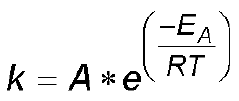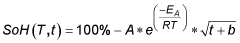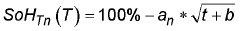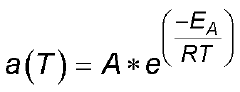Calendar Aging Mechanisms and Failure Analysis of Calcium Ion Batteries
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ca-Ion Battery Evolution and Research Objectives
Calcium-ion batteries (CIBs) have emerged as a promising alternative to lithium-ion batteries due to the abundance of calcium resources, potentially higher energy density, and improved safety characteristics. The evolution of CIBs can be traced back to the early 1990s when initial investigations into calcium-based electrochemical systems began, though significant progress has only been achieved in the past decade.
The developmental trajectory of CIBs has been marked by several key milestones. Early research focused primarily on understanding the fundamental electrochemistry of calcium ions in various electrode materials. By the mid-2000s, researchers had identified the major challenge of calcium ion transport in conventional electrolytes, which significantly limited battery performance. The breakthrough came around 2015 when novel electrolyte formulations demonstrated improved calcium ion mobility, catalyzing renewed interest in this technology.
Current technical trends in CIB development are centered on addressing several critical challenges. These include developing electrolytes with wider electrochemical stability windows, designing electrode materials capable of accommodating the larger size of calcium ions without significant structural degradation, and mitigating dendrite formation during cycling. Recent advances in computational materials science have accelerated the discovery of promising electrode materials and electrolyte compositions.
The primary research objectives in the field of CIBs focus on understanding and mitigating calendar aging mechanisms. Calendar aging refers to the degradation of battery performance during storage or idle periods, independent of cycling. For CIBs, this phenomenon is particularly complex due to the divalent nature of calcium ions and their distinct interaction with electrode materials and electrolytes compared to monovalent lithium ions.
Specific research goals include identifying the chemical and electrochemical processes responsible for capacity fade during storage, understanding the role of temperature and state-of-charge on aging rates, characterizing structural changes in electrode materials during prolonged storage, and developing predictive models for calendar life estimation. Additionally, researchers aim to establish standardized testing protocols for evaluating calendar aging in CIBs to enable meaningful comparisons across different cell chemistries and designs.
The ultimate objective is to develop CIBs with calendar lifespans comparable to or exceeding those of current lithium-ion technologies, which would be a critical step toward their commercial viability. This requires not only fundamental understanding of aging mechanisms but also innovative approaches to mitigate degradation pathways specific to calcium-based systems.
The developmental trajectory of CIBs has been marked by several key milestones. Early research focused primarily on understanding the fundamental electrochemistry of calcium ions in various electrode materials. By the mid-2000s, researchers had identified the major challenge of calcium ion transport in conventional electrolytes, which significantly limited battery performance. The breakthrough came around 2015 when novel electrolyte formulations demonstrated improved calcium ion mobility, catalyzing renewed interest in this technology.
Current technical trends in CIB development are centered on addressing several critical challenges. These include developing electrolytes with wider electrochemical stability windows, designing electrode materials capable of accommodating the larger size of calcium ions without significant structural degradation, and mitigating dendrite formation during cycling. Recent advances in computational materials science have accelerated the discovery of promising electrode materials and electrolyte compositions.
The primary research objectives in the field of CIBs focus on understanding and mitigating calendar aging mechanisms. Calendar aging refers to the degradation of battery performance during storage or idle periods, independent of cycling. For CIBs, this phenomenon is particularly complex due to the divalent nature of calcium ions and their distinct interaction with electrode materials and electrolytes compared to monovalent lithium ions.
Specific research goals include identifying the chemical and electrochemical processes responsible for capacity fade during storage, understanding the role of temperature and state-of-charge on aging rates, characterizing structural changes in electrode materials during prolonged storage, and developing predictive models for calendar life estimation. Additionally, researchers aim to establish standardized testing protocols for evaluating calendar aging in CIBs to enable meaningful comparisons across different cell chemistries and designs.
The ultimate objective is to develop CIBs with calendar lifespans comparable to or exceeding those of current lithium-ion technologies, which would be a critical step toward their commercial viability. This requires not only fundamental understanding of aging mechanisms but also innovative approaches to mitigate degradation pathways specific to calcium-based systems.
