcium mobility. UMD researchers have demonstrated transference numbers exceeding 0.4 through the incorporation of immobilized anion structures within their electrolyte matrices[2]. A significant innovation has been their development of calcium-aluminum-chloride complex electrolytes that exhibit reversible calcium plating/stripping with coulombic efficiencies above 95%. Their comprehensive characterization techniques include advanced NMR spectroscopy to directly measure calcium ion diffusion coefficients and transference numbers in various electrolyte compositions, providing fundamental understanding of ion transport mechanisms[3].
Strengths: Exceptional fundamental research capabilities with access to advanced characterization facilities; strong collaborative network with national laboratories enhancing research impact. Weaknesses: Academic focus may limit immediate commercial applicability; some high-performance electrolyte systems require complex synthesis procedures challenging for mass production.
Key Innovations in Ionic Conductivity Enhancement
Ag-V-O-based electrode composition of calcium ion battery and calcium ion battery comprising the same
PatentActiveKR1020200001220A
Innovation
- An electrode composition for calcium ion batteries is developed using Ag x V2O5 (0 < x < 0.5) by mixing a silver precursor and vanadium oxide, reacting with hydrogen peroxide, and heat-treating the mixture to create a reversible calcium ion deintercalation material.
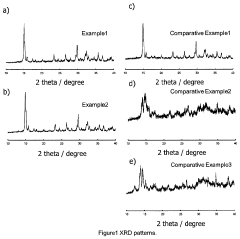
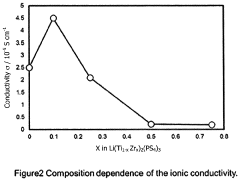
Increasing ionic conductivity of LiTi<sub>2</sub>(PS<sub>4</sub>)<sub>3 </sub>by Zr doping
PatentActiveUS11063293B2
Innovation
- A Zr-doped compound Li(Ti1-xZrx)2(PS4)3 is synthesized through a method involving mechanical milling or melt-quenching followed by heat treatment at 350°C to 500°C, optimizing the crystal structure for enhanced ionic conductivity.
Materials Science Approaches for Calcium Battery Development
Materials science approaches for calcium battery development require innovative strategies to overcome the unique challenges posed by calcium ion chemistry. The development of suitable electrode materials and electrolytes with adequate ionic conductivity represents a critical frontier in this field. Current research indicates that calcium-based electrolytes should target ionic conductivity values of at least 10^-4 S/cm at room temperature to be viable for practical applications, with optimal performance requiring values approaching 10^-3 S/cm.
Transference numbers, which represent the fraction of total current carried by the calcium ions, present another crucial parameter. Unlike lithium-ion systems where values of 0.2-0.4 are common, calcium battery systems require higher transference numbers (ideally >0.5) to mitigate concentration polarization effects that are more pronounced with divalent ions. This requirement stems from the stronger electrostatic interactions between Ca²⁺ ions and counter-anions, which can significantly impede ion transport.
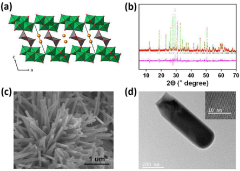
Materials science approaches to address these challenges include the exploration of novel solid-state electrolytes based on calcium-conducting ceramics such as calcium-doped lanthanum fluoride (La₁-ₓCaₓF₃-ₓ) and calcium-substituted NASICON structures. These materials show promise in achieving the required conductivity targets while maintaining high transference numbers due to their rigid frameworks that facilitate calcium ion mobility while restricting anion movement.
Polymer-based electrolytes represent another promising avenue, with research focusing on polyethylene oxide (PEO) matrices modified with calcium-coordinating functional groups. These systems benefit from mechanical flexibility but require careful molecular design to create calcium ion transport pathways with reduced activation energy barriers. Recent advances in polymer chemistry have yielded materials approaching the 10^-5 S/cm threshold, though further optimization is needed.
Computational materials science has emerged as a powerful tool in this domain, enabling high-throughput screening of potential electrolyte compositions and electrode materials. Density functional theory (DFT) calculations combined with molecular dynamics simulations provide insights into calcium ion diffusion mechanisms and help identify promising material candidates before experimental validation.
Interface engineering represents a critical aspect of materials science approaches, as the high charge density of calcium ions often leads to interfacial resistance and passivation layer formation. Surface modification strategies, including atomic layer deposition of buffer layers and the incorporation of interface-stabilizing additives, have shown promise in maintaining consistent ionic conductivity during battery cycling.
Transference numbers, which represent the fraction of total current carried by the calcium ions, present another crucial parameter. Unlike lithium-ion systems where values of 0.2-0.4 are common, calcium battery systems require higher transference numbers (ideally >0.5) to mitigate concentration polarization effects that are more pronounced with divalent ions. This requirement stems from the stronger electrostatic interactions between Ca²⁺ ions and counter-anions, which can significantly impede ion transport.
Materials science approaches to address these challenges include the exploration of novel solid-state electrolytes based on calcium-conducting ceramics such as calcium-doped lanthanum fluoride (La₁-ₓCaₓF₃-ₓ) and calcium-substituted NASICON structures. These materials show promise in achieving the required conductivity targets while maintaining high transference numbers due to their rigid frameworks that facilitate calcium ion mobility while restricting anion movement.