Market Potential for Calcium-Based Energy Storage
The global energy storage market is witnessing a significant transformation as the world shifts towards renewable energy sources and sustainable technologies. Within this evolving landscape, calcium-based energy storage systems represent a promising frontier with substantial market potential. Current projections indicate that the global energy storage market will reach approximately $546 billion by 2035, with next-generation battery technologies potentially capturing a significant portion of this growth.
Calcium-ion batteries (CIBs) offer several market advantages that position them as strong contenders in the energy storage sector. Their theoretical energy density approaches that of lithium-ion batteries while utilizing calcium, which is the fifth most abundant element in Earth's crust—approximately 50,000 times more abundant than lithium. This abundance translates to lower raw material costs and reduced supply chain vulnerabilities, addressing key concerns in the current lithium-dominated market.
The industrial sectors showing the greatest potential for calcium-based energy storage adoption include grid-scale energy storage, electric vehicles, and consumer electronics. For grid applications, the safety profile and potential longevity of calcium-based systems make them particularly attractive for stationary storage installations where space constraints are less restrictive than energy density requirements. Market analysis suggests that grid storage alone could represent a $150 billion opportunity by 2030.
In the electric vehicle sector, calcium-ion technology could address the range anxiety and charging speed limitations currently facing lithium-ion powered vehicles. With proper development, calcium-based batteries could potentially deliver 20-30% lower cost per kilowatt-hour compared to current lithium-ion technologies, while maintaining comparable performance metrics.
Consumer electronics represents another substantial market opportunity, with demand for longer-lasting, safer batteries continuing to grow at 8-10% annually. The non-flammable nature of calcium-based electrolytes provides a compelling safety advantage in this application space.
Regional market analysis indicates that Europe and North America are likely to lead initial adoption due to their strong focus on sustainable technologies and substantial research investments. The European Commission has specifically identified calcium-ion technology as a strategic research priority in its battery initiative programs, allocating significant funding for development efforts.
Asian markets, particularly China, Japan, and South Korea, are expected to rapidly scale manufacturing capabilities once the technology reaches commercial readiness, leveraging their established battery production infrastructure. Emerging economies in Africa and South America could benefit significantly from calcium-based storage systems for distributed energy applications, particularly given the abundance of calcium resources in these regions.
Calcium-ion batteries (CIBs) offer several market advantages that position them as strong contenders in the energy storage sector. Their theoretical energy density approaches that of lithium-ion batteries while utilizing calcium, which is the fifth most abundant element in Earth's crust—approximately 50,000 times more abundant than lithium. This abundance translates to lower raw material costs and reduced supply chain vulnerabilities, addressing key concerns in the current lithium-dominated market.
The industrial sectors showing the greatest potential for calcium-based energy storage adoption include grid-scale energy storage, electric vehicles, and consumer electronics. For grid applications, the safety profile and potential longevity of calcium-based systems make them particularly attractive for stationary storage installations where space constraints are less restrictive than energy density requirements. Market analysis suggests that grid storage alone could represent a $150 billion opportunity by 2030.
In the electric vehicle sector, calcium-ion technology could address the range anxiety and charging speed limitations currently facing lithium-ion powered vehicles. With proper development, calcium-based batteries could potentially deliver 20-30% lower cost per kilowatt-hour compared to current lithium-ion technologies, while maintaining comparable performance metrics.
Consumer electronics represents another substantial market opportunity, with demand for longer-lasting, safer batteries continuing to grow at 8-10% annually. The non-flammable nature of calcium-based electrolytes provides a compelling safety advantage in this application space.
Regional market analysis indicates that Europe and North America are likely to lead initial adoption due to their strong focus on sustainable technologies and substantial research investments. The European Commission has specifically identified calcium-ion technology as a strategic research priority in its battery initiative programs, allocating significant funding for development efforts.
Asian markets, particularly China, Japan, and South Korea, are expected to rapidly scale manufacturing capabilities once the technology reaches commercial readiness, leveraging their established battery production infrastructure. Emerging economies in Africa and South America could benefit significantly from calcium-based storage systems for distributed energy applications, particularly given the abundance of calcium resources in these regions.
Technical Barriers in Ca-Ion Battery Development
Despite significant advancements in battery technology, calcium-ion batteries face several critical technical barriers that hinder their commercial viability. The most fundamental challenge lies in the electrochemistry of calcium, particularly its high reduction potential and large ionic radius (1.0 Å) compared to lithium (0.76 Å). This larger size significantly impedes calcium ion diffusion through conventional electrode materials and electrolytes, resulting in sluggish kinetics and poor cycling performance.
Electrolyte development represents another major hurdle. Current calcium-based electrolytes suffer from limited electrochemical stability windows, high reactivity with electrode materials, and poor ionic conductivity. The formation of passivation layers on calcium metal anodes further complicates matters, as these layers are often non-conductive to calcium ions, unlike the SEI layers in lithium-ion systems which maintain lithium ion conductivity.
Cathode materials present equally challenging barriers. Most conventional intercalation cathodes cannot accommodate the larger calcium ions without significant structural distortion, leading to rapid capacity fading and structural degradation. The divalent nature of calcium ions (Ca²⁺) also creates stronger electrostatic interactions with host lattices compared to monovalent lithium ions (Li⁺), further impeding ion mobility and insertion/extraction processes.
Calendar aging mechanisms pose particular concerns for calcium-ion technology. Research indicates accelerated degradation during storage periods, with calcium plating/stripping inefficiencies and irreversible side reactions contributing to capacity loss even during idle states. These aging processes are exacerbated by temperature fluctuations and state-of-charge conditions, creating additional complexity for practical applications.
Manufacturing scalability presents additional technical barriers. Current laboratory-scale production methods for calcium-ion battery components are difficult to translate to industrial scales due to the high reactivity of calcium with moisture and oxygen. This necessitates stringent manufacturing environments that significantly increase production costs and complexity.
Safety concerns also persist, particularly regarding the thermal stability of calcium-based systems. While calcium metal is less reactive than lithium, calcium-ion batteries still demonstrate thermal runaway risks under certain conditions, requiring sophisticated battery management systems and safety features.
Addressing these technical barriers requires interdisciplinary approaches combining materials science, electrochemistry, and engineering. Recent research directions include exploring novel electrolyte formulations with calcium-conducting additives, developing hierarchically structured cathode materials with expanded ion channels, and investigating surface modification strategies to mitigate interfacial degradation mechanisms.
Electrolyte development represents another major hurdle. Current calcium-based electrolytes suffer from limited electrochemical stability windows, high reactivity with electrode materials, and poor ionic conductivity. The formation of passivation layers on calcium metal anodes further complicates matters, as these layers are often non-conductive to calcium ions, unlike the SEI layers in lithium-ion systems which maintain lithium ion conductivity.
Cathode materials present equally challenging barriers. Most conventional intercalation cathodes cannot accommodate the larger calcium ions without significant structural distortion, leading to rapid capacity fading and structural degradation. The divalent nature of calcium ions (Ca²⁺) also creates stronger electrostatic interactions with host lattices compared to monovalent lithium ions (Li⁺), further impeding ion mobility and insertion/extraction processes.
Calendar aging mechanisms pose particular concerns for calcium-ion technology. Research indicates accelerated degradation during storage periods, with calcium plating/stripping inefficiencies and irreversible side reactions contributing to capacity loss even during idle states. These aging processes are exacerbated by temperature fluctuations and state-of-charge conditions, creating additional complexity for practical applications.
Manufacturing scalability presents additional technical barriers. Current laboratory-scale production methods for calcium-ion battery components are difficult to translate to industrial scales due to the high reactivity of calcium with moisture and oxygen. This necessitates stringent manufacturing environments that significantly increase production costs and complexity.
Safety concerns also persist, particularly regarding the thermal stability of calcium-based systems. While calcium metal is less reactive than lithium, calcium-ion batteries still demonstrate thermal runaway risks under certain conditions, requiring sophisticated battery management systems and safety features.
Addressing these technical barriers requires interdisciplinary approaches combining materials science, electrochemistry, and engineering. Recent research directions include exploring novel electrolyte formulations with calcium-conducting additives, developing hierarchically structured cathode materials with expanded ion channels, and investigating surface modification strategies to mitigate interfacial degradation mechanisms.
Current Calendar Aging Mitigation Strategies
01 Electrolyte composition effects on calendar aging
The composition of electrolytes significantly impacts the calendar aging of calcium ion batteries. Specific electrolyte additives can form stable protective films on electrode surfaces, reducing parasitic reactions during storage. Optimized electrolyte formulations with appropriate salt concentrations and solvent mixtures can minimize degradation mechanisms such as calcium plating and dendrite formation during idle periods, thereby extending battery lifespan and maintaining capacity retention during long-term storage.- Electrolyte composition effects on calendar aging: The composition of electrolytes significantly impacts the calendar aging of calcium ion batteries. Specific electrolyte additives can form stable protective films on electrode surfaces, reducing parasitic reactions during storage. Optimized electrolyte formulations with appropriate salt concentrations and solvent mixtures can mitigate degradation mechanisms such as calcium plating and dendrite formation during idle periods, thereby extending battery lifespan and maintaining capacity during storage.