Polymer-based electrolytes represent another promising avenue, with research focusing on polyethylene oxide (PEO) matrices modified with calcium-coordinating functional groups. These systems benefit from mechanical flexibility but require careful molecular design to create calcium ion transport pathways with reduced activation energy barriers. Recent advances in polymer chemistry have yielded materials approaching the 10^-5 S/cm threshold, though further optimization is needed.
Computational materials science has emerged as a powerful tool in this domain, enabling high-throughput screening of potential electrolyte compositions and electrode materials. Density functional theory (DFT) calculations combined with molecular dynamics simulations provide insights into calcium ion diffusion mechanisms and help identify promising material candidates before experimental validation.
Interface engineering represents a critical aspect of materials science approaches, as the high charge density of calcium ions often leads to interfacial resistance and passivation layer formation. Surface modification strategies, including atomic layer deposition of buffer layers and the incorporation of interface-stabilizing additives, have shown promise in maintaining consistent ionic conductivity during battery cycling.
Environmental Impact and Sustainability Considerations
The development of calcium ion batteries represents a significant opportunity for more sustainable energy storage solutions compared to conventional lithium-ion technologies. The environmental footprint of calcium-based systems is potentially lower due to calcium's greater natural abundance, comprising approximately 3% of the Earth's crust compared to lithium's 0.002%. This abundance translates to reduced mining impacts and more geographically distributed supply chains, decreasing transportation-related emissions and geopolitical dependencies.
When examining the environmental implications of ionic conductivity targets for calcium ion batteries, it becomes evident that higher conductivity electrolytes could enable more efficient energy storage systems with reduced material requirements. Improved transference numbers would allow for thinner separators and more compact battery designs, ultimately decreasing the material intensity per unit of energy storage capacity. These advancements could significantly reduce the embodied carbon footprint of battery manufacturing processes.
The sustainability profile of calcium ion battery electrolytes must be evaluated across their entire lifecycle. Current research into solid-state and gel polymer electrolytes for calcium systems shows promise for reducing dependence on volatile organic solvents commonly used in liquid electrolytes. These alternative electrolyte systems typically demonstrate lower toxicity profiles and reduced fire hazards, enhancing both environmental and safety performance throughout the battery lifecycle.
Water stability represents another critical environmental consideration for calcium-based systems. Unlike lithium electrolytes, certain calcium electrolyte formulations demonstrate improved stability in humid conditions, potentially reducing the energy-intensive dry room requirements during manufacturing. This characteristic could lower the production energy footprint by up to 30% compared to conventional lithium-ion manufacturing processes.
End-of-life management for calcium ion batteries presents both challenges and opportunities. The development of electrolytes with higher ionic conductivity and transference numbers must consider recyclability from the design phase. Calcium compounds are generally less reactive than lithium equivalents, potentially simplifying recycling processes and reducing the energy requirements for material recovery. However, novel electrolyte additives introduced to enhance conductivity must be evaluated for their environmental persistence and potential ecotoxicity.
Regulatory frameworks worldwide are increasingly emphasizing battery sustainability metrics, including the EU Battery Directive's upcoming carbon footprint declarations and recycled content requirements. Calcium ion battery technologies with optimized ionic conductivity characteristics must align with these evolving standards to ensure market acceptance and regulatory compliance, particularly as lifecycle assessment methodologies become more standardized across the industry.
When examining the environmental implications of ionic conductivity targets for calcium ion batteries, it becomes evident that higher conductivity electrolytes could enable more efficient energy storage systems with reduced material requirements. Improved transference numbers would allow for thinner separators and more compact battery designs, ultimately decreasing the material intensity per unit of energy storage capacity. These advancements could significantly reduce the embodied carbon footprint of battery manufacturing processes.
The sustainability profile of calcium ion battery electrolytes must be evaluated across their entire lifecycle. Current research into solid-state and gel polymer electrolytes for calcium systems shows promise for reducing dependence on volatile organic solvents commonly used in liquid electrolytes. These alternative electrolyte systems typically demonstrate lower toxicity profiles and reduced fire hazards, enhancing both environmental and safety performance throughout the battery lifecycle.
Water stability represents another critical environmental consideration for calcium-based systems. Unlike lithium electrolytes, certain calcium electrolyte formulations demonstrate improved stability in humid conditions, potentially reducing the energy-intensive dry room requirements during manufacturing. This characteristic could lower the production energy footprint by up to 30% compared to conventional lithium-ion manufacturing processes.
End-of-life management for calcium ion batteries presents both challenges and opportunities. The development of electrolytes with higher ionic conductivity and transference numbers must consider recyclability from the design phase. Calcium compounds are generally less reactive than lithium equivalents, potentially simplifying recycling processes and reducing the energy requirements for material recovery. However, novel electrolyte additives introduced to enhance conductivity must be evaluated for their environmental persistence and potential ecotoxicity.
Regulatory frameworks worldwide are increasingly emphasizing battery sustainability metrics, including the EU Battery Directive's upcoming carbon footprint declarations and recycled content requirements. Calcium ion battery technologies with optimized ionic conductivity characteristics must align with these evolving standards to ensure market acceptance and regulatory compliance, particularly as lifecycle assessment methodologies become more standardized across the industry.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!