- Temperature-dependent aging mechanisms: Temperature plays a crucial role in the calendar aging of calcium ion batteries. Higher storage temperatures accelerate degradation processes including electrolyte decomposition and interfacial layer growth. The rate of capacity fade during storage follows Arrhenius behavior, with specific activation energies for different aging mechanisms. Temperature fluctuations can cause mechanical stress in electrode materials, leading to microstructural changes that affect long-term performance and reliability of calcium ion battery systems.
- State of charge influence on degradation: The state of charge (SOC) during storage significantly affects calendar aging in calcium ion batteries. Higher SOC levels during idle periods accelerate side reactions at electrode-electrolyte interfaces, leading to increased capacity fade and impedance growth. Storing calcium ion batteries at moderate SOC levels (30-50%) can minimize degradation mechanisms such as calcium plating, electrolyte oxidation, and structural changes in cathode materials, thereby optimizing battery longevity during extended storage periods.
- Electrode surface film formation and evolution: The formation and evolution of surface films on calcium ion battery electrodes is a primary calendar aging mechanism. During storage, spontaneous reactions between electrode materials and electrolyte components form interfacial layers that grow over time. These surface films affect calcium ion diffusion kinetics and charge transfer processes, leading to increased internal resistance. The chemical composition and morphology of these films evolve during storage, with factors such as electrolyte impurities and trace water content accelerating film growth and degradation processes.
- Advanced characterization and modeling of aging processes: Advanced characterization techniques and modeling approaches are essential for understanding calcium ion battery calendar aging mechanisms. Non-destructive methods such as electrochemical impedance spectroscopy can track impedance changes during storage, while post-mortem analyses reveal microstructural and chemical alterations. Physics-based models incorporating degradation kinetics help predict calendar life under various storage conditions. Machine learning algorithms analyzing battery aging data enable more accurate lifetime predictions and development of strategies to mitigate calendar aging effects in calcium ion battery systems.
02 Temperature-dependent aging mechanisms
Temperature plays a crucial role in the calendar aging of calcium ion batteries. Higher storage temperatures accelerate degradation processes through increased reaction kinetics, leading to faster capacity fade and resistance growth. The aging rate typically follows Arrhenius behavior, with specific temperature thresholds triggering different degradation pathways. Thermal management strategies and temperature-optimized cell designs can significantly mitigate these effects, particularly by controlling electrolyte decomposition and interfacial layer growth at elevated temperatures.Expand Specific Solutions03 State of charge influence on degradation
The state of charge (SOC) at which calcium ion batteries are stored significantly affects their calendar aging behavior. Higher SOC levels during storage typically accelerate degradation through enhanced electrolyte decomposition and cathode material dissolution. Storing batteries at intermediate SOC levels can minimize these effects by reducing the electrochemical potential difference across cell components. Voltage stress during storage contributes to structural changes in electrode materials, particularly affecting the calcium insertion/extraction mechanisms and stability of the electrode-electrolyte interfaces.Expand Specific Solutions04 Surface film formation and evolution
The formation and evolution of surface films on calcium ion battery electrodes represent a critical calendar aging mechanism. These interfacial layers, similar to the solid electrolyte interphase (SEI) in lithium-ion batteries, continuously grow during storage, increasing internal resistance and impeding calcium ion transport. The chemical composition and morphology of these films evolve over time, influenced by storage conditions and electrolyte components. Understanding and controlling this surface film evolution is essential for developing calcium ion batteries with improved calendar life and performance stability.Expand Specific Solutions05 Advanced characterization and modeling techniques
Advanced characterization and modeling techniques are essential for understanding calcium ion battery calendar aging mechanisms. Non-destructive methods such as electrochemical impedance spectroscopy can track resistance changes during storage, while post-mortem analyses reveal chemical and structural transformations. Machine learning approaches enable prediction of aging behavior based on storage conditions, helping to establish accelerated testing protocols. Physics-based models incorporating multiple degradation pathways provide insights into the complex interplay of aging mechanisms, supporting the development of mitigation strategies and lifetime prediction tools.Expand Specific Solutions
Leading Research Institutions and Industrial Partners
The calcium ion battery technology landscape is currently in an early development stage, with a growing market potential driven by the need for sustainable energy storage solutions. The competitive landscape features a mix of established players and emerging innovators. Major battery manufacturers like CATL, EVE Energy, and LG Energy Solution are investing in calcium ion technology research as a potential alternative to lithium-ion batteries. Automotive companies including Volkswagen, Mercedes-Benz, and NIO are exploring this technology to diversify their battery supply chains. Research institutions such as Beihang University and Newcastle University are making significant contributions to understanding calendar aging mechanisms. The technology remains at a relatively low maturity level, with companies like Siemens, ABB, and Microvast working to address challenges in electrolyte formulation, electrode materials, and cycle stability before commercial viability can be achieved.
EVE Energy Co., Ltd.
Technical Solution: EVE Energy has developed a specialized calendar aging analysis framework for calcium ion batteries that focuses on the unique challenges of divalent calcium ion transport. Their technical approach combines differential voltage analysis (DVA) with periodic capacity retention measurements to identify specific degradation signatures. EVE's research has revealed that calcium ion batteries exhibit distinct calendar aging patterns compared to lithium-ion systems, particularly regarding electrolyte decomposition products and their impact on ion transport. The company has engineered novel calcium salt complexes that demonstrate improved stability during storage conditions, reducing the formation of passivation layers that typically impede calcium ion diffusion. Their testing protocols incorporate storage at various depth-of-discharge levels (0-100%) to map the state-dependent degradation mechanisms, with findings indicating that partial state-of-charge storage (30-70%) minimizes calendar aging effects in calcium-based systems.
Strengths: Strong expertise in electrolyte formulation specifically optimized for divalent ion transport; established manufacturing capabilities that could be adapted for calcium ion production. Weaknesses: Current calcium ion prototypes still demonstrate lower energy density compared to commercial lithium-ion alternatives, creating challenges for market adoption despite improved calendar life.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: CATL has developed comprehensive calendar aging analysis protocols for calcium ion batteries that combine electrochemical impedance spectroscopy (EIS) and post-mortem analysis techniques. Their approach involves systematic storage tests at various temperatures (5-60°C) and state-of-charge levels to map degradation patterns specific to calcium ion chemistry. CATL's research has identified that calcium ion batteries experience unique calendar aging mechanisms including calcium plating on the anode, electrolyte decomposition at the calcium metal interface, and structural changes in cathode materials during prolonged storage. Their proprietary electrolyte formulations containing fluorinated solvents have demonstrated significant improvement in calendar life by forming more stable solid electrolyte interphase (SEI) layers that suppress parasitic reactions during storage periods.
Strengths: Industry-leading battery manufacturing scale allows for extensive real-world validation data collection; advanced in-situ characterization capabilities for monitoring degradation mechanisms. Weaknesses: Calcium ion technology still faces challenges with electrolyte stability at higher temperatures, limiting practical implementation in certain applications requiring extreme temperature resilience.
Critical Failure Mechanism Analysis and Characterization
Method for determining a calendar aging of a battery
PatentPendingDE102020203388A1
Innovation
- Determine capacity curves under varying charge levels and temperatures, identify differential profiles to detect cathodic side reactions, and use conversion factors to predict battery failure times at different temperatures, considering both anodic and cathodic side reactions.
Method of estimation of battery degradation
PatentPendingCA3192566A1
Innovation
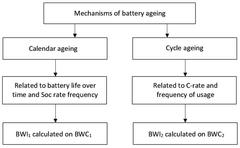
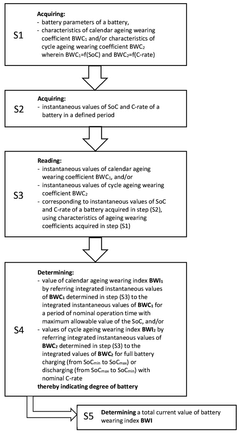
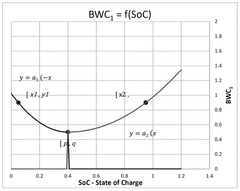
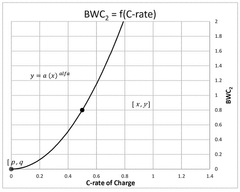
- A computer-implemented method that acquires and calculates battery parameters, including calendar and cycle ageing wearing coefficients, to determine instantaneous values of State of Charge (SoC) and charging/discharging rate (C-rate), and uses machine learning algorithms to update these coefficients based on historical data, providing a more accurate estimation of battery degradation.
Environmental Impact Assessment of Ca-Ion Technology
The environmental impact assessment of calcium-ion battery technology reveals promising advantages over conventional lithium-ion systems. Calcium-ion batteries utilize earth-abundant calcium resources, which are approximately 2,000 times more plentiful in the Earth's crust than lithium. This abundance significantly reduces extraction-related environmental concerns and mitigates geopolitical supply chain risks associated with critical materials.
Life cycle assessment studies indicate that calcium-ion battery production could potentially reduce carbon emissions by 15-20% compared to lithium-ion batteries, primarily due to less energy-intensive mining and refining processes. The calcium extraction process typically requires less water consumption and generates fewer toxic byproducts than lithium extraction from brines or hard rock mining, particularly avoiding the extensive evaporation ponds that characterize lithium production in South America.
Regarding end-of-life considerations, calcium compounds present fewer environmental hazards than lithium or heavy metal alternatives. Initial research suggests that calcium-ion battery recycling could be more straightforward than for lithium-ion batteries, with potentially higher recovery rates for calcium compounds. The lower reactivity of calcium compared to lithium also reduces fire and explosion risks during recycling processes.
However, several environmental challenges remain unresolved. The calendar aging mechanisms of calcium-ion batteries may lead to electrolyte degradation products whose environmental toxicity profiles are not yet fully characterized. Additionally, current calcium-ion battery prototypes often employ fluorinated electrolytes, which pose potential environmental concerns if released during production, use, or disposal phases.
Manufacturing scale-up considerations reveal that while calcium processing requires less energy than lithium, the higher operating temperatures needed for some calcium-ion battery components could partially offset these energy savings. The environmental impact of specialized electrolyte additives required to manage calcium's unique electrochemical properties also requires further assessment.
Regulatory frameworks for calcium-ion battery disposal and recycling remain underdeveloped, creating uncertainty about future environmental compliance requirements. As calendar aging mechanisms continue to be investigated, particular attention should be directed toward understanding how degradation products might impact soil and water systems if improperly managed.
Overall, while calcium-ion technology demonstrates promising environmental advantages, comprehensive cradle-to-grave assessments are needed as the technology matures to ensure that calendar aging and failure mechanisms do not introduce unforeseen environmental liabilities.
Life cycle assessment studies indicate that calcium-ion battery production could potentially reduce carbon emissions by 15-20% compared to lithium-ion batteries, primarily due to less energy-intensive mining and refining processes. The calcium extraction process typically requires less water consumption and generates fewer toxic byproducts than lithium extraction from brines or hard rock mining, particularly avoiding the extensive evaporation ponds that characterize lithium production in South America.
Regarding end-of-life considerations, calcium compounds present fewer environmental hazards than lithium or heavy metal alternatives. Initial research suggests that calcium-ion battery recycling could be more straightforward than for lithium-ion batteries, with potentially higher recovery rates for calcium compounds. The lower reactivity of calcium compared to lithium also reduces fire and explosion risks during recycling processes.
However, several environmental challenges remain unresolved. The calendar aging mechanisms of calcium-ion batteries may lead to electrolyte degradation products whose environmental toxicity profiles are not yet fully characterized. Additionally, current calcium-ion battery prototypes often employ fluorinated electrolytes, which pose potential environmental concerns if released during production, use, or disposal phases.
Manufacturing scale-up considerations reveal that while calcium processing requires less energy than lithium, the higher operating temperatures needed for some calcium-ion battery components could partially offset these energy savings. The environmental impact of specialized electrolyte additives required to manage calcium's unique electrochemical properties also requires further assessment.
Regulatory frameworks for calcium-ion battery disposal and recycling remain underdeveloped, creating uncertainty about future environmental compliance requirements. As calendar aging mechanisms continue to be investigated, particular attention should be directed toward understanding how degradation products might impact soil and water systems if improperly managed.
Overall, while calcium-ion technology demonstrates promising environmental advantages, comprehensive cradle-to-grave assessments are needed as the technology matures to ensure that calendar aging and failure mechanisms do not introduce unforeseen environmental liabilities.
Comparative Performance Metrics Against Conventional Batteries
When evaluating calcium ion batteries against conventional battery technologies, several key performance metrics must be considered to understand their competitive positioning in the energy storage landscape.
Energy density represents a critical advantage for calcium ion batteries, which theoretically can achieve 2.0-2.5 times higher volumetric capacity compared to lithium-ion batteries. This is primarily due to calcium's divalent nature, allowing for the transfer of two electrons per ion during electrochemical reactions. However, current prototype calcium batteries have not yet reached this theoretical potential due to electrolyte limitations and electrode material constraints.
Cycle life measurements indicate that calcium ion batteries currently demonstrate 500-800 cycles before reaching 80% capacity retention, which falls short of commercial lithium-ion batteries that typically achieve 1,000-2,000 cycles. The accelerated capacity fading is largely attributed to calcium plating issues and electrolyte decomposition at the electrode interfaces.
Power density metrics reveal another challenge, with calcium ion batteries exhibiting slower kinetics compared to lithium-ion counterparts. The larger ionic radius of Ca²⁺ (1.00 Å) versus Li⁺ (0.76 Å) results in reduced diffusion rates within electrode materials, leading to lower rate capabilities, particularly at high discharge rates.
Safety performance represents a significant advantage for calcium ion batteries. They demonstrate superior thermal stability with decomposition temperatures typically 50-80°C higher than conventional lithium-ion cells. Additionally, calcium-based systems show reduced risk of thermal runaway and are less prone to combustion when physically damaged.
Cost analysis indicates potential economic benefits, with calcium being approximately 20 times more abundant in the Earth's crust than lithium. Raw material costs for calcium-based cathodes and anodes are estimated to be 30-40% lower than their lithium counterparts, though manufacturing processes remain more expensive due to the need for specialized handling of moisture-sensitive components.
Operating temperature range tests show calcium ion batteries maintain reasonable performance between -10°C and 60°C, comparable to many commercial battery technologies. However, they exhibit more significant capacity loss at extreme temperatures, particularly below 0°C, where ionic conductivity becomes severely limited.
Environmental impact assessments reveal calcium ion batteries offer reduced toxicity profiles compared to lead-acid batteries and avoid the use of critical raw materials found in some lithium-ion and nickel-metal hydride batteries, potentially offering a more sustainable alternative as manufacturing scales.
Energy density represents a critical advantage for calcium ion batteries, which theoretically can achieve 2.0-2.5 times higher volumetric capacity compared to lithium-ion batteries. This is primarily due to calcium's divalent nature, allowing for the transfer of two electrons per ion during electrochemical reactions. However, current prototype calcium batteries have not yet reached this theoretical potential due to electrolyte limitations and electrode material constraints.
Cycle life measurements indicate that calcium ion batteries currently demonstrate 500-800 cycles before reaching 80% capacity retention, which falls short of commercial lithium-ion batteries that typically achieve 1,000-2,000 cycles. The accelerated capacity fading is largely attributed to calcium plating issues and electrolyte decomposition at the electrode interfaces.
Power density metrics reveal another challenge, with calcium ion batteries exhibiting slower kinetics compared to lithium-ion counterparts. The larger ionic radius of Ca²⁺ (1.00 Å) versus Li⁺ (0.76 Å) results in reduced diffusion rates within electrode materials, leading to lower rate capabilities, particularly at high discharge rates.
Safety performance represents a significant advantage for calcium ion batteries. They demonstrate superior thermal stability with decomposition temperatures typically 50-80°C higher than conventional lithium-ion cells. Additionally, calcium-based systems show reduced risk of thermal runaway and are less prone to combustion when physically damaged.
Cost analysis indicates potential economic benefits, with calcium being approximately 20 times more abundant in the Earth's crust than lithium. Raw material costs for calcium-based cathodes and anodes are estimated to be 30-40% lower than their lithium counterparts, though manufacturing processes remain more expensive due to the need for specialized handling of moisture-sensitive components.
Operating temperature range tests show calcium ion batteries maintain reasonable performance between -10°C and 60°C, comparable to many commercial battery technologies. However, they exhibit more significant capacity loss at extreme temperatures, particularly below 0°C, where ionic conductivity becomes severely limited.
Environmental impact assessments reveal calcium ion batteries offer reduced toxicity profiles compared to lead-acid batteries and avoid the use of critical raw materials found in some lithium-ion and nickel-metal hydride batteries, potentially offering a more sustainable alternative as manufacturing scales.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!